Introduction
Lung cancer is one of the most prevalent types of
cancers worldwide and is the leading cause of cancer-related
mortalities. Based on the GLOBOCAN 2008 estimates, lung cancer was
the leading type of cancer in males, comprising 17% of total novel
cancer cases and 23% of total cancer-related mortalities. The
mortality rate for lung cancer among females in developing
countries accounts for 11% of the total female cancer-related
mortalities, second to breast cancer (1). To date, surgery, radiotherapy and
chemotherapy are the predominant treatment strategies for lung
cancer; however, the efficacy of these therapies is limited and may
result in a number of side effects. Over the past decade, the
mortality rates of lung cancer remain high, with the 5-year
survival rate <15% (2).
Therefore, investigating novel therapeutic targets is a demanding
task.
In previous years, traditional Chinese medicine has
drawn great attention for use as chemotherapeutic agents against
malignant tumors. Traditional Chinese medicines have a number of
advantages, including fewer side effects and combination treatment
to increase efficiency (3,4). Diterpene acid is one of the numerous
traditional Chinese medicines that has a long history of
recordation and clinical application (5). Diterpene acid has antiviral,
anti-inflammatory, antitumor, antifungal and immunosuppressive
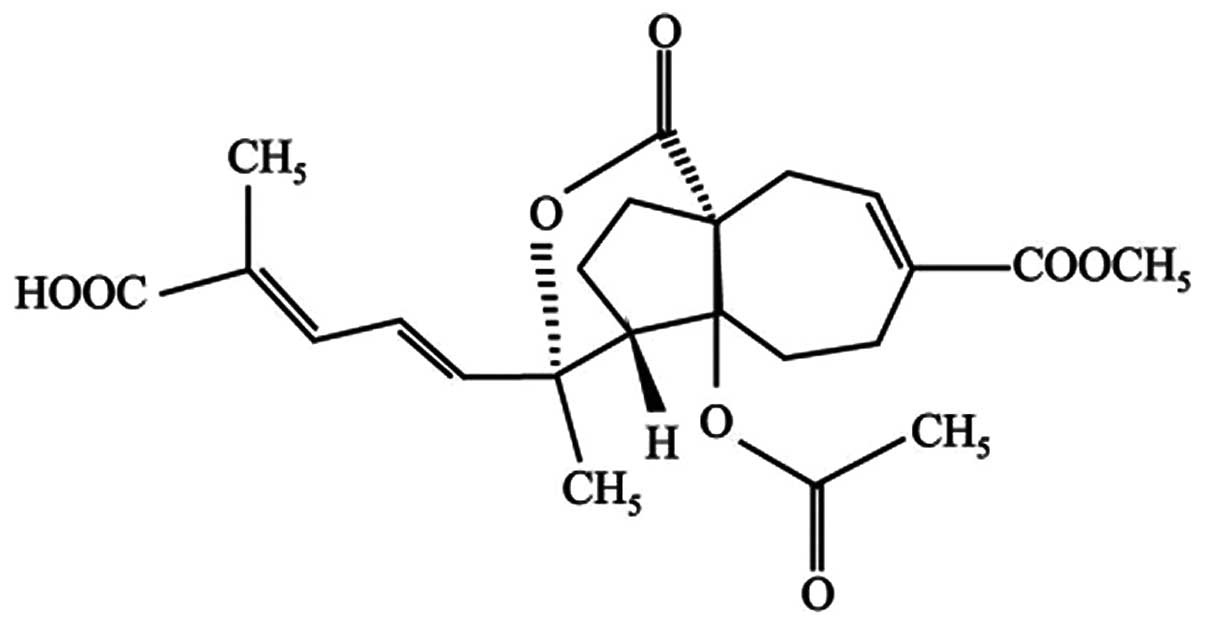
effects (6–9). Pseudolaric acid B (PAB; Fig. 1) is a diterpene acid isolated from
the root and trunk bark of Pseudolarix kaempferi and
possesses multiple biological and pharmacological activities,
including antifungal, antifertility, antitumor, anti-inflammatory
and anti-angiogenic properties (10–15).
The antitumor effect of PAB has been confirmed in
multiple cancer cell lines. In human HeLa cervical carcinoma cells,
PAB promotes apoptosis via activating c-Jun N-terminal kinases
(JNK), protein kinase C (PKC) and caspase-3, downregulating
extracellular signal-regulated kinases (ERK) and Bcl-2 as well as
upregulates the P53 and Bax proteins (16,17).
