Introduction
The airway mucociliary clearance system is essential
for innate lung defence. The major components of this system are
ciliary movement and airway surface liquid, which is composed of
water, electrolytes and macromolecules. Appropriate mucin secretion
ensures the removal of inhaled foreign objects, including
particulates and pathogens. Mucus hypersecretion is a hallmark of
various pulmonary inflammatory diseases, including chronic
obstructive disease (COPD), asthma and cystic fibrosis. The
gel-forming mucin 5ac (MUC5AC) is primarily synthesised by goblet
cells. Airway goblet cell hyperplasia is the primary pathology in
asthma and COPD (1). However, the
oversecretion of gel-forming mucin, particularly MUC5AC, is
generally observed in those patients who succumb to a severe asthma
attack or an acute exacerbation of COPD (2).
Neutrophil elastase (NE) is primarily synthesised
and released by neutrophils, which have been implicated in various
mucus hypersecretory diseases. NE has been reported to be
associated with goblet cell metaplasia (3) and decreased airway mucociliary
clearance ability. NE induces robust MUC5AC production in human
airway epithelial cells, and the upregulation of MUC5AC gene
expression is mediated by the epidermal growth-factor receptor
(EGFR) signalling pathway (4,5).
Furthermore, NE downregulates the expression of CD40, CD80 and
CD86, which promotes the maturation of dendritic cells in COPD
patients (6).
Annexins are a family of membrane binding proteins
that regulate membrane organisation, membrane trafficking and
Ca2+-related cellular processes (7). Annexin II (ANXII) is expressed in
numerous cells, but is more highly expressed in cells that are
poorly differentiated than in well-differentiated cells. ANXII has
been implicated in the fusion of secretory vesicles and target
membranes in several studies (8,9). In
eukaryotes, ANXII exists either as a soluble monomer (p36) or as a
tetrameric complex (p90) with its specific ligand, S100A10.
According to a study on chromaffin cells, the prevention of ANXII
tetramer formation markedly inhibited the exocytosis of
noradrenaline secretory granules (SGs) (10). Studies have demonstrated that a
synthetic peptide bound to the NH2-terminal of ANXII,
containing the protein kinase-C (PKC) phosphorylation site,
inhibits catecholamine secretion when microinjected into chromaffin
cells (11). This finding suggests
a close correlation between ANXII activation and the
phosphorylation of PKC (12). A
study on rat lung epithelial cells revealed a time-dependent
increase in ANXII expression following stimulation with acrolein
(13). ANXII has been identified
to correlate with N-ethylmaleimide sensitive factor attachment
protein receptors (SNAREs) in stimulated chromaffin cells (10). However, whether ANXII mediates the
exocytosis of MUC5AC SGs has not been investigated. Based on the
evidence mentioned above, we hypothesized that ANXII mediated
membrane fusion between MUC5AC SGs and the plasma membrane. We
designed cellular study in vitro and attempted to reveal the
specific mechanisms involved.
Materials and methods
Cells, reagents and antibodies
16HBE human bronchial epithelial cells were
purchased from Guangzhou Respiratory Institute (Guangzhou, China).
Human NE (hNE) was purchased from Elastin Products Company
(Owensville, MO, USA). All antibodies used for western blotting and
immunocytochemistry, including mouse anti-human ANXII (ab54771),
mouse anti-human mucin5ac (ab3649), fluorescein isothiocyanate
(FITC)-conjugated goat polyclonal secondary antibody to mouse IgG
(ab6785) and horseradish peroxidase (HRP)-conjugated goat
polyclonal secondary antibody to mouse IgG (ab6789), were purchased
from Abcam (Cambridge, MA, USA). The transfection reagent, FuGENE
HD, was acquired from Roche (Basel, Switzerland).
Cell culture and treatment
16HBE cells were propagated in Dulbecco’s modified
Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum
(FBS), 50 U/ml penicillin and 100 μg/ml streptomycin in a 37°C, 5%
CO2 incubator. The 16HBE cells were plated in 6×60-mm
culture dishes at a density of ~2×106 cells/ml and
cultured in a 37°C, 5% CO2 incubator to allow the cells
to attach. Following serum-starvation for 24 h, 16HBE cells were
divided into the following groups: i) The untreated group, which
was grown in serum-free DMEM to determine the basal level of MUC5AC
secretion and the basal expression level and intracellular
distribution of ANXII. ii) The NE-stimulated group, which was
treated with 8 μg/ml hNE for 4 h. iii) The NE-treated and control
siRNA-transfected group, which was transfected with the negative
control siRNA and subsequently treated with 8 μg/ml hNE in
serum-free DMEM for 4 h. iv) The NE-treated and ANXII
siRNA-transfected group, which was transfected with ANXII siRNA and
subsequently treated with 8 μg/ml hNE in serum-free DMEM for 4 h.
v) The PKC inhibitor- and NE-treated group, which was preincubated
with the PKC inhibitor bisindolylmaleimide I (500 nmol/l) for 15
min and subsequently treated with hNE in serum-free DMEM for 4
h.
