Introduction
Inflammatory response is a major defense mechanism
against pathogens and chemical or mechanical injury. This mechanism
is mediated by inflammatory cells, including macrophages. Activated
macrophages produce reactive oxygen species (ROS) and nitric oxide
(NO) and cause substantial oxidant injury to surrounding tissue
(1). Chronic inflammation is known
to contribute to cancer (2).
Oxidative stress-induced neuron injury induces a variety of
neurodegenerative diseases, including Alzheimer’s disease,
Parkinson’s disease and cerebral ischemia (3).
The bacterial peptidoglycan, recognized by toll-like
receptor (TLR) 2 on monocytes/macrophages, induces inflammatory
responses by activating MAPKs and NF-κB (4). Macrophages are important in host
defense mechanisms against tissue injury and microbial invasion and
are also involved in various processes in autoimmune disease,
infection and inflammatory disorders (5). Lipopolysaccharide (LPS) is a potent
macrophage activator that binds to the TLR4 on the macrophage cell
surface. LPS stimulation of macrophages produces various
pro-inflammatory cytokines as well as prostaglandin E2 and nitric
oxide (NO) (6).
Rhus verniciflua Stokes (RVS) has
traditionally been used as an ingredient in East Asian medicine for
the treatment of gastritis, stomach cancer and atherosclerosis. The
compounds identified from RVS are as follows: gallic acid,
protocatechuic acid, quercetin, fustin, fisetin, sulfuretin and
butein (7). RVS protects against
oxidative damage by scavenging ROS (8), causes antiproliferative activity and
anticancer and anti-inflammatory effects (9).
The effect of RVS on LPS-induced inflammatory
responses in the RAW264.7 mouse macrophage cell line was
investigated in this study. We examined whether ethanol (EtOH)
extract from RVS herbal medicine suppresses the LPS-induced
inflammatory responses in RAW264.7. We also analyzed whether RVS
exhibits anti-proliferative activity regulating intracellular
molecules associated with cell survival and apoptosis.
Materials and methods
Cell culture
RAW264.7 mouse macrophage cells were obtained from
the Korean Cell Line Bank (Seoul, Korea). The cells were cultured
in Dulbecco’s modified Eagle’s medium supplemented with 10%
heat-inactivated fetal bovine serum and 1% antibiotics at 37°C in a
5% CO2 humidified incubator.
Extraction of RVS
RVS used in this study was purchased from Omniherb
(Gyeongsangbuk-do, Korea). A 100 g ground powder was extracted
twice from the wood and fruit with 80% v/v ethanol (Duksan
Pharmaceutical Co. Ltd., Korea) using an Ultra-sonicator (Branson,
Danbury, CT, USA) for 30 min at room temperature. Alcoholic extract
was filtered through a 0.22 μm filter, evaporated at 40°C and
freeze-dried. The extract yield of RVS was 13.7% w/w.
Cell proliferation assay
The cell proliferation rate was determined using the
WST assay following RVS treatment. The WST assay is based on the
cleavage of yellow tetrazolium salt to purple formazan crystals by
metabolically active cells.
RAW264.7 cells (1×104cells/well) were
seeded into 96-well plates, incubated overnight and treated with
RVS for 24 h. WST solution (10 μl) was added to 100 μl cell culture
medium and the plates were incubated for 2 h. Optical density was
determined at 490 nm using an ELISA reader (Molecular Devices,
Sunnyvale, CA, USA).
Cell death assay
Cell death was determined using trypan blue assay
following RVS treatment. Trypan blue selectively stains dead cells.
RAW264.7 cells were treated with RVS for 12 and 24 h. Cells were
suspended and stained with trypan blue solution (Sigma-Aldrich, St.
Louis, MO, USA). The cell number was quantified using a
hemocytometer.
Cell surface observation
Cells were seeded into 60-mm culture dishes at a
density of 3×105 cells/dish. The following day, cells
were treated with RVS for 12 h. The cell surface was imaged using a
camera (Olympus Corporation, Tokyo, Japan) attached to a light
microscope.