In human melanoma A375-S2 cells, PAB inhibits proliferation and
induces apoptosis by arresting the cell cycle at the G2/M
checkpoint, upregulating P53 and Bax levels and downregulating
expression of Bcl-2 and Bcl-xl proteins (18). In human AGS gastric cancer cells,
PAB inhibits cell growth in a time- and dose-dependent manner by
arresting the cells at the G2/M phase, downregulating the CDC2 and
Bcl-2 proteins and activating caspase-3 (19). In Bel-7402 human hepatocellular
carcinoma cells, PAB induces apoptosis through caspase-3 activation
and cell cycle inhibition at the G2/M phase (20). In MDA-MB-468 human breast cancer
cells, PAB inhibits cell growth by downregulating the hypoxia
inducible factor-1α protein (15).
In MCF-7 human breast cancer cells, PAB induced apoptosis by
activating JNK, inactivating ERK and upregulating P21 and P53
protein expression (21,22). In murine fibrosarcoma L929 cells,
PAB promotes proliferation, inhibits apoptosis and arrests the cell
cycle at the G2/M phase by downregulating the Bcl-2 and CDC2
proteins (23,24). In HL-60 human leukemia cells, PAB
inhibits proliferation and induces apoptosis by arresting cells at
the G2/M phase of the cell cycle and activating of caspase-3
(25). In DU145 hormone-refractory
prostate cancer cells, PAB induced apoptosis by activating
caspase-3 and -9 and downregulating Bcl-2 (26). In U87 glioblastoma cells, PAB
inhibits proliferation and induces apoptosis by arresting cell
cycle at the G2/M phase, activating caspase-3, upregulating P53 and
Bax protein and downregulating Bcl-2 (27).
However, there have been no studies concerning the
antitumor effect of PAB in A549 human lung cancer cells. In the
present study, the potential roles of PAB in proliferation and
apoptosis in A549 lung cancer cells were investigated.
Materials and methods
Reagents
Pseudolaric acid B (PAB, product no. 110880-200502),
which was purchased from Liaoning North Yaojian Technology and
Developing Company (Liaoning, China), was dissolved in
dimethylsulfoxide (DMSO) and stored at −20°C.
Cell culture
The A549 human lung cancer cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% fetal calf serum (Solarbio Science and
Technology, Beijing, China). Cells were cultured in a 37°C, 5%
CO2/95% air environment. The medium was changed everyday
and the cells were digested using 0.25% trypsin.
MTT assay
A549 cells (1.0×104/well) were plated in
96-well plates and cultured overnight. Cells were incubated with 0,
5, 10, 20, 40 and 80 μmol/l PAB for 24, 48 and 72 h, respectively.
Briefly, 10 μl of 5 mg/ml MTT (Sigma, St. Louis, MO, USA) solution
was added to each well and incubated for 4 h at 37°C, then the
supernatant was removed and DMSO (100 μl) was added to dissolve the
formazan crystals. Absorbance was measured at 570 nm with an
enzyme-linked immunosorbent assay plate reader (Model 550, Bio-Rad,
Hercules, CA USA). The experiment was repeated three times.
Assessment of morphological changes
A549 cells (5.0×105) were seeded on
slides in a 6-well plate and cultured overnight. The cells were
treated with 0.20 μmol/l PAB and incubated at 37.0°C and 5%
CO2 for 24 h. Cells were washed with cold
phosphate-buffered saline (PBS) twice, fixed with methanol and
glacial acetic acid (3:1) for 15 min and stained with Hoechst 33342
for 30 min (Sigma). Morphological changes were observed using
fluorescence microscopy (Nikon, Tokyo, Japan).
Assessment of apoptosis
Cells were treated with various concentrations of
PAB (0, 5, 10, 20, 40 and 80 μmol/l) and incubated at 37.0°C and 5%
CO2 overnight. Cells were collected, centrifuged at 155
× g for 5 min, washed twice with cold PBS and resuspended using 1X
binding buffer, producing a final concentration of
1.0×106 cells/ml. Suspension buffer (100 μl) was
transferred to a test tube, 5 μl Annexin V-fluorescein
isothiocyanate (FITC) and 10 μl propidium iodide (PI) were added
and mixed. Following 15 min staining at room temperature, another
400 μl 1X binding buffer was added. Cell apoptosis was examined
using a BD FACScan flow cytometry system (Franklin Lakes, NJ,
USA).