Small interfering RNA (siRNA) preparation
and transfection
ANXII-specific siRNA and the vector
pGC-silencer-U6/Neo/green fluorescent protein (GFP)/ANXII (target
shRNA sequence 5′-GGTCTGAATTCAAGAGAAA-3′) were synthesised and
packaged by GeneChem (Shanghai, China). A base sequence containing
a similar GC content was inserted into the vector as a negative
control. Prior to transfection, 16HBE cells in the exponential
growth phase were plated at a density of ~2×106 cells/ml
and incubated in the culture dishes for 12 h. Following washing
with phosphate-buffered saline (PBS) three times in order to avoid
any interference by the antibiotics and the serum, the 16HBE cells
were transfected using FuGENE HD with either ANXII siRNA or the
negative control vector according to the manufacturer’s
instructions. siRNA concentrations were based on dose-response
studies (data not shown).
Reverse transcription (RT) and
quantitative polymerase chain reaction analysis (qPCR)
Total RNA was extracted from 16HBE cells in each
group using TRIzol. The extraction was confirmed by RNA
electrophoresis on a 1.5% agarose gel, and an absorbance
(A260/280) value of 1.8–2.0 was deemed acceptable. The
reverse transcription followed the specifications provided in the
iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). The
synthesised cDNA was prepared for qPCR. qPCR was performed using
the iQ SYBR Green supermix (Bio-Rad) with PCR primers in an iCycler
(Bio-Rad). In order to quantify the expression of ANXII mRNA,
β-actin mRNA served as an internal control. All primers used for
qPCR are listed in Table I. The
qPCR curves were analysed using the CFX Manager™ software (Bio-Rad)
in order to obtain threshold cycle (Ct) values for each sample. The
mRNA expression level was calculated based on a generated standard
curve.
 | Table IPrimers for real-time quantitative
PCR. |
Table I
Primers for real-time quantitative
PCR.
| Forward primer | Reverse primer |
|---|
| β-actin |
5′-TGGCACCCAGCACAATGAA-3′ |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
| Annexin II |
5′-ACTTTGATGCTGAGCGGGATG-3′ |
5′-CGAAGGCAATATCCTGTCTCTGTG-3′ |
Western blotting to detect ANXII
protein
The expression of ANXII protein in each group was
detected by western blotting. The cells were washed with PBS three
times and lysed on ice for 20 min using a lysis buffer containing
10 mmol/l Tris (pH 7.4), 1% sodium dodecyl sulphate (SDS), 1 mmol/l
sodium orthovanadate and cOmplete ULTRA protease inhibitors (Roche
ID: 05892970001; Roche, Basel, Switzerland). In order to remove the
nuclei and intact cells, the lysis products were centrifuged at
21,773 × g (1–14K centrifuge; Sigma Laborzentrifugen GmbH, Osterode
am Harz, Germany) for 15 min at 4°C. The supernatants were
standardised for equal protein concentration using the instructions
in the Bicinchoninic Acid Protein Assay kit (Beyotime, Beijing,
China). The samples were subsequently boiled for 5 min in water.
Following separation with SDS-polyacrylamide gel electrophoresis,
the proteins were transferred onto polyvinylidene difluoride (PVDF)
membranes (Bio-Rad). The PVDF membranes were incubated with
anti-ANXII (dilution, 1:100) and anti-β-actin primary antibodies
(dilution, 1:1,000) overnight at room temperature. Following
washing with PBS with tween-20 three times for 15 min, the PVDF
membranes were incubated with the secondary antibody,
HRP-conjugated goat anti-mouse IgG, at a 1:2,000 dilution for 2 h.
The blots were visualised using enhanced chemiluminescence
according to the manufacturer’s instructions (KeyGen, Nanjing,
China). The intensity of each band was measured using the Fluor-S
MultiImager and Quantity-One software (Bio-Rad). The ANXII protein
expression level was normalised to that of β-actin.
