Introduction
Chemotherapy remains an important approach for
cancer therapy. However, development of drug resistance during
chemotherapy constitutes a predominant challenge in cancer
treatment (1). A previous study
demonstrated that nuclear factor-κB (NF-κB) activation is one
mechanism by which tumors become resistant to chemotherapeutic
agents (2).
The NF-κB family contains five members: c-Rel, RelA
(p65), RelB, p50 and p52. In its inactive state, NF-κB resides in
the cytoplasm due to inhibitory binding by the IκBa protein. Upon
activation, IκBa undergoes phosphorylation and
ubiquitination-dependent degradation, leading to p65 nuclear
translocation and binding to a specific consensus sequence in the
DNA, which then activates transcription of target genes. Previous
studies have shown that NF-κB may be activated by anticancer
chemotherapeutic compounds in a number of cancer cell lines
(3–5) and activation of NF-κB attenuates
apoptosis by regulating inhibitors of apoptosis, including c-IAP1,
c-IAP2, TRAF1, TRAF2, survivin and Bcl-xL (5–7).
Thus, a number of strategies have been employed to enhance
apoptosis induced by chemotherapeutic compounds via NF-κB
inhibition.
Irinotecan (CPT-11) and its more active metabolite,
SN38, are topoisomerase I inhibitors that are efficacious in the
treatment of specific neoplasms, including colorectal cancer
(8). CPT-11 treatment has been
shown to lead to the activation of NF-κB in a variety of human
colorectal cancer cell lines (7).
Several methods of inhibiting NF-κB activation, including antisense
oligonucleotides, proteasome inhibitors and p65 small interfering
RNA (siRNA) have been shown to reverse inducible chemotherapy
resistance to CPT-11 (9–13).
Berberine, a botanical alkaloid derived from a plant
that is used traditionally in Chinese medicine, has been reported
to exhibit multiple biological and pharmacological properties.
Berberine has potential as a chemotherapy adjuvant due to its low
toxicity and anticancer properties (14). A previous study demonstrated that
berberine potentiates apoptosis induced by 5-fluorouracil (5-FU)
and doxorubicin through suppression of NF-κB activation (15). However, the effect of berberine on
CPT-11-induced apoptosis and the underlying mechanisms have not
been investigated. In the present study, berberine was observed to
suppress CPT-11-induced NF-κB activation in a dose-dependent manner
and significantly enhanced the sensitization of HCT116 cells to
CPT-11-induced apoptosis by downregulating the NF-κB target
antiapoptotic genes, c-IAP1, c-IAP2, survivin and Bcl-xL. These
results indicate that berberine enhances chemosensitivity to CPT-11
through the inhibition of NF-κB activation.
Materials and methods
Materials
CPT-11, berberine, sulforhodamine B (SRB),
trichloroacetic acid (TCA), acetic acid, tumor necrosis factor
(TNF)-α, anti-β-actin and dimethylsulfoxide (DMSO) were obtained
from Sigma-Aldrich (St. Louis, MO, USA). TRIzol and cell culture
reagents were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). Antibodies against p65, c-IAP1, c-IAP2,
survivin and Bcl-xL were obtained from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Secondary antibodies for western blotting
were obtained from Amersham Biosciences Biotech. (Piscataway, NJ,
USA). All other reagents were obtained from Sigma-Aldrich unless
stated otherwise.
Transfection of siRNA
Human colon cancer HCT 116 cells were obtained from
ATCC (Manassas, VA, USA) and were seeded at 2×105
cells/ml in 6-well. Cells plates were grown to 50% confluency and
transfected with double-stranded siRNA for NF-κB p65 target
sequence (sense: 5′-CUUCCAAGUUCCUAUAGAAdTdT-3′ and antisense:
3′-dTdTGAAGGUUCAAGGAUAUCUU-5′) or with a siRNA nonspecific control
(Guangzhou Ribobio Co. Ltd., Guangzhou, China). Silencing was
confirmed by the electrophoretic mobility shift assay (EMSA) and
western blotting.
SRB assay
Cytotoxicity was determined by the SRB assay as
described previously (16). Cells
were seeded into 96-well plates and exposed to various
concentrations of berberine with or without CPT-11. Following 48-h
incubation, cells were fixed with TCA for 1 h at 4°C, air-dried and
stained with 0.4% SRB solution for 30 min at room temperature.
Following staining, the SRB solution was removed and cells were
subsequently washed with 1% acetic acid five times. Tris base
solution (10 mM; pH 10.5) was used to dissolve the protein-bound
dye and the plate was agitated on a plate shaker (Nangjing
Changxiang Co. Ltd., Nangjing, China) for 10 min. The
OD570 was determined using a 96-well plate reader (MRX;
Dynex Technologies, Chantilly, VA, USA).