Mitochondrial membrane potential
analysis
Loss of mitochondrial membrane potential is a
specific characteristic of apoptosis. JC-1 is a membrane-permeable
dye widely used for determining mitochondrial membrane potential in
flow cytometry and fluorescent microscopy. Cells were seeded into
60-mm culture dishes at a density of 3×105 cells/dish.
The following day, the cells were treated with RVS for 24 h. The
cells were harvested from each culture dish, washed with PBS,
suspended in PBS containing 2 μM JC-1 and incubated for 30 min at
37°C in the dark. The data were analyzed by FACSCalibur flow
cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Intracellular ROS level measurement
The molecule 2′,7′-dichlorofluorescein diacetate
(DCFH-DA) permeates cells where it is converted into fluorescent
2,7-dichlorofluorescein (DCF) by oxidative substances, revealing
the intracellular production of redox-active substances. DCFH-DA
has been widely used to investigate oxidative damage in intact
cells. Cells were seeded into 35-mm culture dishes containing glass
coverslips. Following various pretreatments, the cells were washed
with PBS and incubated with 20 μM DCFH-DA for 30 min at 37°C in the
dark. Following washing with cold PBS, the fluorescence was
captured by confocal laser scanning microscopy (LSM 510; Carl
Zeiss, Thornwood, NY, USA) and FACSCalibur flow cytometry. DCF
fluorescence was measured at an excitation wavelength of 488 nm and
emission at 515–540 nm.
RNA extraction and reverse
transcription-polymerase chain reaction (RT-PCR)
Cells were harvested by centrifugation (1,500 × g)
and the pellet was washed with ice-cold PBS. RNA was isolated from
the pellet using an Invitrogen Life Technologies kit (Carlsbad, CA,
USA) according to the manufacturer’s instructions. Isolated RNA
content was measured using the NanoDrop ND-1000 spectrophotometer
(NanoDrop Technologies Inc, Wilmington, DE, USA). Total cellular
RNA (2 μg) from each sample was reverse transcribed using cDNA
synthesis kit (Takara, Japan). PCR was conducted in a 20 μl
reaction mixture consisting of DNA template, 10 pM of each
gene-specific primer, 10X Taq buffer, 2.5 mM dNTP mixture and 1
unit of Taq DNA polymerase (Takara). PCR was performed using the
specific primer. The following primers were used: COX-2 sense,
5′-GGAGAGACTATCAAGATAGT-3′ and antisense,
5′-ATGGTCAGTAGACTTTTACA-3′; iNOS sense, 5′-AATGGCAA
CATCAGGTCGGCCATCACT-3′ and antisense,
5′-GCTGTGTGTCACAGAAGTCTCGAACTC-3′; and GAPDH sense,
5′-TGAAGGTCGGTGTGAACGGAT TTGGC-3′ and antisense,
5′-CATGTAGGCCATGAGGTC CACCAC-3′. The sequencing involved 30 cycles
with denaturation at 94°C for 45 sec, annealing at 55°C for 45 sec
and extension at 72°C for 45 sec. The resulting PCR products were
resolved on 1% agarose gels containing ethidium bromide.
Western blot analysis
Cells were lysed in modified RIPA buffer [150 mM
NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), 1
mM EDTA, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM NaF, 1 mM
Na3VO4, and protease inhibitor mixture]. The
lysates were cleared by centrifugation at 10,000 × g for 15 min and
the supernatants were collected. The protein concentration was
quantified using a Bio-Rad Bradford protein assay (Bio-rad,
Hercules, CA, USA). Equal amounts of protein lysates were used for
western blot analyses with the indicated antibodies (p-AKT, p-ERK,
p-JNK, p-p38, p-NFκB, α-tubulin, PARP, Pro-caspase-9, cleaved
caspase-3, Bcl-xL, Bcl-2, Bax, LC3). Immunoreactive protein bands
were detected with an EZ-Western Detection kit (Daeillab service
Co., Ltd., Seoul, Korea).