Cell immunochemistry and laser confocal
microscopy
The direct visual observation of ANXII and
intracellular MUC5AC protein were performed using immunochemistry
and laser confocal microscopy. 16HBE cells were plated at a density
of 2×105 cells/ml in 24-well plates on a glass coverslip
in each well. Following culturing in a serum- and antibiotic-free
environment for 12 h, the cells were washed three times with PBS.
The cells were fixed with 4% paraformaldehyde for 15 min and washed
again with PBS. The fixed 16HBE cells were permeabilised with 0.1%
Triton X-100 in PBS for 10 min and washed three times with PBS. The
cells were subsequently blocked in 5% goat serum for 60 min and
incubated with mouse anti-MUC5AC (1:500 dilution) or mouse
anti-ANXII (1:50 dilution) overnight. Following three washes with
PBS, the slides were incubated with the secondary antibody,
FITC-linked goat anti-mouse IgG (dilution, 1:1,000), for 60 min.
The cells were washed three times with PBS and embedded in 50%
glycerol. The 16HBE cells were visualised using a confocal
microscope (TCS-SP2, Leica Microsystems, Wetzlar, Germany).
Representative images were captured with the incorporated digital
camera and subsequently processed with Adobe Photoshop 7.0 (Adobe
Systems Inc., Beijing, China).
Enzyme-linked immunosorbent assay (ELISA)
for MUC5AC in the cell supernatant
Secreted MUC5AC in the 16HBE cell culture
supernatant was assessed by ELISA. The culture supernatants (50
μl/well) were added to a 96-well plate and incubated at 40°C until
dry. Following washing and blocking the wells, the mouse monoclonal
antibody against MUC5AC (dilution, 1:200) was incubated in the
wells for 1 h. The plates were washed three times with PBS and
incubated with 100 μl/well HRP-conjugated goat anti-mouse IgG at a
1:5,000 dilution. After 1 h, the plates were washed three times
with PBS. The colour reaction was performed using an HRP solution
and was stopped with H2SO4. The absorbance
was read at 450 nm and the results were expressed as the ratio of
MUC5AC to the standard.
Statistical analysis
Data were reported as the mean ± standard deviation.