Western blot analysis
Cells were treated with 0, 5, 20 and 80 μmol/l PAB
solution for 24 h. The cells were then harvested (cell number,
>5×106/ml) and washed twice with cold PBS. Western
blot analysis was performed. Briefly, the cell pellets were
resuspended in lysis buffer at 4°C for 1 h. Following
centrifugation at 22,378 × g for 20 min (2K15C, Sigma), the
supernatant was collected and stored at −80°C. A total of 40 μg
protein was separated using 10% SDS-PAGE and transferred to a
polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). The
membrane was blocked with 5% non-fat milk and incubated overnight
at 4°C with antibodies against Bcl-2 (1:500), Bcl-xl (1:500), Bax
(1:1,000), Bad (1:1,000) (all Cell Signaling Technology, Inc.,
Beverly, MA, USA) and β-actin (1:500; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). Following incubation with horseradish
peroxidase-conjugated anti-mouse IgG (Santa Cruz Biotechnology,
Inc.) at 37°C for 2 h, proteins were visualized using enhanced
chemiluminescence (Pierce Biotechnology Inc., Rockford, IL, USA)
and detected using BioImaging Systems (UVP Inc., Upland, CA,
USA).
Statistical analysis
The statistical package SPSS version 11.0 (SPSS,
Chicago, IL, USA) was used for all analysis. All values are
expressed as the mean ± standard deviation. Differences between
groups were compared with Student’s t-test and P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of PAB on proliferation of A549
lung cancer cells
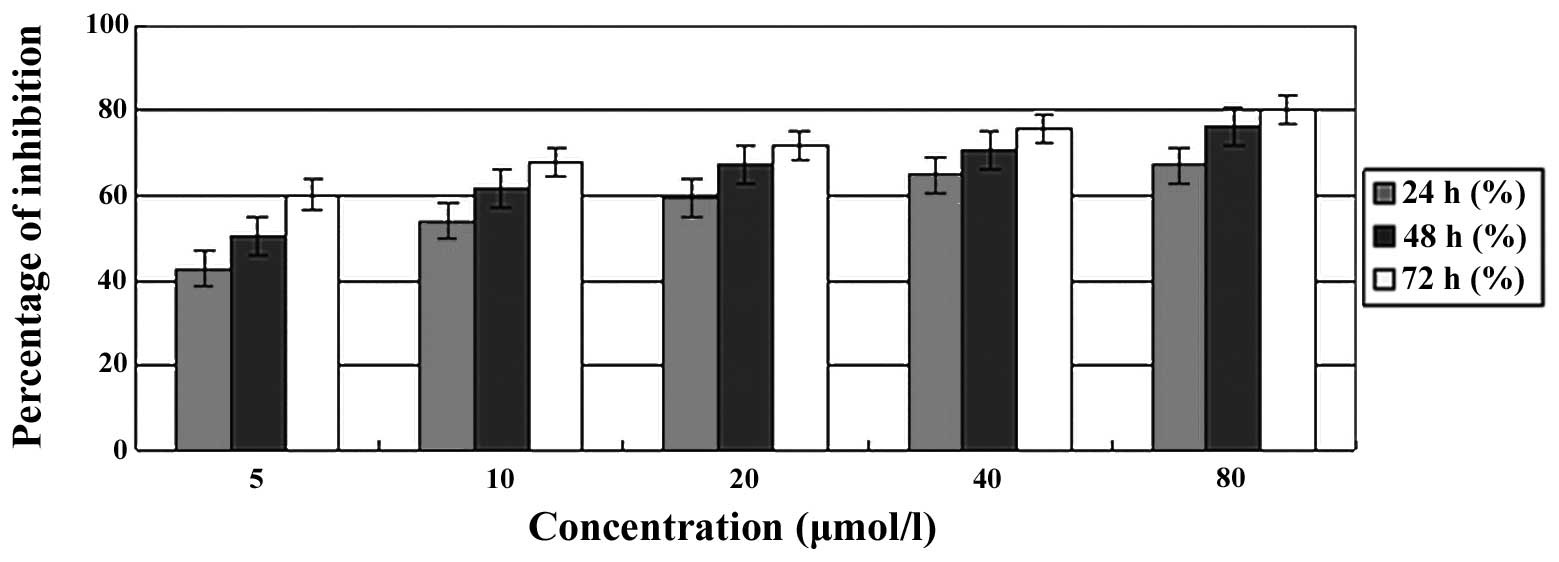
To detect the effect of PAB on the proliferation of
A549 cells, the cells were treated with different doses of PAB
ranging from 0, 5, 10, 20, 40 and 80 μmol/l for the indicated times
(24, 48 or 72 h). The cell growth inhibition ratio was observed to
increase with the PAB concentration and incubation time (Fig. 2), suggesting that PAB significantly
inhibits A549 cell growth in a dose- and time-dependent manner.