Statistical analysis
The experiments were performed in triplicate. The
data are expressed as the means ± standard deviation (SD). SDs for
all measured biological parameters are displayed in the appropriate
figures. Student’s t-test was used for single variable comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of RVS on cell viability
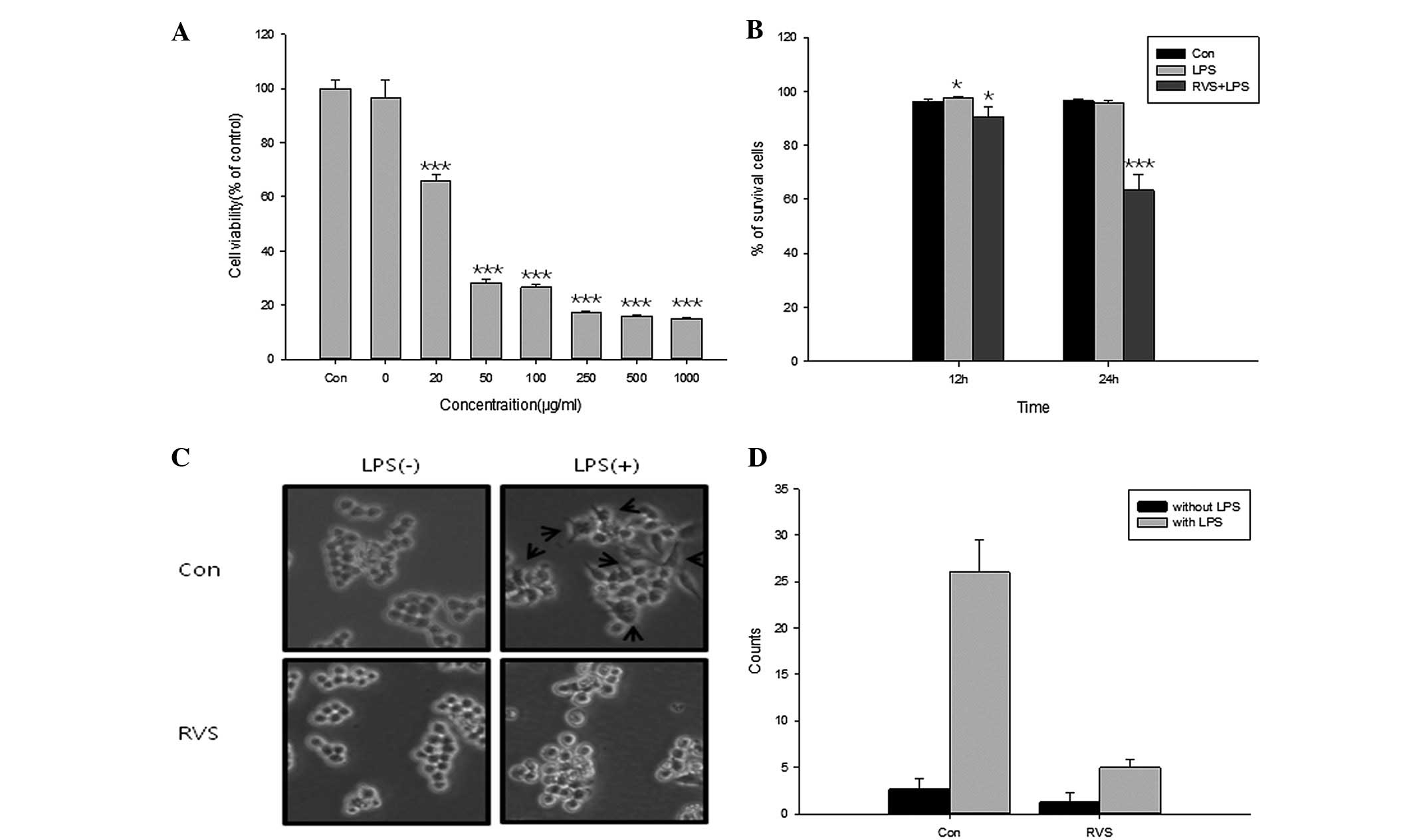
Anti-proliferative effects of RVS were determined in
RAW264.7 mouse macrophage cells using a WST assay (Fig. 1A). Cells were treated with RVS at
concentrations between 0 and 1,000 μg/ml and 1 μg/ml LPS for 12 h.
LPS alone did not show proliferative activity in RAW264.7 cells.