Western blotting
HCT116 cells were seeded into 6-well plates and
exposed to the indicated concentrations of berberine with or
without CPT-11. Following treatment, cells were harvested using
lysis buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM
EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM
Na3VO4, 1 mM β-glycerophosphate and 1:1,000
protease inhibitors]. Protein concentrations were determined by the
bicinchoninic acid method. Total protein (25 μg) was separated
using 8–12% sodium dodecyl sulfate-polyacrylamide gels and
transferred to nitrocellulose blotting membranes. Monoclonal
antibodies against p65 (1:2,000), c-IAP1 (1:500), c-IAP2 (1:500) or
β-actin (1:8,000) were used. Immunopositive bands were visualized
using the Amersham ECL™ Plus Western Blotting Detection kit (GE
Healthcare, Piscataway, NJ, USA).
EMSA
EMSA was performed as previously described (17,18).
Biotin 3′ end-labeled DNA probes containing the NF-κB consensus
site, 3′-TCAACTCCCCTGAAAGGGTCCG-5′ and
5′-AGTTGAGGGGACTTTCCCAGGC-3′, were purchased from Invitrogen Life
Technologies. EMSA was performed using the LightShift
Chemiluminescent EMSA kit (Pierce, Rockford, IL, USA). NF-κB
activation induced by TNF-α is considered as a positive control.
Cells were treated with 20 ng/ml TNF-α for 30 min, TNF-α for 30 min
plus 100X unlabeled NF-κB probes or cells were exposed to unlabeled
NF-κB probes. Briefly, the nuclear proteins were incubated in 1X
binding buffer, 50 ng/μl poly(dI-dC), 0.05% NP-40, 5 mM
MgCl2, 50 mM KCl, 2.5% glycerol and ddH2O for
20 min at room temperature in a total volume of 20 μl. The reaction
mixture was separated in a 6% nondenaturing polyacrylamide gel and
transferred to a positively charged nylon membrane. The membrane
was cross-linked and the biotin-labeled DNA was detected by
chemiluminescence.
Hoechst 33258 staining
Hoescht 33258 staining was performed as previously
described (19). Cells were
treated with different concentrations of berberine (2.5–10 μM) for
48 h with or without 20 μM CPT-11 for 24 h and the cells were fixed
with fixation fluid and washed with phosphate-buffered saline
(PBS). Subsequently, Hoechst 33258 was used to stain the cells for
5 min and the cells were then washed with PBS three times. Cell
apoptosis was observed by fluorescent microscopy (Olympus, Tokyo,
Japan).
Assessment of apoptotic and necrotic cell
death
Apoptotic and necrotic cell death was assessed as
previously described (20,21). The effect of berberine on
CPT-11-induced apoptosis was evaluated by Annexin V/propidium
iodide (PI) double staining and flow cytometry. HCT-116 cells were
incubated with 10 μM berberine for 48 h and/or 20 μM CPT-11 for 24
h. Cells were harvested and resuspended in binding buffer [10 Mm
HEPES (pH 7.4), 150 mM NaCl, 5 mM KCl, 1 mM MgCl2 and
1.8 mM CaCl2) followed by Annexin V-flurescein
isothiocyanate (FITC) and PI. The percentages of viable, early
apoptotic, late apoptotic and necrotic cells were analyzed with a
FACScan flow cytometer (Becton Dickinson, San Jose, CA, USA).
Statistical analysis
Statistical analysis between groups was performed by
unpaired Student's t-test with SigmaPlot 10.0 software (Systat
Software, Inc., San Jose, CA, USA). Data are presented as the mean
± SEM. P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of CPT-11 on cell viability in
HCT116 cells
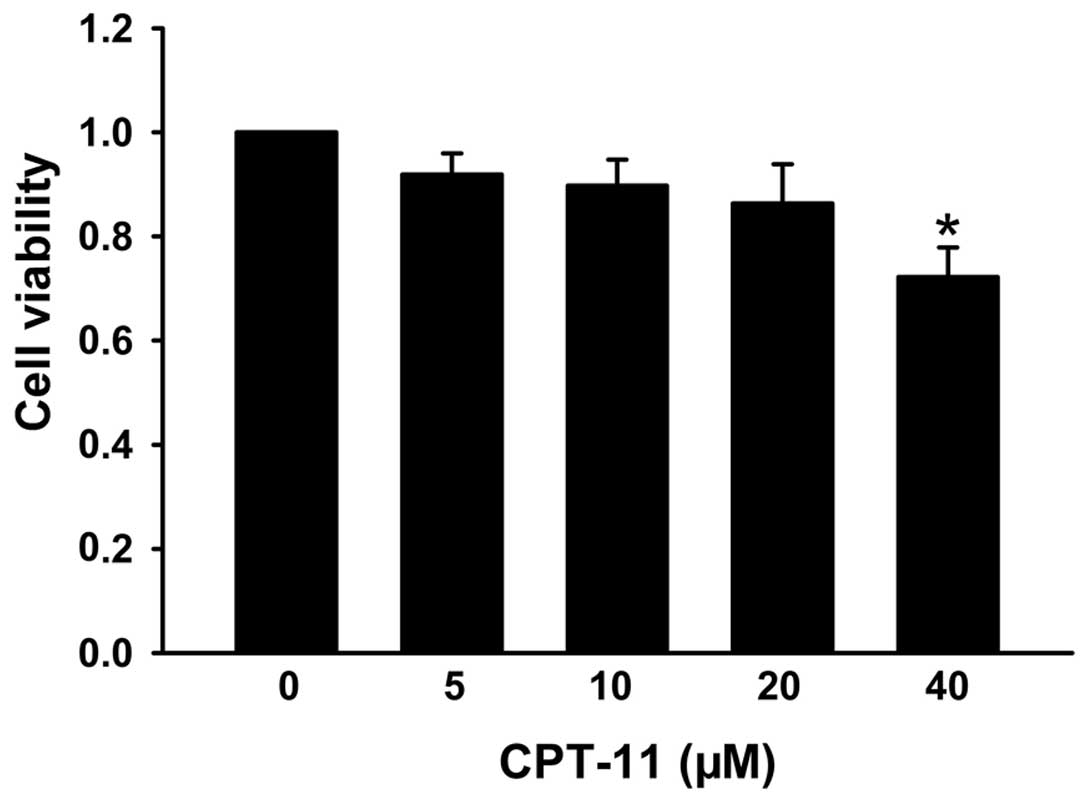
The SRB assay was used to detect the cytotoxic
effects of CPT-11 in the HCT116 colon cancer cell line. Cells were
treated with CPT-11 for 24 h at concentrations of 5, 10, 20, 40 μM.