Effect of PAB on A549 cell
morphology
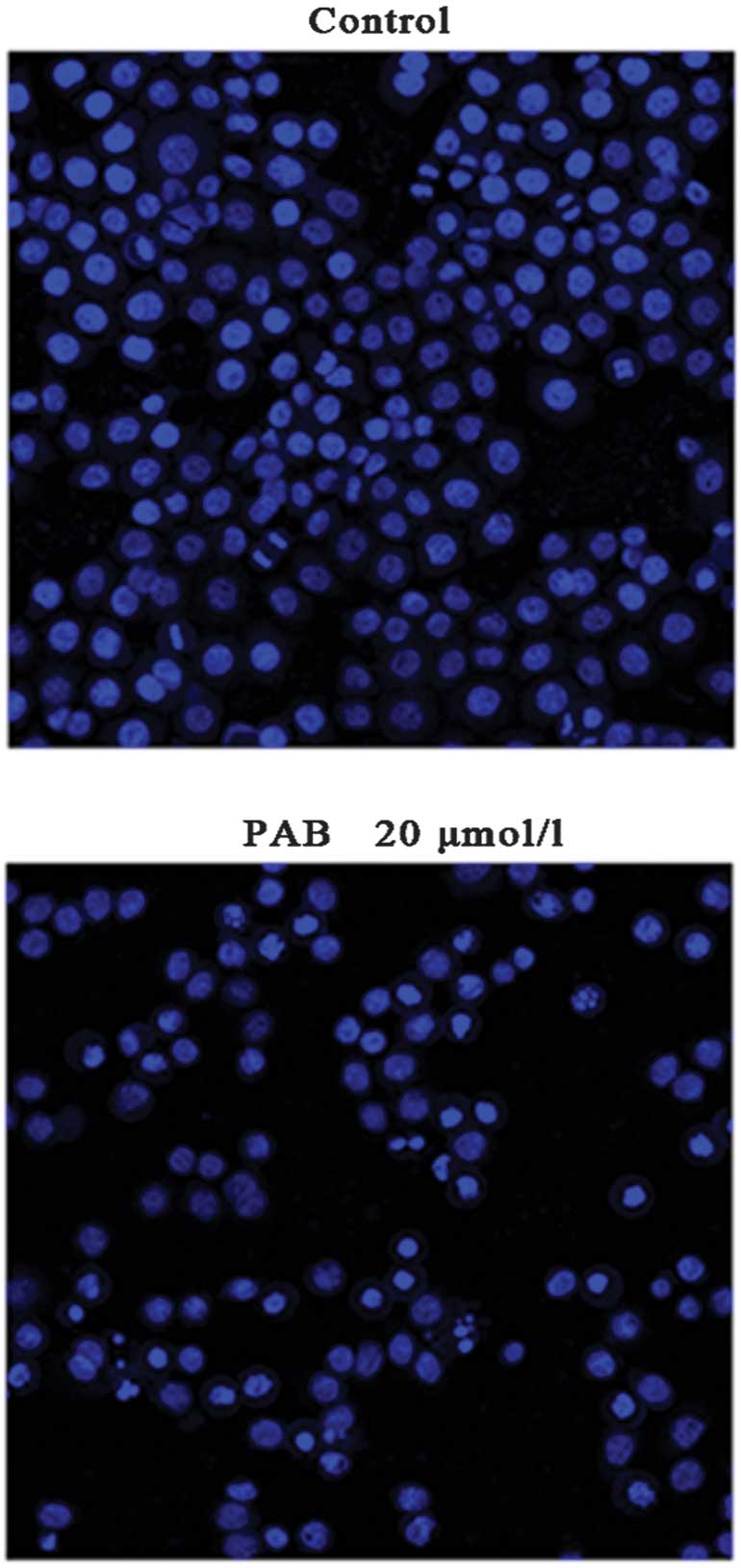
Morphological changes were observed using
fluorescence microscopy (Nikon). A549 cells treated with 20 μmol/l
PAB for 24 h were stained with Hoechst 33342. Cells treated with 20
μmol/l PAB for 24 h exhibited karyorrhexis and apoptotic body
formation following Hoechst 33342 staining (Fig. 3). Morphologically, PAB is capable
of inducing apoptosis in A549 cells.
