Introduction
Dexamethasone is a potent synthetic member of the
glucocorticoid class of steroid hormones (1). Dexamethasone is reported to have
major effects on a number of organ systems, including the
cardiovascular, endocrine, gastrointestinal, ophthalmic and
musculoskeletal systems (2).
Dexamethasone is a potent modulator of osteogenic differentiation
and previous studies have shown that dexamethasone increased the
bone formation capacity of osteoprogenitor cells in vitro
(3,4). Furthermore, it was reported that
dexamethasone acts synergistically with growth factors (5). Fibroblast growth factor-2 (FGF-2) is
reported to have numerous biological activities, including
stimulation of cell growth, migration, angiogenesis, wound healing,
tissue repair, differentiation and morphogenesis (5). In addition, FGF-2 is known to play a
critical role in bone growth and development (6–8).
A previous report showed that dexamethasone and
FGF-2 are required to maintain cell propagation, alkaline
phosphatase (ALP) expression and osteocalcin secretion and act
synergistically. However, protein content is FGF-2-dependent and
mineralization is dexamethasone-dependent (9). Controversial opinions exist with
regard to the combined effects of dexamethasone and FGF-2 in
proliferation, differentiation and mineralization. FGF-2, in
combination with dexamethasone, were previously found to stimulate
the proliferation and osteoblastic differentiation of human adipose
tissue-derived mesenchymal stromal cells (10). In another study, dexamethasone
increased mineralization, while FGF-2 blocked this activity and the
addition of dexamethasone to FGF-2 did not alter FGF-2-associated
inhibition (5).
The current study aimed to examine the
dose-dependent impact of dexamethasone and FGF-2 on the
differentiation of osteoprecursor cells. The ALP test was performed
to assess differentiation and protein expression associated with
bone formation, including bone morphogenetic protein receptor-IA
(BMPRIA) and bone morphogenetic protein receptor-II (BMPRII) and
phosphor-Smad1/5/8 (pSmad1/5/8) was evaluated by western blot
analysis.
Materials and methods
Cell culture
Murine calvarial osteoprecursor cells (MC3T3-E1)
were plated and cultures were maintained in α-minimum essential
medium (αMEM) supplemented with 10% fetal bovine serum, antibiotics
[penicillin 100 U/ml and streptomycin 100 μg/ml (all Invitrogen
Life Technologies, Carlsbad, CA, USA)], 50 μg/ml ascorbic acid and
10 mM β-glycerophosphate (both Sigma-Aldrich, St. Louis, MO, USA).
Cells were stimulated with dexamethasone and FGF-2 at a final
concentration of 10 nM (D1) to 100 nM (D2) for dexamethasone and 2
ng/ml (F1) to 20 ng/ml (F2) for FGF-2. The cultures were maintained
in a humidified atmosphere with 5% CO2 and 95% air at
37°C.
Protein measurement
Cells were incubated in αMEM in the presence of
ascorbic acid and β-glycerophosphate for two days. Protein content
was determined based on the Bradford method, using the Coomassie
protein assay reagent in comparison with a series of bovine serum
albumin as internal standards (11). The absorbance was recorded at 595
nm using the microplate spectrophotometer system (BioTek, Winooski,
VT, USA) and results are presented as the percentage of control
values.
ALP activity assays
The ALP assay for osteoblast differentiation was
performed after incubation. MC3T3-E1 murine calvarial
preosteoblasts were lysed with a buffer containing 10 mM Tris-HCl
(pH 7.4) and 0.2% Triton X-100 and were then sonicated for 20 sec
at 4°C. Samples were incubated with 10 mM p-nitrophenylphosphate as
a substrate in 100 mM glycine buffer (pH 10.5) containing 1 mM
MgCl2 at 37°C in a water bath. The absorbance at 405 nm
was measured using a microplate reader (BioTek) and ALP activities
were normalized with respect to total protein content (12,13).
Western blot analysis
Osteoprecusor cells were washed twice with ice-cold
phosphate-buffered saline and solubilized with a lysis buffer. The
lysates were centrifuged at 16,000 × g for 20 min at 4°C to remove
the nuclear pellet. The supernatants were boiled in a sodium
dodecyl sulfate sample buffer containing β-mercaptoethanol. Equal
amounts extracts were separated by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
polyvinylidene fluoride microporous membranes (Immobilon-P
membranes; Millipore Corporation, Billerica, MA, USA). The
membranes were blocked for >1 h in 0.1% (v/v) phosphate-buffered
saline and Tween-20 containing 5% (w/v) powdered milk. Each
membrane was probed with the desired antibodies diluted in the same
buffer at the recommended concentrations. Each membrane was
incubated with horseradish peroxidase-conjugated secondary antibody
and then the washed blot was developed using enhanced
chemiluminescence detection kits (14,15).