Fig. 1 shows that CPT-11 did not
affect the viability of HCT116 cells below 40 μM.
Inhibition of NF-κB activation enhances
chemosensitivity to CPT-11 in HCT116 cells
It has been shown that inducible resistance to
CPT-11 may be reversed by inhibiting NF-κB (9–13).
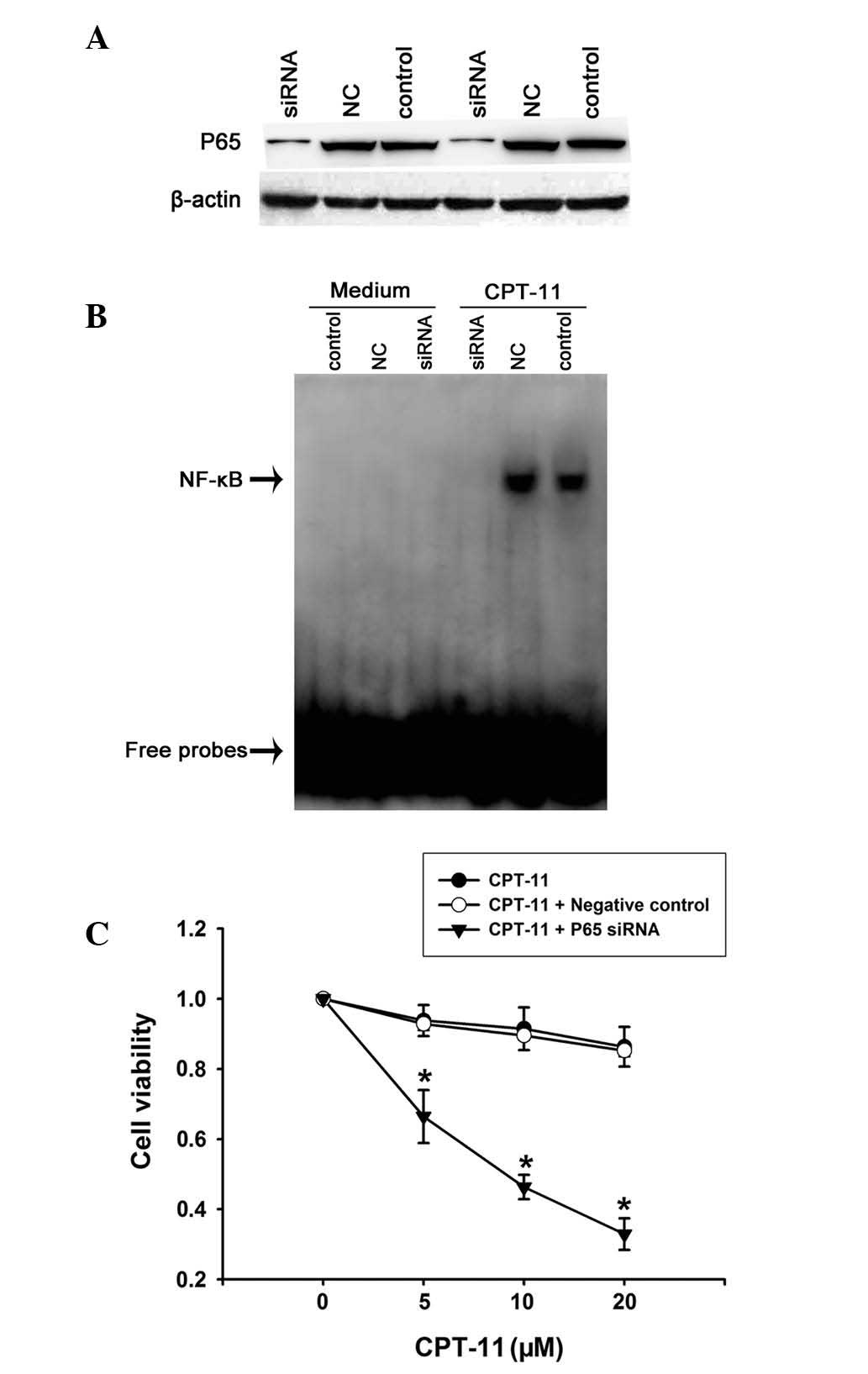
To confirm this effect, p65 siRNA was used in the present study.