All data were analysed with the SPSS 17.0 statistical package (SPSS
Inc., Chicago, IL, USA). The analysis of one-way analysis of
variance with Student-Newman-Keuls q-test was used to compare the
levels of difference between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
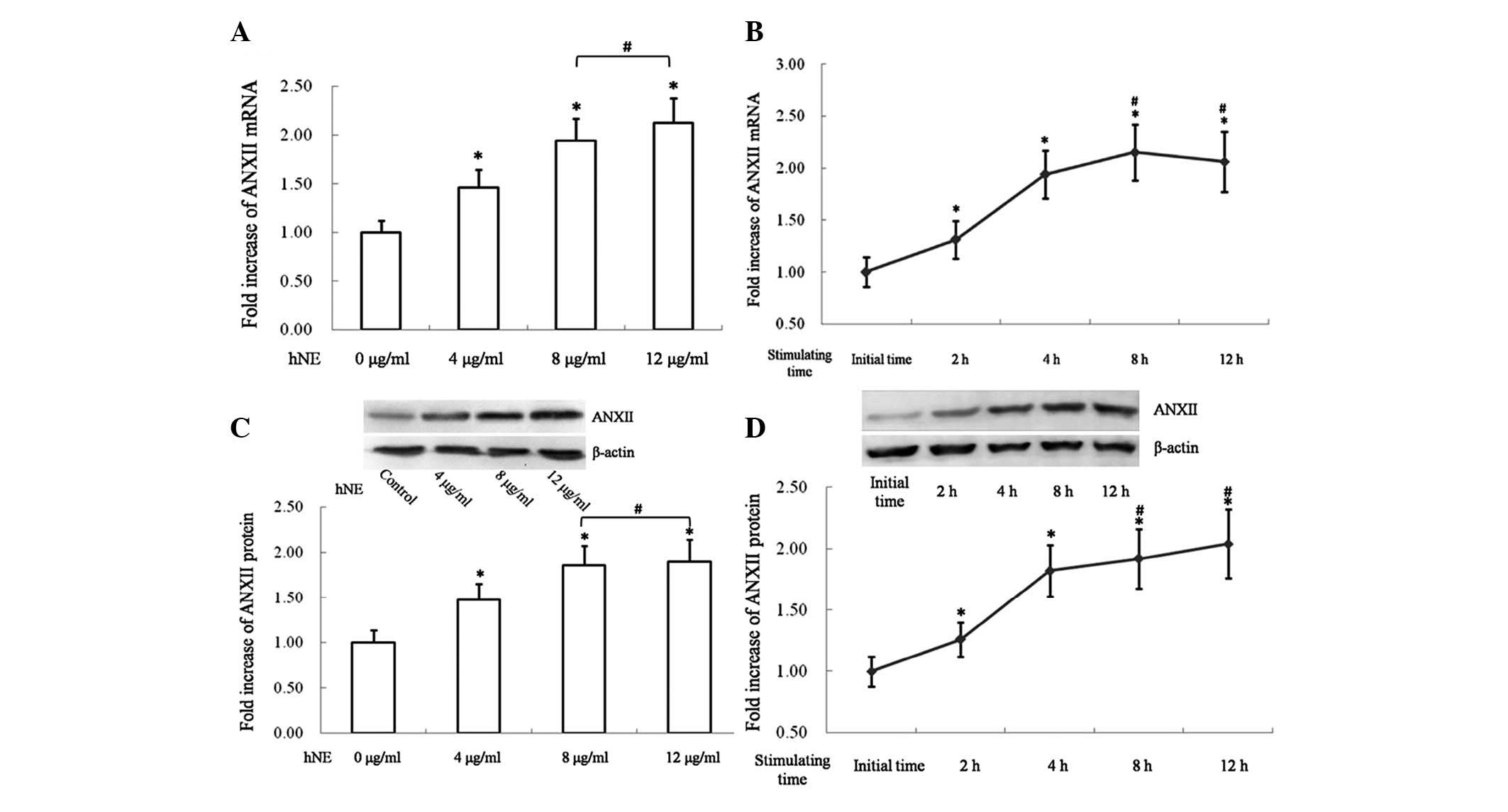
NE increases ANXII expression in 16HBE
cells
The mRNA expression of ANXII was investigated in
16HBE cells following treatment with hNE. Trypan staining was used
to evaluate the viability of 16HBE cells following treatment with
hNE. Further research depended on >95% cell viability. As shown
in Fig. 1A and B, the normalized
ANXII mRNA level exhibited a dose- and time-dependent increase
following stimulation with hNE. However, a higher dose of 12 μg/ml
hNE or extending the stimulation time to >4 h failed to induce a
significantly higher transcription of ANXII in 16HBE cells
(Fig. 1A and B).
Furthermore, ANXII protein in 16HBE cells was
detected by western blotting. The expression levels of ANXII in
16HBE cells were normalized by β-actin. As shown in Fig. 1C a concentration of hNE ranging
from 4 to 12 μg/ml significantly increased the synthesis of ANXII
in 16HBE cells. However, no significant differences were observed
between 16HBE cells stimulated by 8 or 12 μg/ml hNE in the
synthesis level of ANXII (Fig.
1C). Stimulation of hNE increased the expression of ANXII in
16HBE cells in a time-dependent manner. However, extending the
exposure time to >8 h failed to induce a further increase of
ANXII expression compared with the 4 h exposure group (Fig. 1D).
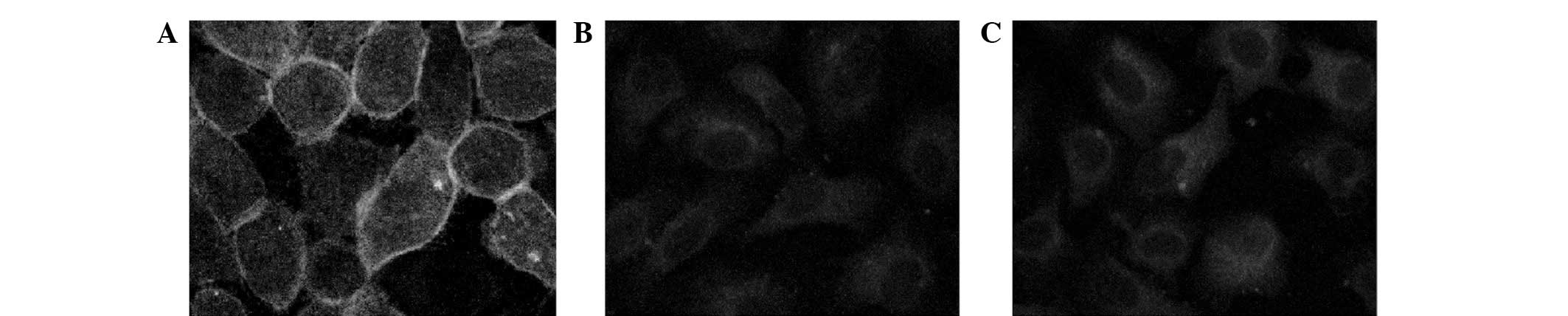
ANXII is recruited to the cell membrane
upon stimulation with NE
As mentioned previously, NE upregulated the
expression of ANXII. To further investigate the distribution of
ANXII protein in stimulated 16HBE cells, ANXII was visualised by
cell immunochemistry. Immunoreactivity was performed using laser
confocal microscopy and Leica Confocal Software. ANXII was
recruited to the plasma membrane in 16HBE cells treated for 4 h
with 8 μg/ml NE but not in the untreated control cells (Fig. 2A and B). The phosphorylation of