However, RVS significantly inhibited cell proliferation at
concentrations between 50 and 1,000 μg/ml, indicating that RVS
inhibits the growth of RAW264.7 cells. Cell death rate was
determined using a trypan blue assay following RVS treatment
(Fig. 1B). At 24 h, RVS
significantly decreased the percentage of surviving cells. In
addtion, changes in cellular morphology under LPS and RVS treatment
were observed (Fig. 1C). Untreated
RAW264.7 cells are circular, however, under LPS-stimulated
conditions, the cells presented as an irregular shape and became
elongated. Microscopic examination of cell cultures showed a
reversal of LPS-induced alteration in cell morphology when treated
with RVS. Fig. 1D shows the number
of cell surface changes under LPS and/or RVS treatment in RAW 264.7
cells. RVS significantly decreased the number of cell surface
changes induced by LPS. These results indicate that RVS inhibits
the proliferation of RAW264.7 cells and blocks the LPS-induced
activation of RAW264.7 cells.
RVS decreases iNOS and COX-2 mRNA
expression in RAW264.7 cells
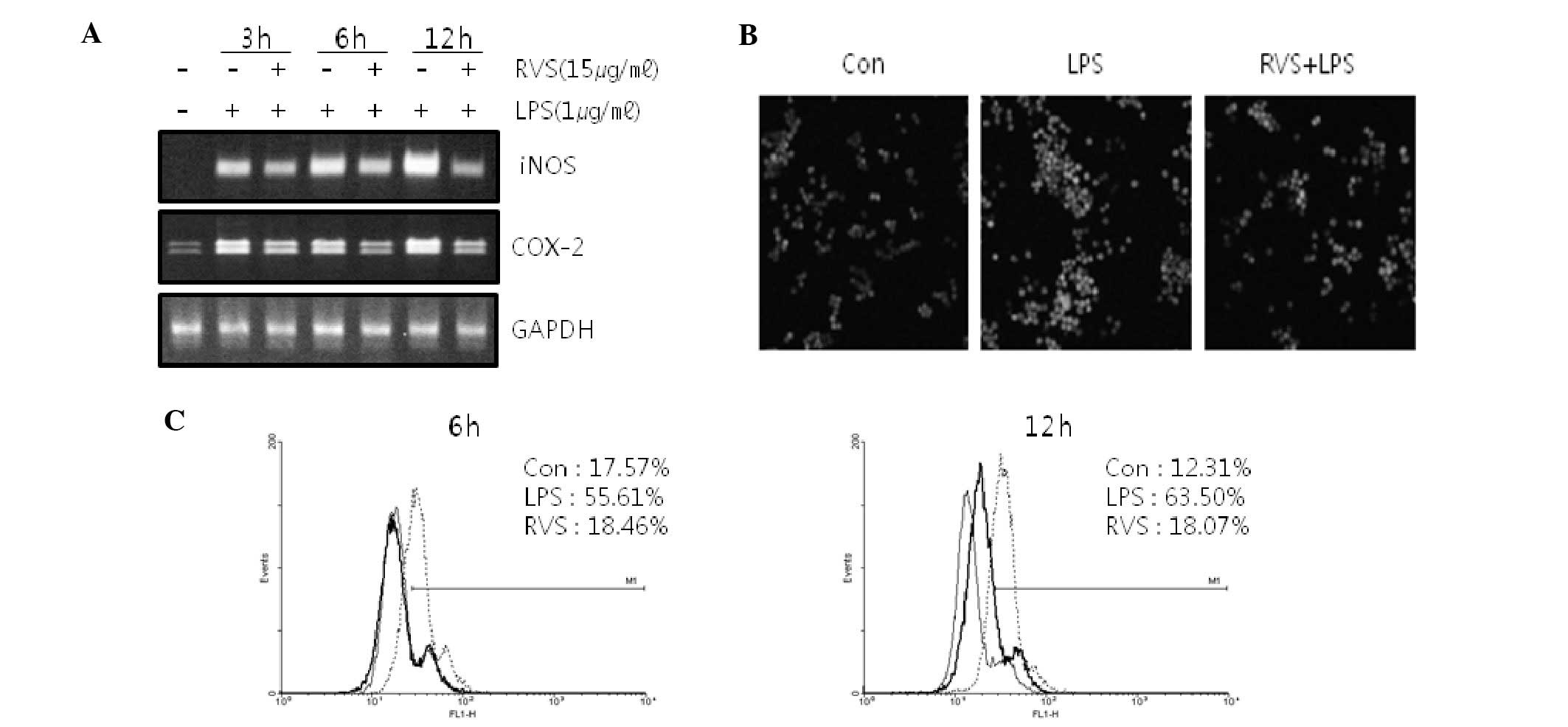
Since NO and ROS are mediators in inflammatory
reactions, iNOS mRNA and COX-2 mRNA expression in RAW264.7 cells
was measured. RVS suppressed iNOS mRNA and COX-2 mRNA expression
induced by LPS in RAW264.7 cells (Fig.