HCT116 cells were transfected with p65 siRNA for 48 h, followed by
treatment with 20 μM CPT-11 for 2 h. Western blot analysis
confirmed that the expression of p65 was reduced in cells treated
with p65 siRNA relative to their untreated counterparts and siRNA
negative controls (Fig. 2A).
Consistent with the downregulation of p65 expression, NF-κB
activation was shown by EMSA to be significantly inhibited by the
siRNAs (Fig. 2B).
To address whether the inhibition of NF-κB enhances
chemosensitivity to CPT-11 in HCT116 cells, the effect of p65 siRNA
on cell viability of CPT-11-treated cells was determined. Following
48-h transfection with p65 siRNA, HCT116 cells were incubated with
CPT-11 (5–20 μM) for 24 h. The siRNA studies showed that p65 siRNA
suppresses CPT-11-induced NF-κB activation and enhances the
chemosensitivity to CPT-11 in HCT116 cells (Fig. 2C). This is in agreement with the
hypothesis that inducible resistance to CPT-11 may be reversed by
inhibition of NF-κB.
Berberine enhances chemosensitivity to
CPT-11 in HCT116 cells
The results in Fig.
2 confirmed that the inhibition of NF-κB reverses inducible
resistance to CPT-11. Berberine has been reported to inhibit NF-κB
activation in several cell lines (15). Thus, the effect of berberine on the
cytotoxicity of CPT-11 in HCT116 cells was investigated.
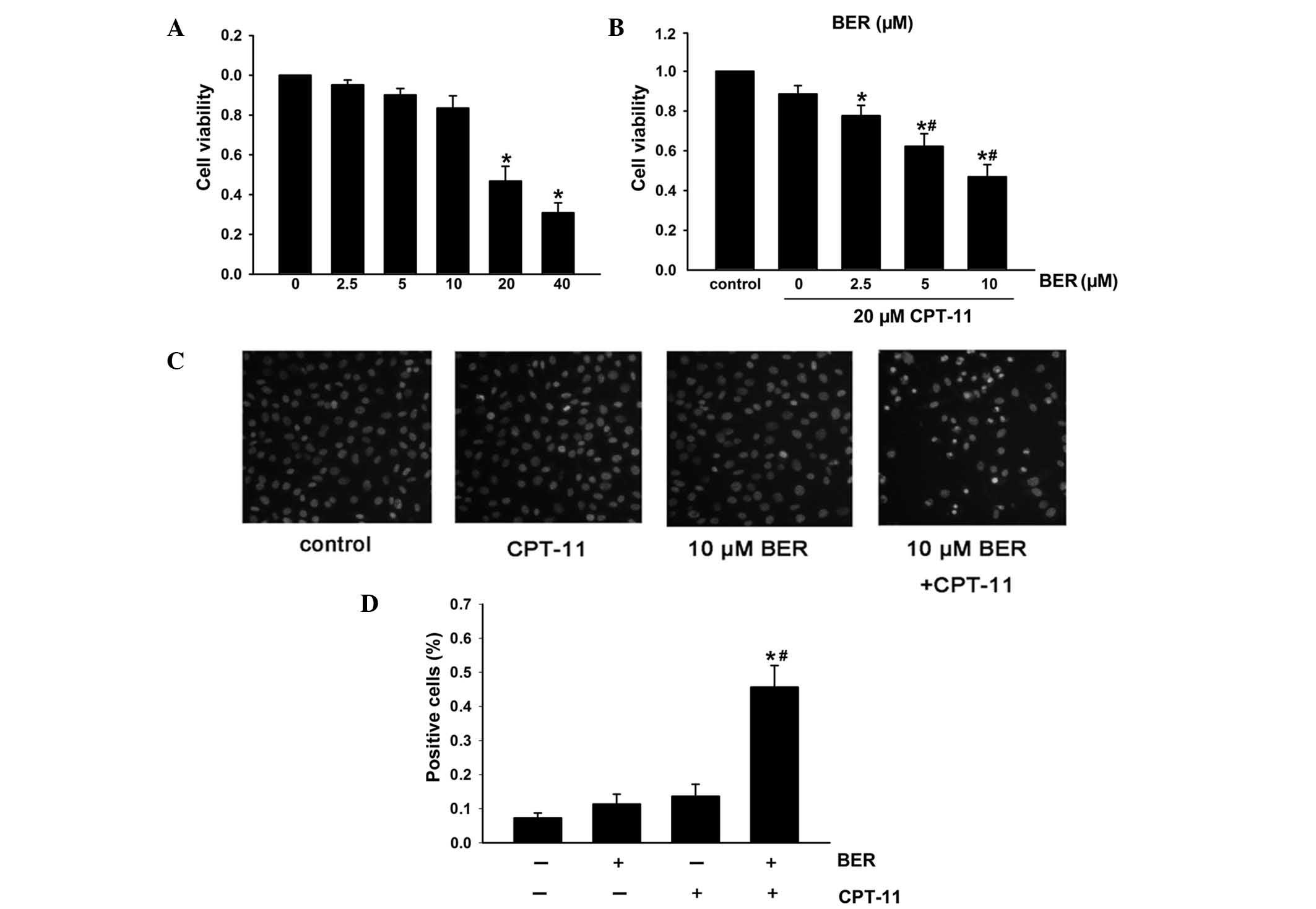
The effect of berberine on the cell viability in
HCT116 cells was observed. Fig. 3A
shows that berberine had no effect on cell viability up to a
concentration of 20 μM; thus, the non-cytotoxic concentrations of
berberine, between 2.5 and 10 μM were used in the subsequent