ANXII and the formation of an ANXII heterotetrameric complex (p90),
which has been demonstrated to be more efficient than monomeric
p36, has been reported to be dependent on the activation of PKC
(14). Therefore, it was
investigated whether PKC participated in the redistribution of
ANXII in stimulated 16HBE cells. 16HBE cells were preincubated with
the PKC inhibitor bisindolylmaleimide I (500 nmol/l) and
subsequently stimulated with NE, as described previously. Images
were captured using a laser confocal microscope (Fig. 2C). Pretreatment with
bisindolylmaleimide I markedly reduced the recruitment of ANXII to
the cell membrane in NE-stimulated 16HBE cells.
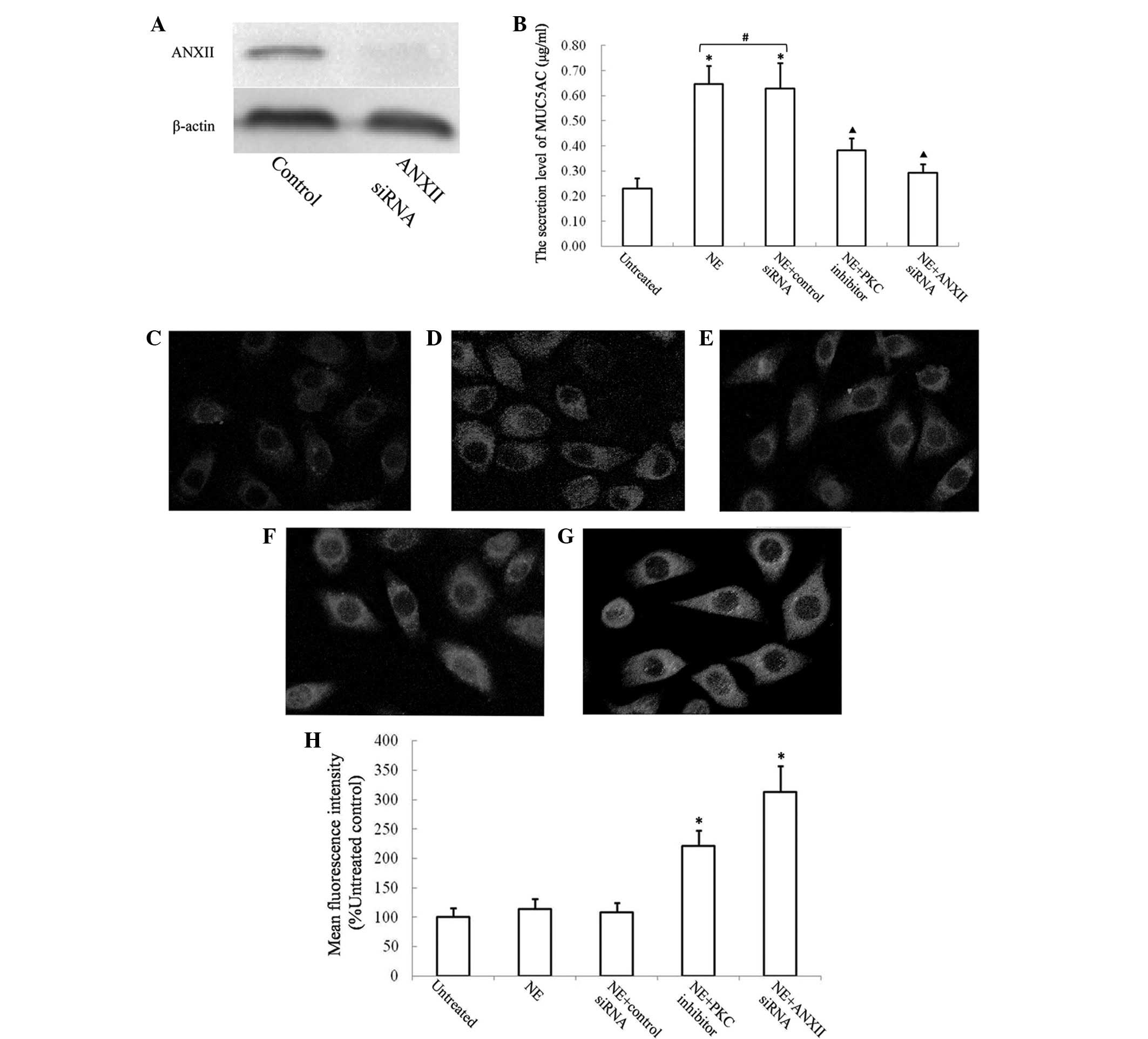
ANXII is required for MUC5AC secretion in
16HBE cells
In order to investigate whether ANXII is required
for the secretion of MUC5AC, a specific siRNA that targets ANXII
was designed. The downregulation of ANXII in 16HBE cells using
ANXII siRNA was verified by western blotting (Fig. 3A). The secretion of MUC5AC into the
cell culture supernatant was measured by ELISA. NE increased MUC5AC
secretion by ~3-fold after 4 h. 16HBE cells transfected with ANXII
siRNA secreted significantly less MUC5AC following stimulation with
NE (Fig. 3B). Treatment with
bisindolylmaleimide I partially reduced the secretion of MUC5AC in
NE-stimulated 16HBE cells. The MUC5AC retained in the endochylema
was visualised by cell immunochemistry. The retained MUC5AC in the
16HBE cells was quantified using the fluorescence intensity ratio
and was compared with the untreated control (Fig. 3C–G). 16HBE cells transfected with
ANXII siRNA exhibited poor levels of MUC5AC secretion following
incubation with NE, and the retained MUC5AC level was significantly
higher in the endochylema compared with that in the NE-treated
group (Fig. 3H, P<0.05).
Discussion
Mucus hypersecretion in the human airway is a
pathology present in several respiratory diseases, including asthma
and COPD. Excessive mucus production in the airways has been linked
to an increase in morbidity and mortality in patients with
respiratory diseases (15). In
asthma and COPD, increased numbers of goblet cells correlates with