2A), suggesting that RVS suppresses inflammatory reactions.
RVS decreases ROS level in RAW264.7
cells
ROS levels were measured using confocal microscopy
(Fig. 2B) and FACS analysis
(Fig. 2C) stained with DCFH-DA.
Following LPS treatment, cellular ROS levels were increased.
However, RVS co-treatment inhibited ROS generation induced by LPS
in a time-dependent manner.
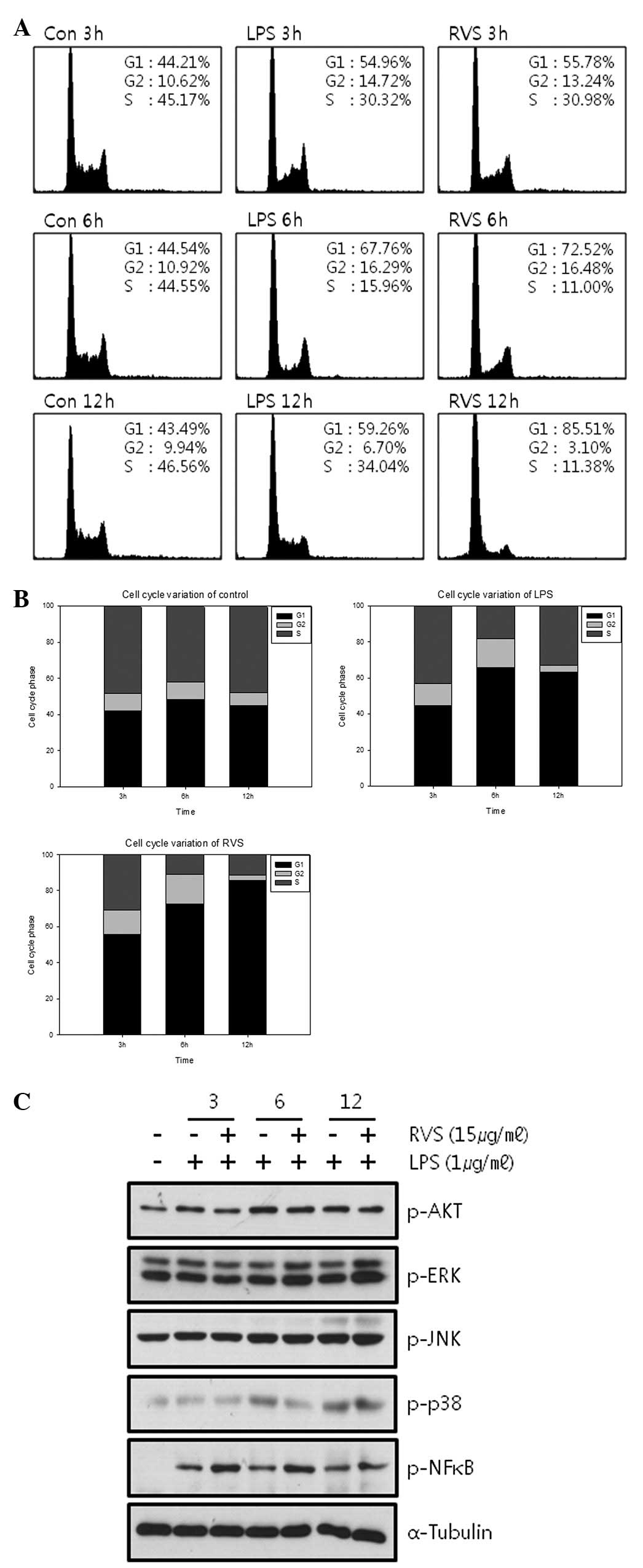
RVS affects the cell cycle
Cell cycle changes induced by RVS were analyzed by
FACs analysis. RVS caused G1 arrest at 6 and 12 h in a
time-dependent manner (Fig. 3A and
B). The expression of intracellular molecules associated with
cell proliferation was measured by western blot analysis (Fig. 3C). RVS failed to decrease the
phosphorylation of AKT, ERK, JNK, p38 and NF-κB.