expriments. To observe the effect of berberine on the cytotoxicity
of CPT-11 cells, cells were incubated with berberine (2.5–10 μM)
for 48 h, followed by incubation with 20 μM CPT-11 for 24 h. The
results in Fig. 3B demonstrated
that the chemosensitivity to CPT-11 was enhanced by berberine,
leading to a significant increase in the inhibition rate from
11.42±2.3% (20 μM CPT-11 alone) to 49.06±3.8% (10 μM berberine plus
20 μM CPT-11). Cell apoptosis was detected using Hoechst 33258 and
Annexin V/PI staining and the results were consistent with the SRB
assay. As shown in Fig. 3C,
combination treatment of berberine and CPT-11 in HCT116 cells
resulted in an increased number of cells with condensed and
fragmented nuclei than CPT-11 or berberine treatment alone. Similar
results were confirmed by Annexin V/PI staining (Fig. 3D). These results indicate that
berberine enhances the sensitization of HCT116 cells to
CPT-11-induced apoptosis.
Berberine inhibits NF-κB activation
induced by CPT-11
Previous studies have shown that the NF-κB pathway
is activated in HCT116 cells in response to CPT-11 treatment
(7) and that colon cancer cells
become more sensitive to CPT-11 by abrogating this activation
(9–13). Thus, the results in Fig. 3 indicated that berberine may
enhance the cytotoxicity of CPT-11 through the suppression of NF-κB
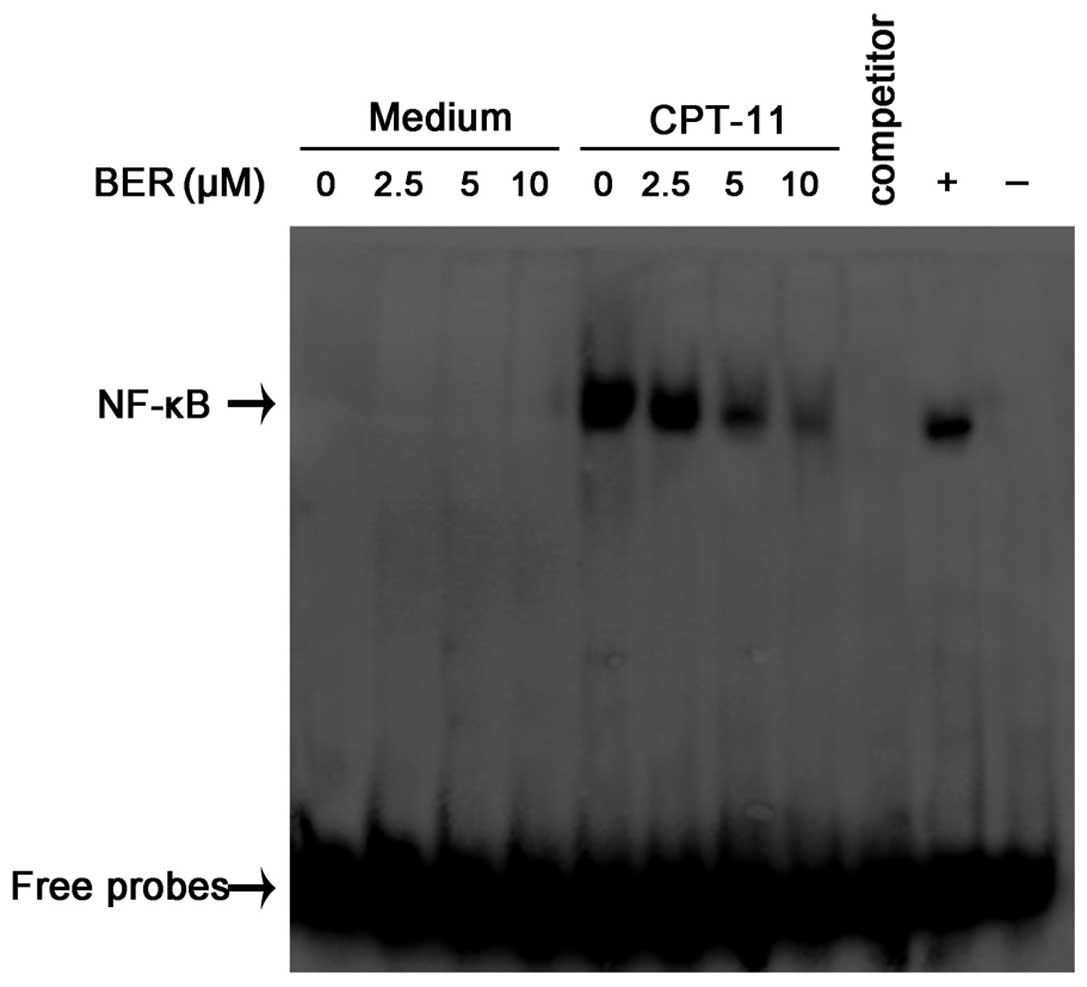
activation. EMSA was used to determine the effect of berberine on
CPT-11-induced NF-κB activation. HCT116 cells were pretreated with
varying concentrations of berberine (2.5–10 μM) for 48 h and
exposed to 20 μM CPT-11 for 2 h. Fig.