excessive mucus production. Goblet cells are capable of rapidly
secreting mucus in response to certain stimuli in order to form a
mucus layer that lines the airways. Highly glycosylated forms of
mucin, MUC5AC in particular, form a mucus gel and may lead to
severe airway obstruction (16).
As several studies have reported, the translocation
and exocytosis of MUC5AC SGs is a complicated process with an
obscure intrinsic regulatory mechanism. The PKC-dependent
phosphorylation of myristorylated alanine-rich C-kinase substrate
(MARCKS) has been demonstrated to be involved in the translocation
of MUC5AC SGs (17,18). Several proteins that promote mucus
secretion, including interleukin (IL)-1β, IL-6, monocyte
chemoattractant protein-1 and tumour necrosis factor-α, improve
MUC5AC hypersecretion through the activation of MARCKS. The
exogenous attenuation of the function of MARCKS may decrease airway
mucin secretion (18).
ANXII, also termed ANXA2, is widely expressed in
eukaryotic cells and is a calcium- and phospholipid-binding protein
that mediates essential cellular processes, in particular membrane
trafficking events. Deep-etch electron microscopy has revealed a
crosslink between the SGs and the plasma membrane formed by ANXII
in stimulated neuroendocrine cells (19). Direct evidence has been obtained
for the role of ANXII in exocytosis.
ANXII exists either as a monomer (p36) or as a
section of a heterotetrameric complex (p90) with S100A10, a protein
of 11 kDa, which is referred to as p11. In the heterotetrameric p90
complex, the central S100A10 dimer links two ANXII chains in a
highly symmetrical manner, creating a scaffold that is capable of
bridging the opposing membrane surfaces. Several observations have
suggested that the ANXII-S100A10 heterotetrameric complex targets
the cell surface and the cortical cytoskeleton (20). In neuroendocrine cells, ANXII
promotes monosialotetrahexosylganglioside-containing lipid
microdomains that are required for calcium-related exocytosis
(12). Soluble SNAREs present at
the plasma membrane have been reported to be the membrane fusion
sites for vesicle exocytosis (21,22).