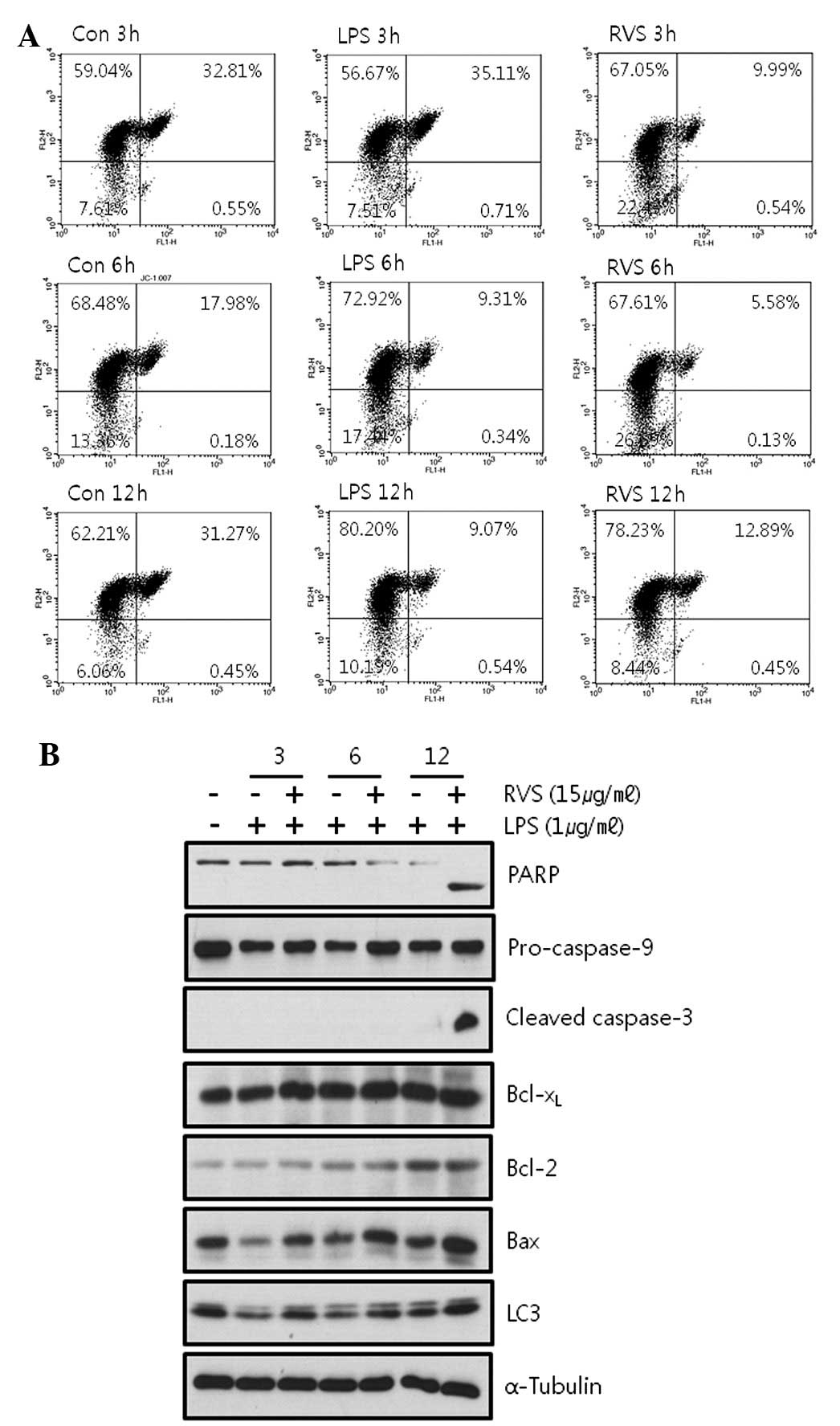
RVS induces cell apoptosis via a
mitochondrial-independent pathway
Loss of the mitochondrial membrane potential (ΔΨ) is
a hallmark for apoptosis. The mitochondrial permeability transition
is an important step in the induction of cell apoptosis. During
this process, several key events occur in the mitochondria,
including the release of caspase activators such as cytochrome
c, changes in electron transport and loss of mitochondrial
transmembrane potential. JC-1 selectively enters the mitochondria
and reversibly changes color from red to green as the membrane
potential decreases. Thus, cells were stained with JC-1 and FACS
analysis was used to determine whether mitochondrial membrane
potential is decreased by RVS. It was observed that RVS did not
decrease mitochondrial membrane potential in RAW264.7 cells
(Fig. 4A). LPS alone showed a more
stable mitochondrial membrane potential (green fluorescence, 9.61%
at 12 h) than the control (green fluorescence, 31.72% at 12 h). RVS
failed to alter this stability induced by LPS (green fluorescence,
13.34% at 12 h) indicating that RVS induces cell death via a
mitochondrial-independent pathway.
In addition, RVS was confirmed to regulate the
expression of apoptosis-related molecules. RVS induced cleavage of
apoptotic products, PARP and caspase-3, indicating that RVS induces
apoptosis (Fig 4B).
Discussion
In the current study, EtOH-extracted RVS was found
to suppress LPS-induced inflammatory responses in the RAW264.7
mouse macrophage cell line. Inflammation is a host protection
method against pathogens and is stimulated by diverse microbial
products (10). Pro-inflammatory
cytokines have been reported to aggravate the severity of multiple
inflammatory diseases (11).
Diverse inflammatory agents are known to activate NF-κB and