4 shows that berberine alone had no effect on NF-κB activation,
but it suppressed CPT-11-induced NF-κB activation in a
dose-dependent manner in HCT116 cells. These results indicate that
berberine effectively blocks the activation of NF-κB induced by
exposure to CPT-11 and enhances chemosensitivity to CPT-11 in
HCT116 cells.
Berberine decreases c-IAP1, c-IAP2,
survivin and Bcl-xL expression induced by CPT-11
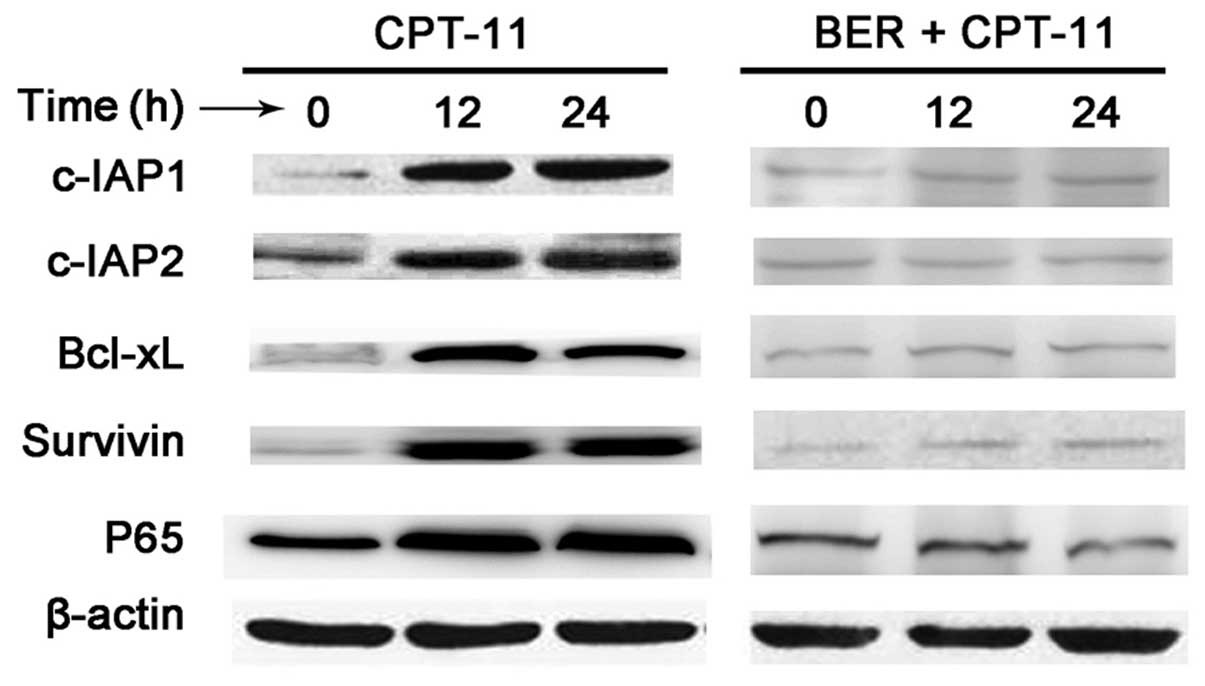
As NF-κB regulates antiapoptotic proteins, including
c-IAP1, c-IAP2, survivin and Bcl-xL, the effect of berberine on
CPT-11-induced expression of these antiapoptotic proteins was
observed. CPT-11 was observed to induce the expression of the
antiapoptotic proteins c-IAP1, c-IAP2, survivin and Bcl-xL
(Fig. 5) and berberine treatment
blocked the expression of these proteins. This result indicates
that berberine increases the cytotoxicity of CPT-11 by
downregulating NF-κB target of antiapoptotic genes.