ANXII has been revealed to colocalise with SNAP-23, which is
abundant in non-neuronal cells and is responsible for the secretion
of mucin granules (23).
Therefore, it was investigated whether ANXII was responsible for
MUC5AC secretion. There is evidence that reactive oxygen species,
inflammation and hypoxia activate the expression of ANXII (24,25).
It was demonstrated that following stimulation with NE, ANXII mRNA
transcription and protein levels increased by ~2-fold at their peak
levels. Immunohistochemistry on the NE-stimulated 16HBE cells
allowed the visualisation of the distribution of ANXII in
stimulated hypersecretion cells. Similar to what has been reported
in chromaffin cells (10), it was
established that ANXII is recruited to the cell membrane in
stimulated 16HBE cells. As demonstrated in mesangial cells in
vitro, ANXII is phosphorylated by PKC (14) and a synthetic peptide corresponding
to the NH2-terminus of ANXII, which contains the PKC
phosphorylation site and inhibits catecholamine secretion in
chromaffin cells (26). Similarly,
bisindolylmaleimide I was used to inhibit the phosphorylation of
ANXII and revealed an attenuation in the peripheral recruitment of
ANXII in NE-stimulated 16HBE cells. These data suggest that the
induction of ANXII expression and its recruitment to the cell
membrane in 16HBE cells upon NE stimulation is dependent on the PKC
pathway.
Despite 20 years of extensive study, the precise
function of ANXII remains unknown. In particular, the role of ANXII
in non-neuroendocrine cell secretion has yet to be clarified.
According to a previous study, NE increases the novel synthesis of
MUC5AC following treatments that last >4 h, primarily by
enhancing MUC5AC mRNA stability (27). It was established that 4 h was the
adequate stimulation time to investigate secretion of MUC5AC
granules induced by NE (28). A
specific siRNA targeting ANXII was synthesised to inhibit the
endogenous production of ANXII in 16HBE cells. Using these methods,
it was determined that ANXII is essential for MUC5AC secretion.
The present study provides evidence of ANXII
involvement in the mechanisms of MUC5AC secretion in airway
epithelial cells. It may aid in the understanding of the specific
mechanism of MUC5AC secretion and provide a novel therapy for the
management of local and systemic diseases in mucus
hypersecretion.
Acknowledgements
This study was supported by grant from the National
Nature Science Foundation of China (grant no. 81370111) and the
China-Russia Cooperation Research Foundation (grant no.
31211120168).
References
|
1
|
Ordonez CL, Khashayar R, Wong HH, et al:
Mild and moderate asthma is associated with airway goblet cell
hyperplasia and abnormalities in mucin gene expression. Am J Respir
Crit Care Med. 163:517–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Turner J and Jones CE: Regulation of mucin
expression in respiratory diseases. Biochem Soc Trans. 37:877–881.
2009. View Article : Google Scholar
|
|
3
|
Voynow JA, Fischer BM, Malarkey DE, et al:
Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am
J Physiol Lung Cell Mol Physiol. 287:L1293–1302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kohri K, Ueki IF and Nadel JA: Neutrophil
elastase induces mucin production by ligand-dependent epidermal
growth factor receptor activation. Am J Physiol Lung Cell Mol
Physiol. 283:L531–L540. 2002.PubMed/NCBI
|
|
5
|
Jiang DP, Li Q, Yang J, Perelman JM,
Kolosov VP and Zhou XD: Scutellarin attenuates
human-neutrophil-elastase-induced mucus production by inhibiting
the PKC-ERK signaling pathway in vitro and in vivo.
Am J Chin Med. 39:1193–1206. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roghanian A, Drost EM, MacNee W, Howie SE
and Sallenave JM: Inflammatory lung secretions inhibit dendritic
cell maturation and function via neutrophil elastase. Am J Respir
Crit Care Med. 174:1189–1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Benz J and Hofmann A: Annexins: from
structure to function. Biol Chem. 378:177–183. 1997.PubMed/NCBI
|
|
8
|
Paumet F, Rahimian V and Rothman JE: The
specificity of SNARE-dependent fusion is encoded in the SNARE
motif. Proc Natl Acad Sci USA. 101:3376–3380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chattopadhyay S, Sun P, Wang P, Abonyo B,
Cross NL and Liu L: Fusion of lamellar body with plasma membrane is
driven by the dual action of annexin II tetramer and arachidonic
acid. J Biol Chem. 278:39675–39683. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Umbrecht-Jenck E, Demais V, Calco V,
Bailly Y, Bader MF and Chasserot-Golaz S: S100A10-mediated
translocation of annexin-A2 to SNARE proteins in adrenergic
chromaffin cells undergoing exocytosis. Traffic. 11:958–971. 2010.