activation induces inflammation and increases cell survival and
tumor cell transformation (12).
MAPK pathways are associated with inflammation, for example, the
ERK pathway is activated by inflammation (13).
Results of the present study indicate that RVS
effectively inhibits growth stimulation and the activation of
RAW264.7 cells induced by LPS. RVS significantly inhibited cell
growth at concentrations between 50 and 1,000 μg/ml and induced
cell death at 15 μg/ml (24 h). In addition, RVS negated
morphological changes of RAW264.7 cells induced by LPS. RVS
decreased intracellular ROS levels and suppressed iNOS and COX-2
mRNA expression induced by LPS. RVS failed to decrease
mitochondrial membrane potential but cleaved caspase-3 and PARP
indicating that RVS induces apoptosis via a
mitochondrial-independent pathway.
Since RVS has an anti-inflammatory effect it may be
used for the treatment of inflammatory diseases, including
rheumatoid arthritis and asthma (14). Transformation of a normal cell into
a tumor cell is closely associated with chronic inflammation
(15), therefore, RVS may
represent a useful compound for cancer prevention.
Acknowledgements
This study was supported by a grant from the
Traditional Korean Medicine R and D Project, Ministry of Health and
Welfare, Republic of Korea (no. B110043).
References
|
1
|
Oh PS, Lee SJ and Lim KT: Glycoprotein
isolated from Rhus verniciflua Stokes inhibits
inflammation-related protein and nitric oxide production in
LPS-stimulated RAW 264.7 cells. Biol Pharm Bull. 30:111–116.
2007.
|
|
2
|
Hofseth LJ and Wargovich MJ: Inflammation,
cancer and targets of ginseng. J Nutr. 137(1 Suppl): S183–S185.
2007.
|
|
3
|
Liu Q, Kou JP and Yu BY: Ginsenoside Rg1
protects against hydrogen peroxide-induced cell death in PC12 cells
via inhibiting NF-κB activation. Neurochem Int. 58:119–125.