Mouse antibodies against BMPRIA, BMPRII and pSmad1/5/8 and
horseradish peroxidase secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA) and Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Statistical analysis
Results are expressed as means ± standard deviations
of the experiments. Two-way analysis of variance (ANOVA) with
post-hoc tests were performed to determine the combination effects
of dexamethasone and FGF-2 using a commercially available program
(SPSS 12 for Windows; SPSS Inc., Chicago, IL, USA). A one-way ANOVA
was used to compare data within the same groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Protein measurement
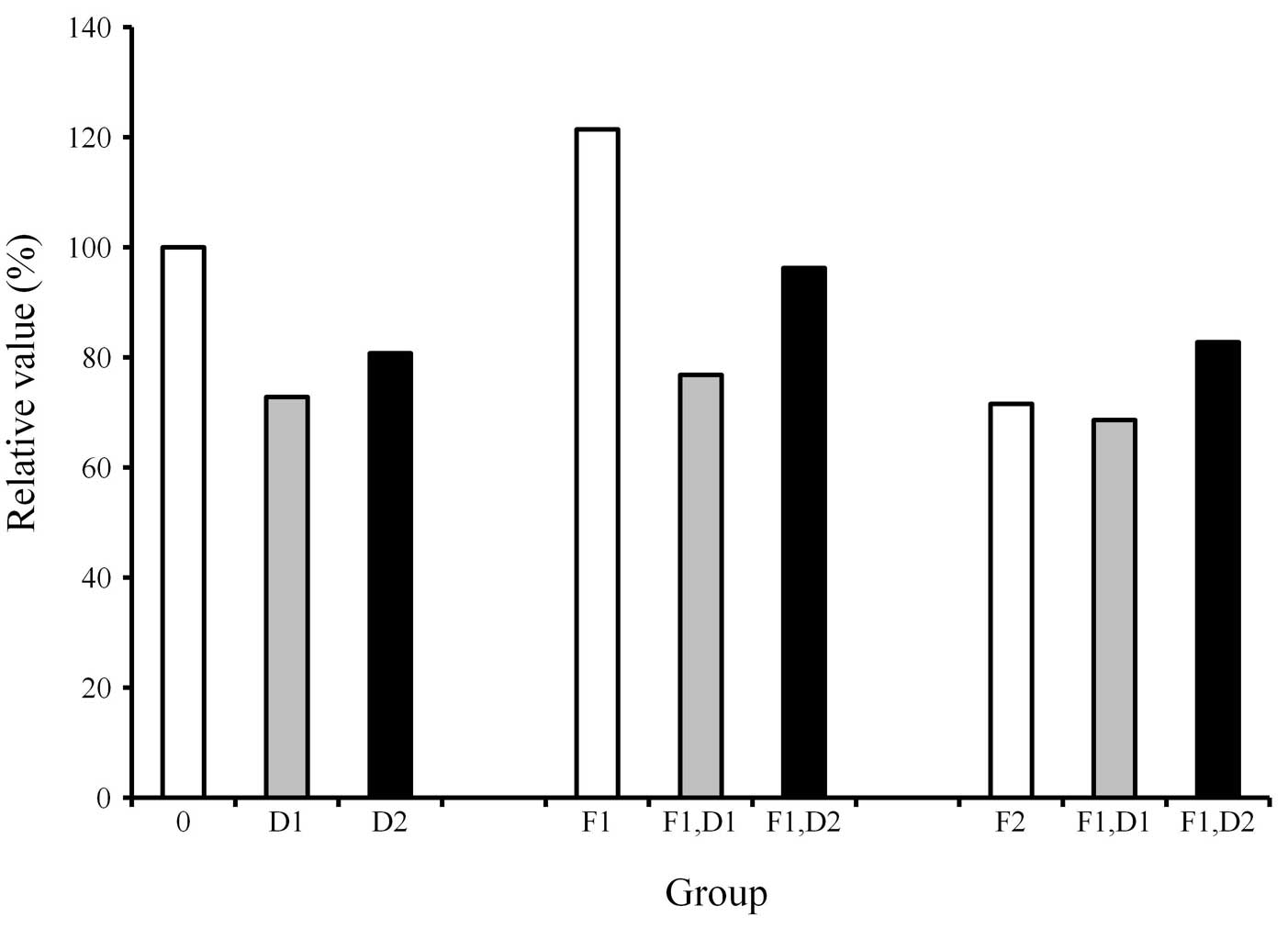
The protein content in each culture plate was
determined (Fig. 1). The results
showed that the protein content of the cultures grown with the
osteogenic differentiation media in the presence of FGF-2 at 2
ng/ml (F1) was increased compared with that of the control.
However, the addition of dexamethasone to the cultures uniformly
showed a decrease of protein content when compared with the
dexamethasone-unloaded group.