View Article : Google Scholar
|
|
11
|
Chasserot-Golaz S, Vitale N, Sagot I,
Delouche B, Dirrig S, Pradel LA, Henry JP, Aunis D and Bader MF:
Annexin II in exocytosis: catecholamine secretion requires the
translocation of p36 to the subplasmalemmal region in chromaffin
cells. J Cell Biol. 133:1217–1236. 1996. View Article : Google Scholar
|
|
12
|
Chasserot-Golaz S, Vitale N,
Umbrecht-Jenck E, Knight D, Gerke V and Bader MF: Annexin 2
promotes the formation of lipid microdomains required for
calcium-regulated exocytosis of dense-core vesicles. Mol Biol Cell.
16:1108–1119. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sarkar P and Hayes BE: Proteomic profiling
of rat lung epithelial cells induced by acrolein. Life Sci.
85:188–195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oudinet JP, Russo-Marie F, Cavadore JC and
Rothhut B: Protein kinase C-dependent phosphorylation of annexins I
and II in mesangial cells. Biochem J. 292:63–68. 1993.PubMed/NCBI
|
|
15
|
Vestbo J: Epidemiological studies in mucus
hypersecretion. Novartis Found Symp. 248:3–19. 277–282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rogers DF: Physiology of airway mucus
secretion and pathophysiology of hypersecretion. Respir Care.
52:1134–1149. 2007.PubMed/NCBI
|
|
17
|
Foster WM, Adler KB, Crews AL, Potts EN,
Fischer BM and Voynow JA: MARCKS-related peptide modulates in
vivo the secretion of airway Muc5ac. Am J Physiol Lung Cell Mol
Physiol. 299:L345–352. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JA, Crews AL, Lampe WR, Fang S, Park
J and Adler KB: Protein kinase C delta regulates airway mucin
secretion via phosphorylation of MARCKS protein. Am J Pathol.
171:1822–1830. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Senda T, Okabe T, Matsuda M and Fujita H:
Quick-freeze, deep-etch visualization of exocytosis in anterior
pituitary secretory cells: localization and possible roles of actin
and annexin II. Cell Tissue Res. 277:51–60. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deora AB, Kreitzer G, Jacovina AT and
Hajjar KA: An annexin 2 phosphorylation switch mediates
p11-dependent translocation of annexin 2 to the cell surface. J
Biol Chem. 279:43411–43418. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behrendorff N, Dolai S, Hong W, Gaisano HY
and Thorn P: Vesicle-associated membrane protein 8 (VAMP8) is a
SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein
receptor) selectively required for sequential granule-to-granule
fusion. J Biol Chem. 286:29627–29634. 2011. View Article : Google Scholar
|
|
22
|
Jones LC, Moussa L, Fulcher ML, et al:
VAMP8 is a vesicle SNARE that regulates mucin secretion in airway
goblet cells. J Physiol. 590:545–562. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang P, Chintagari NR, Gou D, Su L and Liu
L: Physical and functional interactions of SNAP-23 with annexin A2.
Am J Respir Cell Mol Biol. 37:467–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Madureira PA, Hill R, Miller VA,
Giacomantonio C, Lee PW and Waisman DM: Annexin A2 is a novel
cellular redox regulatory protein involved in tumorigenesis.
Oncotarget. 2:1075–1093. 2011.
|
|
25
|
Genetos DC, Wong A, Watari S and Yellowley
CE: Hypoxia increases Annexin A2 expression in osteoblastic cells
via VEGF and ERK. Bone. 47:1013–1019. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chasserot-Golaz S, Vitale N, Sagot I, et
al: Annexin II in exocytosis: catecholamine secretion requires the
translocation of p36 to the subplasmalemmal region in chromaffin
cells. J Cell Biol. 133:1217–1236. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Voynow JA, Young LR, Wang Y, Horger T,
Rose MC and Fischer BM: Neutrophil elastase increases MUC5AC mRNA
and protein expression in respiratory epithelial cells. Am J
Physiol. 276:L835–843. 1999.PubMed/NCBI
|
|
28
|
Zhou J, Perelman JM, Kolosov VP and Zhou
X: Neutrophil elastase induces MUC5AC secretion via
protease-activated receptor 2. Mol Cell Biochem. 377:75–85. 2013.
View Article : Google Scholar : PubMed/NCBI
|