Effect of PAB on A549 cell apoptosis
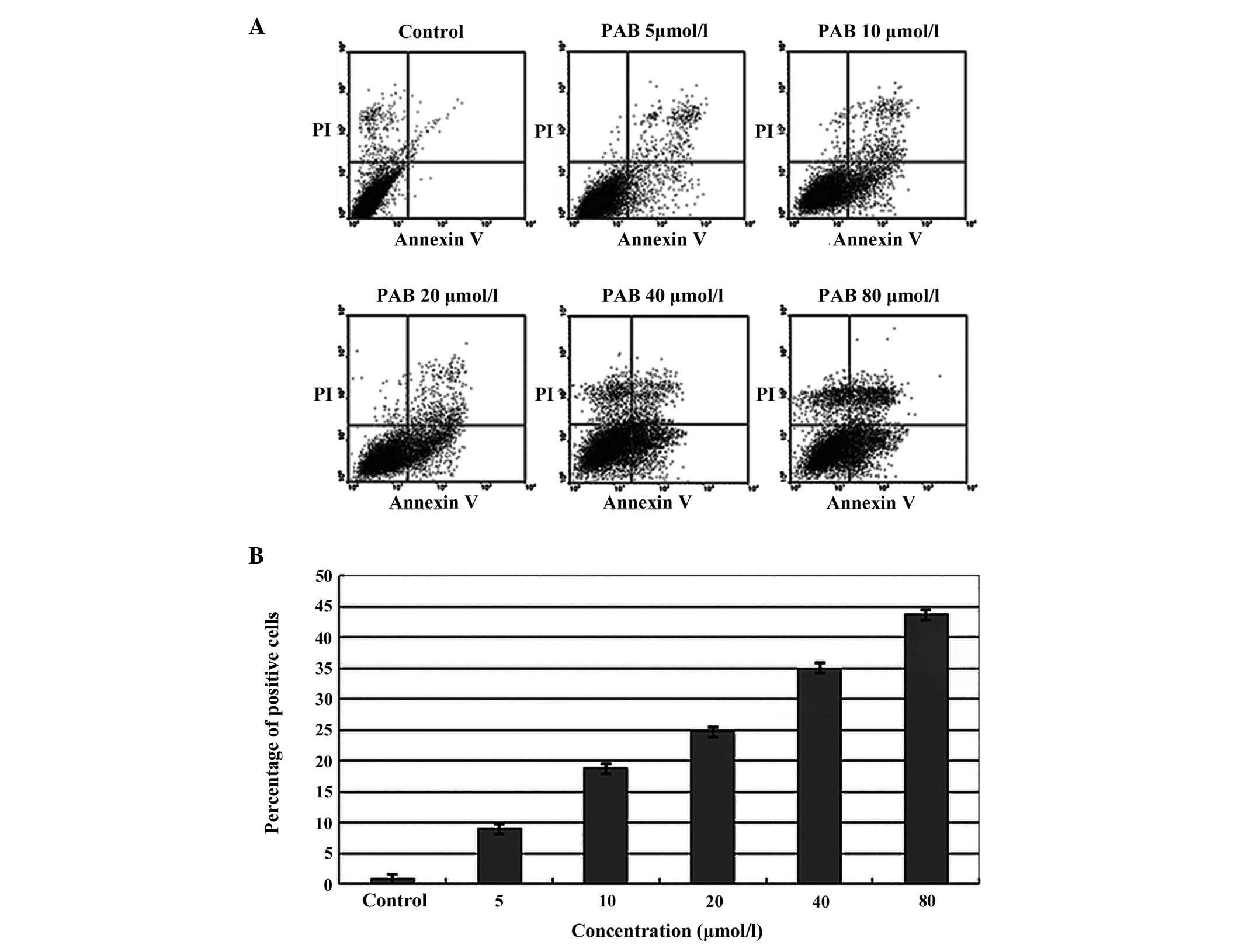
A549 cells were treated with varying concentrations
of PAB (0, 5, 10, 20, 40 and 80 μmol/l) overnight. The cells were
stained with an Annexin-V/PI kit and cell apoptosis was examined
using BD flow cytometry. The apoptosis rates were 8.95, 18.71,
24.66, 35.02 and 43.64%, respectively, in PAB treatment cells and
the rate was 0.80% in the control cells without PAB treatment.
These results suggested that PAB induces A549 cell apoptosis
(Fig. 4).