2011.PubMed/NCBI
|
|
4
|
Ahn JY, Choi IS, Shim JY, Yun EK, Yun YS,
Jeong G and Song JY: The immunomodulator ginsan induces resistance
to experimental sepsis by inhibiting Toll-like receptor-mediated
inflammatory signals. Eur J Immunol. 36:37–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park HJ, Han ES, Park DK, Lee C and Lee
KW: An extract of Phellinus linteus grown on germinated
brown rice inhibits inflammation markers in RAW264.7 macrophages by
suppressing inflammatory cytokines, chemokines and mediators and
up-regulating antioxidant activity. J Med Food. 13:1468–1477.
2010.
|
|
6
|
Yang JH, Suh SJ, Lu Y, Li X, Lee YK, Chang
YC, Na MK, Choi JH, Kim CH, Son JK and Chang HW: Anti-inflammatory
activity of ethylacetate fraction of Cliona celata.
Immunopharmacol Immunotoxicol. 33:373–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jung CH, Jun CY, Lee S, Park CH, Cho K and
Ko SG: Rhus verniciflua stokes extract: radical scavenging
activities and protective effects on
H2O2-induced cytotoxicity in macrophage RAW
264.7 cell lines. Biol Pharm Bull. 29:1603–1637. 2006. View Article : Google Scholar
|
|
8
|
Jung CH, Kim JH, Hong MH, Seog HM, Oh SH,
Lee PJ, Kim GJ, Kim HM, Um JY and Ko SG: Phenolic-rich fraction
from Rhus verniciflua Stokes (RVS) suppress inflammatory
response via NF-κB and JNK pathway in lipopolysaccharide-induced
RAW 264.7 macrophages. J Ethnopharmacol. 110:490–497. 2007.
|
|
9
|
Hong MH, Kim JH, Lee SY, Go HY, Kim JH,
Shin YC, Kim SH and Ko SG: Early antiallergic inflammatory effects
of Rhus verniciflua Stokes on human mast cells. Phytother
Res. 24:288–294. 2010.PubMed/NCBI
|
|
10
|
Lee HJ, Maeng K, Dang HT, Kang GJ, Ryou C,
Jung JH, Kang HK, Prchal JT, Yoo ES and Yoon D: Anti-inflammatory
effect of methyl dehydrojasmonate (J2) is mediated by the NF-κB
pathway. J Mol Med (Berl). 89:83–90. 2011.PubMed/NCBI
|
|
11
|
Tang S, Shen XY, Huang HQ, Xu SW, Yu Y,
Zhou CH, Chen SR, Le K, Wang YH and Liu PQ: Cryptotanshinone
suppressed inflammatory cytokines secretion in RAW264.7 macrophages
through inhibition of the NF-κB and MAPK signaling pathways.
Inflammation. 34:111–118. 2011.PubMed/NCBI
|
|
12
|
Reuter S, Prasad S, Phromnoi K, Ravindran
J, Sung B, Yadav VR, Kannappan R, Chaturvedi MM and Aggarwal BB:
Thiocolchicoside exhibits anticancer effects through downregulation
of NF-κB pathway and its regulated gene products linked to
inflammation and cancer. Cancer Prev Res (Phila). 3:1462–1472.
2010.PubMed/NCBI
|
|
13
|
Fan J, Liu K, Zhang Z, Luo T, Xi Z, Song J
and Liu B: Modified Si-Miao-San extract inhibits the release of
inflammatory mediators from lipopolysaccharide-stimulated mouse
macrophages. J Ethnopharmacol. 129:5–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jeong JB and Jeong HJ: Rheosmin, a
naturally occurring phenolic compound inhibits LPS-induced iNOS and
COX-2 expression in RAW264.7 cells by blocking NF-κB activation
pathway. Food Chem Toxicol. 48:2148–2153. 2010.PubMed/NCBI
|
|
15
|
Reuter S, Gupta SC, Chaturvedi MM and
Aggarwal BB: Oxidative stress, inflammation and cancer: how are
they linked? Free Radic Biol Med. 49:1603–1616. 2010. View Article : Google Scholar : PubMed/NCBI
|