Discussion
Consistent with previous studies, the results of the
current study showed that berberine enhances chemosensitivity to
CPT-11 in HCT116 cells by inhibiting NF-κB activation. Berberine
suppressed CPT-11-mediated NF-κB activation in a dose-dependent
manner and potentiated CPT-11-induced apoptosis by downregulating
NF-κB antiapoptotic target genes.
CPT-11 is widely used to treat certain neoplasms,
including colorectal cancer. However, CPT-11 has been reported to
lead to the activation of NF-κB in a variety of human colorectal
cancer cell lines (7). When
activated, NF-κB increases the expression of antiapoptotic genes,
which promote cell survival and block apoptosis. In addition,
colorectal cancer cells tend to be resistant to CPT-11 (5–7). A
number of strategies of NF-κB inhibition, including antisense
oligonucleotides, proteasome inhibitors and p65 siRNA, have been
shown as promising approaches for enhancing CPT-11-induced
apoptosis (9–13). In the present study, inhibition of
NF-κB by p65 siRNA transfection was confirmed to significantly
enhance chemosensitivity to CPT-11 in HCT116 cells. In addition, to
the best of our knowledge, the current study is the first to show
that berberine suppresses CPT-11-induced NF-κB activation in a
dose-dependent manner in HCT116 cells. The results of the SRB
assay, Hoechst 33258 and Annexin V/PI staining demonstrated that
berberine potentiates CPT-11-induced apoptosis. Compared with
previous methods, including the aformentioned methods, berberine is
a simple, available and applicable means to increase CPT-11-induced
apoptosis. The safety of berberine in humans has already been
established and it is widely used as a therapeutic drug for the
treatment of intestinal infections and diarrhea.
NF-κB-mediated antiapoptosis is essentially
dependent on the ability of NF-κB to enhance transcription of
target genes (22,23), including c-IAP1, c-IAP2, survivin
and Bcl-xL, which prevents tumor cell killing effects (22–24).
Thus, in the present study, western blotting was used to detect the
effect of berberine on expression levels of these antiapoptotic
proteins. The results demonstrate that berberine abolished
CPT-11-induced expression of c-IAP1, c-IAP2, survivin and Bcl-xL
(Fig. 5). Therefore, berberine may
potentiate CPT-11-induced apoptosis by downregulating the NF-κB
antiapoptotic target genes.
Myelosuppression and diarrhea are the two major
toxicities associated with CPT-11 treatment and numerous studies
have investigated pharmacological modulation to reduce CPT-11
toxicity. In the current study, concentrations between 2.5 and 10
μM berberine were non-toxic for HCT116 cells while still enhancing
chemosensitivity to CPT-11. Therefore, CPT-11-induced toxicity may
be alleviated by berberine treatment, thus, potentiating anticancer
effects with lower doses of CPT-11. These results are consistent
with those in previous studies, indicating that berberine is a
non-toxic chemoadjuvant that sensitizes tumor cells to CPT-11 by
inhibiting NF-κB. Future studies are likely to include in
vivo studies to reduce CPT-11 toxicity using berberine as a
chemoadjuvant to CPT-11.
By investiagting the mechanism of interplay between
berberine, NF-κB and the cytotoxicity of CPT-11, the present study
provides a basis for the rational choice of natural compounds,
including berberine, in chemotherapy. In addition, the importance
of basic cell biology in developing an integrated approach to
pharmacological intervention is highlighted. The current study also
indicates the possibility that natural compounds, which inhibit
NF-κB activation, may be used to augment the cytotoxicity of
chemotherapeutic agents.
Acknowledgements
This study was supported by grants from the China
Postdoctoral Science Foundation (grant no. 20090461139), the
National Natural Science Foundation of China (grant no. 81001457)
and the Natural Science Foundation of the Provincial Education
Department of Anhui (grant no. KJ2008A167).
References
|
1
|
Sarkar FH and Li Y: Harnessing the fruits
of nature for the development of multi-targeted cancer
therapeutics. Cancer Treat Rev. 35:597–607. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li F and Sethi G: Targeting transcription
factor NF-kappaB to overcome chemoresistance and radioresistance in
cancer therapy. Biochim Biophys Acta. 1805:167–180. 2010.PubMed/NCBI
|
|
3
|
Van Antwerp DJ, Martin SJ, Kafri T, Green
DR and Verma IM: Suppression of TNF-alpha-induced apoptosis by
NF-kappaB. Science. 274:787–789. 1996.PubMed/NCBI
|
|
4
|
Cusack JC Jr, Liu R and Baldwin AS Jr:
Inducible chemoresistance to
7-ethyl-10-[4-(1-piperidino)-1-piperidino]-carbonyloxycamptothecin
(CPT-11) in colorectal cancer cells and a xenograft model is
overcome by inhibition of nuclear factor-kappaB activation. Cancer
Res. 60:2323–2330. 2000.PubMed/NCBI
|
|
5
|
Baldwin AS Jr: Series introduction: the
transcription factor NF-kappaB and human disease. J Clin Investig.