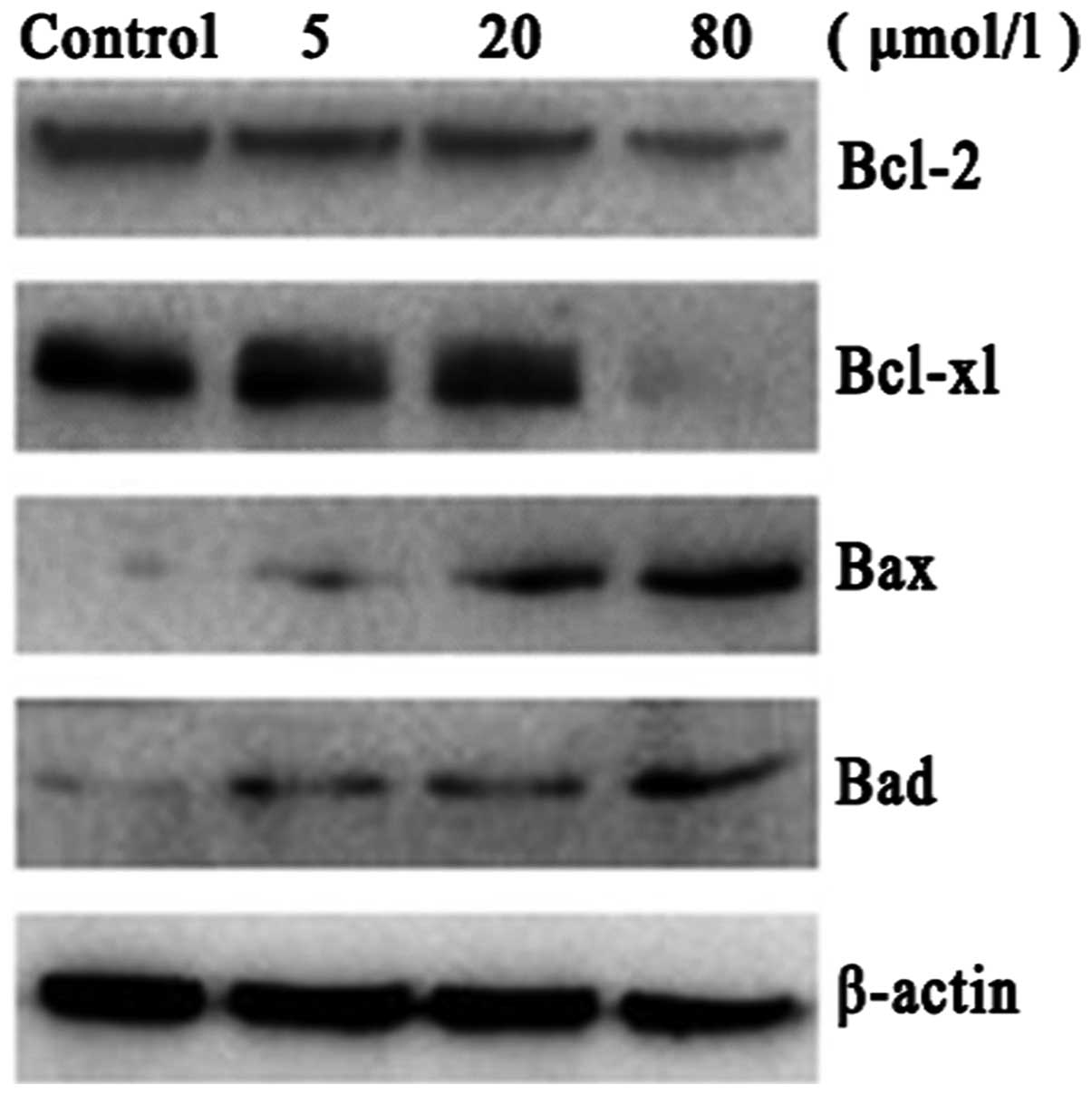
Effect of PAB on the protein levels of
Bcl-2, Bcl-xl, Bax and Bad
To further investigate the mechanism of PAB-induced
apoptosis, the protein levels of a number of Bcl-2 family members
were examined. Bax and Bad protein levels were observed to increase
and Bcl-2 and Bcl-xl protein levels were decreased following PAB
treatment. These results indicated that, at the molecular level,
PAB induces A549 cell apoptosis by regulating Bcl-2, Bcl-xl, Bax
and Bad (Fig. 5).
Discussion
The therapeutic potential of traditional Chinese
medicine has been increasingly recognized by oncologists (3,4). PAB
is a diterpene acid isolated from the root and trunk bark of
Pseudolarix kaempferi and possesses multiple biological and
pharmacological activities, including antifungal, antimicrobial,
antifertility and antiangiogenic properties (10–15).