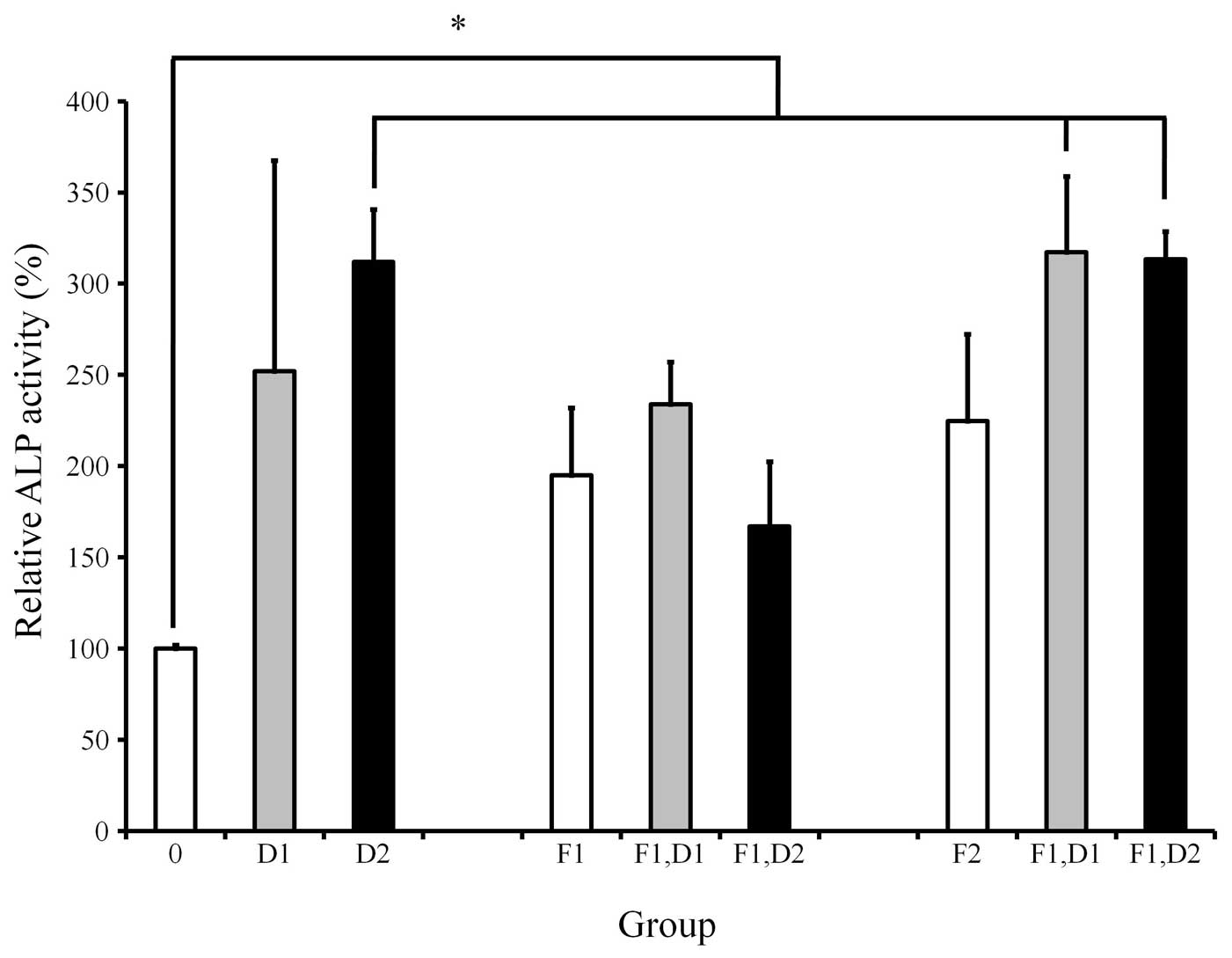
ALP activity assay
ALP activity was increased when cells were treated
with dexamethasone, with the highest value at 100 nM (Fig. 2). Cultures grown in the presence of
20 ng/ml FGF-2 exhibited an increased value of ALP activity when
compared with 10 and 100 nM dexamethasone. Similarly, cultures
grown in the presence of 20 ng/ml FGF-2 and 10 nM dexamethasone
exhibited an increased value of ALP activity when compared with the
10 nM dexamethasone-only group. The addition of 20 ng/ml FGF-2 to
100 nM dexamethasone resulted in an increase of ALP activity in
comparison with that of the 100 nM dexamethasone group. However,
statistically significant differences were not observed
(P>0.05).