107:3–6. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang CY, Mayo MW, Korneluk RG, Goeddel DV
and Baldwin AS Jr: NF-kappaB antiapoptosis: induction of TRAF1 and
TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation.
Science. 281:1680–1683. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu Y and Villalona-Calero MA: Irinotecan:
mechanism of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wasserman E, Sutherland W and Cvitkovic E:
Irinotecan plus oxaliplatin: a promising combination for advanced
colorectal cancer. Clin Colorectal Cancer. 1:149–153. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stahel RA and Zangemeister-Wittke U:
Antisense oligonucleotides for cancer therapy-an overview. Lung
Cancer. 41(Suppl 1): S81–S88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shah SA, Potter MW, McDade TP, Ricciardi
R, Perugini RA, Elliott PJ, Adams J and Callery MP: 26S proteasome
inhibition induces apoptosis and limits growth of human pancreatic
cancer. J Cell Biochem. 82:110–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cusack JC Jr, Liu R, Houston M, Abendroth
K, Elliott PJ, Adams J and Baldwin AS Jr: Enhanced chemosensitivity
to CPT-11 with proteasome inhibitor PS-341: implications for
systemic nuclear factor-kappaB inhibition. Cancer Res.
61:3535–3540. 2001.PubMed/NCBI
|
|
12
|
Guo J, Verma UN, Gaynor RB, Frenkel EP and
Becerra CR: Enhanced chemosensitivity to irinotecan by RNA
interference-mediated down-regulation of the nuclear factor-kappaB
p65 subunit. Clin Cancer Res. 10:3333–3341. 2004. View Article : Google Scholar
|
|
13
|
Lagdec P, Griessinger E, Nawrot MP,
Fenouille N, Colosetti P, Imbert V, Mari M, Hofman P, Czerucka D,
Rousseau D, et al: Pharmacological targeting of NF-kappaB
potentiates the effect of the topoisomerase inhibitor CPT-11 on
colon cancer cells. Br J Cancer. 98:335–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu S, Fang Y, Shen H, Xu W and Li H:
Berberine sensitizes ovarian cancer cells to cisplatin through
miR-21/PDCD4 axis. Acta Biochim Biophys Sin (Shanghai). 45:756–762.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pandey MK, Sung B, Kunnumakkara AB, Sethi
G, Chaturvedi MM and Aggarwal BB: Berberine modifies cysteine 179
of IkappaBalpha kinase, suppresses nuclear factor-kappaB-regulated
antiapoptotic gene products, and potentiates apoptosis. Cancer Res.
68:5370–5379. 2008. View Article : Google Scholar
|
|
16
|
Papazisis KT, Geromichalos GD, Dimitriadis
KA and Kortsaris AH: Optimization of the sulforhodamine B
colorimetric assay. J Immumol Methods. 208:151–158. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hussain AR, Ahmed SO, Ahmed M, Khan OS, Al
Abdulmohsen S, Platanias LC, Al-Kuraya KS and Uddin S: Cross-Talk
between NF-κB and the PI3-Kinase/AKT pathway can be targeted in
primary effusion lymphoma (PEL) cell lines for efficient apoptosis.
PLoS One. 7:e399452012.
|
|
18
|
Hussain AR, Ahmed M, Al-Jomah NA, Khan AS,
Manogaran P, Sultana M, Abubaker J, Platanias LC, Al-Kuraya KS and
Uddin S: Curcumin suppresses constitutive activation of nuclear
factor-kappa B and requires functional Bax to induce apoptosis in
Burkitt's lymphoma cell lines. Mol Cancer Ther. 7:3318–3329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Q, Zhang J, Liu L, Sharma S and Dong
Q: Quercetin potentiates doxorubicin mediated antitumor effects
against liver cancer through p53/Bcl-xl. PLoS One. 7:e517642012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou Z, Xie L, Wei J, Yu L, Qian X, Chen J,
Wang T and Liu B: Synergistic anti-proliferative effects of
gambogic acid with docetaxel in gastrointestinal cancer cell lines.
BMC Complement Altern Med. 12:582012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kawano Y, Nagata M, Kohno T, Ichimiya A,
Iwakiri T, Okumura M and Arimori K: Caffeine increases the
antitumor effect of Cisplatin in human hepatocellular carcinoma
cells. Biol Pharm Bull. 35:400–407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pacifico F and Leonardi A: Role of
NF-kappaB in thyroid cancer. Mol Cell Endocrinol. 321:29–35. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baud V and Karin M: Is NF-kappaB a good
target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov.
8:33–40. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuoharczak J, Simmons MJ, Fan Y and
Gélinas C: To be, or not to be: NF-kappaB is the answer - role of
Rel/NF-kappaB in the regulation of apoptosis. Oncogene.
22:8961–8982. 2003. View Article : Google Scholar : PubMed/NCBI
|