It has been previously confirmed that PAB exhibits an antitumor
effect by inducing apoptosis in various types of cancer (16–27).
Apoptosis is important in homeostasis maintenance through a balance
between cell proliferation and cell death (28). It is well established that cell
apoptosis is closely associated with cancer development and
progression (29). The antitumor
effects of a number of traditional Chinese medicines are based on
their ability to induce apoptosis (30,31).
Bcl-2 family members are apoptosis regulators. Bcl-2
and Bcl-xl inhibit apoptosis while Bax and Bad promote apoptosis
(32). It is reported that a
number of traditional Chinese medicines exhibit their antitumor
effects by simultaneous upregulation of Bax and Bad and
downregulation of Bcl-2 and Bcl-xl (33–35).
In the present study, the effect of PAB on the
proliferation of A549 cells was examined and it was observed that
PAB significantly inhibits A549 cell growth in a time- and
dose-dependent manner. In addition, the morphological changes
induced by PAB were determined and it was identified karyorrhexis
and apoptotic body formation following PAB treatment. Furthermore,
the apoptosis rate using Annexin-V/PI staining was investigated and
it was observed that PAB induced A549 apoptosis in a dose-dependent
manner. Finally, the potential mechanisms by which PAB induces
apoptosis at molecular levels were investigated and PAB was
identified to upregulate pro-apoptosis Bax and Bad proteins and
downregulate pro-survival Bcl-2 and Bcl-xl proteins.
In conclusion, PAB was shown to inhibit A549 cell
proliferation in a time and dose-dependent manner. PAB induced
apoptosis by the upregulation of Bax and Bad and downregulation of
Bcl-2 and Bcl-xl. Thus, PAB may serve as a potent chemotherapeutic
agent against human lung cancer.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Erridge SC, Møller H, Price A and Brewster
D: International comparisons of survival from lung cancer: pitfalls
and warnings. Nat Clin Pract Oncol. 4:570–577. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Efferth T, Li PC, Konkimalla VS and Kaina
B: From traditional Chinese medicine to rational cancer therapy.
Trends Mol Med. 13:353–361. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Harvey AL: Natural products in drug
discovery. Drug Discov Today. 13:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanson JR: Diterpenoids. Nat Product Rep.
26:1156–1171. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng SY, Chuang CT, Wang SK, et al:
Antiviral and anti-inflammatory diterpenoids from the soft coral
Sinularia gyrosa. J Nat Prod. 73:1184–1187. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hsieh TC, Wijeratne EK, Liang JY, et al:
Differential control of growth, cell cycle progression, and
expression of NF-kappa B in human breast cancer cells MCF-7,
MCF-10A, and MDA-MB-231 by ponicidin and oridonin, diterpenoids
from the chinese herb Rabdosia rubescens. Biochem Biophys
Res Commun. 337:224–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SP, Dong L, Wang Y, et al: Antifungal
diterpenoids of Pseudolarix kaempferi, and their
structure-activity relationship study. Bioorg Med Chem.
11:4577–4584. 2003.PubMed/NCBI
|
|
9
|
Duan HQ, Takaishi Y, Momota H, et al:
Immunosuppressive diterpenoids from Tripterygium wilfordii.
J Nat Prod. 62:1522–1525. 1999. View Article : Google Scholar
|
|
10
|
Trost BM, Waser J and Meyer A: Total
synthesis of (−)-pseudolaric acid B. J Am Chem Soc.
130:16424–16434. 2008.
|
|
11
|
Li E, Clark AM and Hufford CD: Antifungal
evaluation of pseudolaric acid B, a major constituent of
Pseudolarix kaempferi. J Nat Prod. 58:57–67. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu B, Chen H, Lei ZY, Yu PF and Xiong B:
Studies on anti-tumour activities of pseudolaric acid-B (PLAB) and
its mechanism of action. J Asian Nat Prod Res. 8:241–252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li T, Wong VK, Yi XQ, et al: Pseudolaric
acid B suppresses T lymphocyte activation through inhibition of
NF-kappa B signaling pathway and p38 phosphorylation. J Cell
Biochem. 108:87–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng AG and Jiang LL: Pseudolaric acid
B-induced apoptosis through p53-dependent pathway in human gastric
carcinoma cells. J Asian Nat Prod Res. 11:142–152. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li MH, Miao ZH, Tan WF, et al: Pseudolaric
acid B inhibits angiogenesis and reduces hypoxia-inducible factor
1alpha by promoting proteasome-mediated degradation. Clin Cancer
Res. 10:8266–8274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gong XF, Wang MW, Wu Z, et al: Pseudolaric
acid B induces apoptosis via activation of c-Jun N-terminal kinase
and caspase-3 in HeLa cells. Exp Mol Med. 36:551–556. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gong XF, Wang MW, Tashiro S, et al:
Involvement of JNK-initiated p53 accumulation and phosphorylation
of p53 in pseudolaric acid B induced cell death. Exp Mol Med.
38:428–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gong XF, Wang MW, Tashiro S, et al:
Pseudolaric acid B induces apoptosis through p53 and Bax/Bcl-2
pathways in human melanoma A375-S2 cells. Arch Pharm Res. 28:68–72.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li KS, Gu XF, Li P, et al: Effect of
pseudolaric acid B on gastric cancer cells: inhibition of
proliferation and induction of apoptosis. World J Gastroenterol.
11:7555–7559. 2005.PubMed/NCBI
|
|
20
|
Wu WY, Guo HZ, Qu GQ, et al: Mechanisms of
pseudolaric acid B-induced apoptosis in Bel-7402 cell lines. Am J
Chin Med. 34:887–899. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu JH, Cui Q, Jiang YY, et al: Pseudolaric
acid B induces apoptosis, senescence, and mitotic arrest in human
breast cancer MCF-7. Acta Pharmacol Sin. 28:1975–1983. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu JH, Wang HJ, Li XR, et al: Protein
tyrosine kinase, JNK, and ERK involvement in pseudolaric acid
B-induced apoptosis of human breast cancer MCF-7 cells. Acta
Pharmacol Sin. 29:1069–1076. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Li X, Tashiro S, et al: Bcl-2 family
proteins were involved in pseudolaric acid B-induced autophagy in
murine fibrosarcoma L929 cells. J Pharmacol Sci. 107:295–302. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qi M, Yao G, Fan S, et al: Pseudolaric
acid B induces mitotic catastrophe followed by apoptotic cell death
in murine fibrosarcoma L929 cells. Eur J Pharmacol. 683:16–26.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma G, Chong L, Li XC, et al: Selective
inhibition of human leukemia cell growth and induction of cell
cycle arrest and apoptosis by pseudolaric acid B. J Cancer Res Clin
Oncol. 136:1333–1340. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao D, Lin F, Wu X, et al: Pseudolaric
acid B induces apoptosis via proteasome-mediated Bcl-2 degradation
in hormone-refractory prostate cancer DU145 cells. Toxicol In
Vitro. 26:595–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khan M, Zheng B, Yi F, et al: Pseudolaric
acid B induces caspase-dependent and caspase-independent apoptosis
in U87 glioblastoma cells. Evid Based Complement Alternat Med.
2012:9575682012.PubMed/NCBI
|
|
28
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 2000. View Article : Google Scholar
|
|
30
|
Huang DM, Shen YC, Wu C, et al:
Investigation of extrinsic and intrinsic apoptosis pathways of new
clerodane diterpenoids in human prostate cancer PC-3 cells. Eur J
Pharmacol. 503:17–24. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheung HY, Cheung SH, Li JL, et al:
Andrographolide isolated from Andrographis paniculata
induces cell cycle arrest and mitochondrial-mediated apoptosis in
human leukemic HL-60 cells. Planta Med. 71:1106–1111.
2005.PubMed/NCBI
|
|
32
|
Korsmeyer SJ: BCL-2 gene family and the
regulation of programmed cell death. Cancer Res. 59(Suppl 7):
1693s–1700s. 1999.PubMed/NCBI
|
|
33
|
Jiao Y, Ge CM, Meng QH, et al:
Dihydroartemisinin is an inhibitor of ovarian cancer cell growth.
Acta Pharmacol Sin. 28:1045–1056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang JB, Qi LL, Zheng SD and Wu TX:
Curcumin induces apoptosis through the mitochondria-mediated
apoptotic pathway in HT-29 cells. J Zhejiang Univ Sci B. 10:93–102.
2009. View Article : Google Scholar
|
|
35
|
Wu SJ, Ng LT, Lin DL, et al: Physalis
peruviana extract induces apoptosis in human Hep G2 cells
through CD95/CD95L system and the mitochondrial signaling
transduction pathway. Cancer Lett. 215:199–208. 2004. View Article : Google Scholar
|