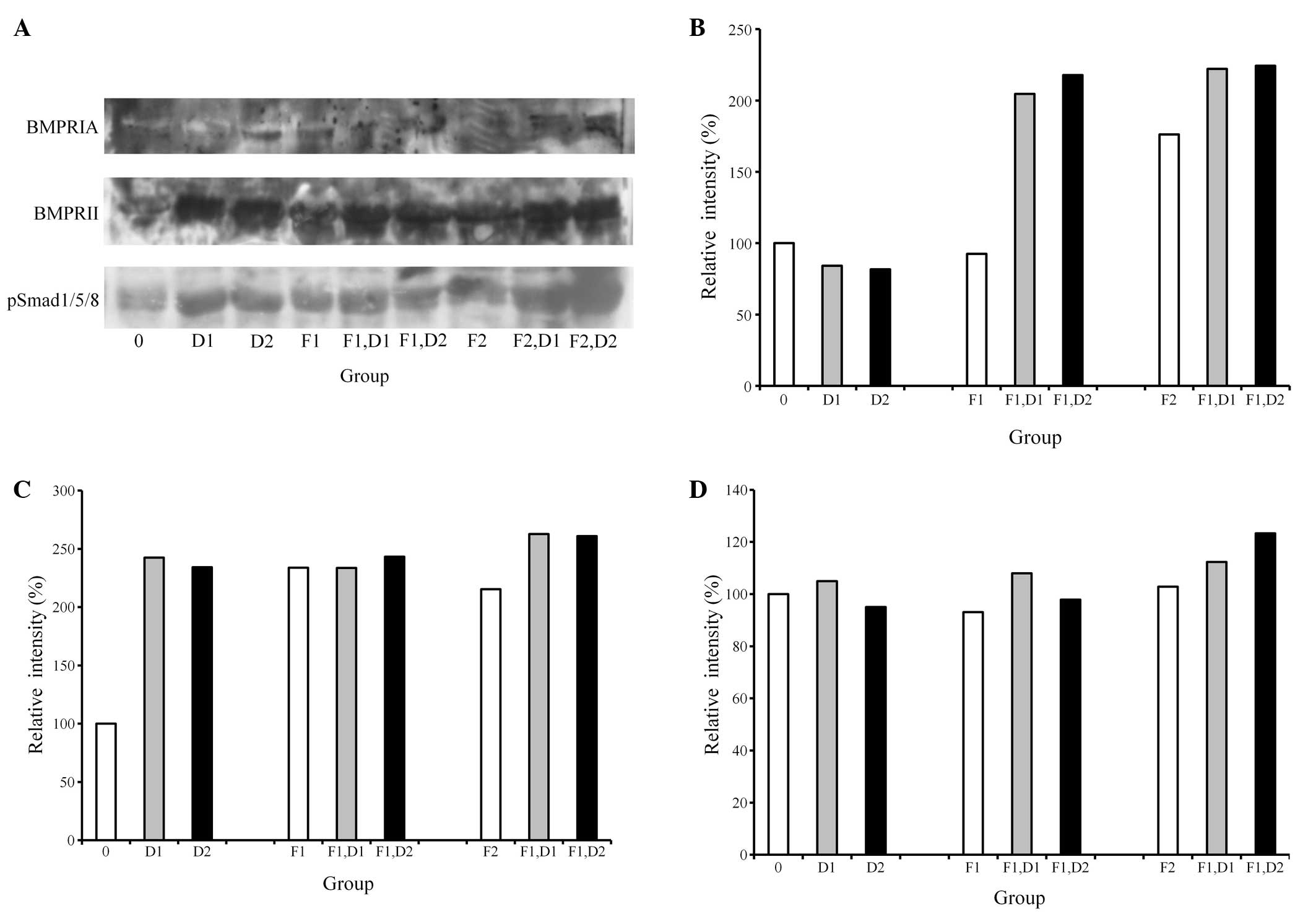
Western blot analysis
A western blot analysis was performed to detect the
protein expression following treatment with dexamethasone and FGF-2
(Fig. 3A). The results showed that
the addition of 20 ng/ml FGF-2 and 100 nM dexamethasone appeared to
increase the expression of BMPRIA and BMPRII (Fig. 3B and C). Similarly, the combination
of 20 ng/ml FGF-2 and 100 nM dexamethasone produced the highest
pSmad1/5/8 expression, yielding 260%, while the untreated control
was considered to be 100% (Fig.
3D).
Discussion
In the current study, the combined effects of
dexamethasone and FGF-2 on protein content, differentiation and
protein expression of osteoblast progenitor cells under
predetermined concentrations (10 and 100 nM dexamethasone; 2 and 20
ng/ml FGF-2) were studied. The study examined the mechanisms of
dexamethasone and FGF-2 on the regulation of the protein expression
in mouse preosteoblastic cells. In addition, evaluations were
conducted to identify whether combinations of dexamethasone and
FGF-2 produced effects additively, synergistically or
competitively.
An increase in protein content was achieved with the
2 ng/ml FGF-2 group under osteogenic differentiation media. This is
in agreement with a previous report demonstrating that FGF-2
affected the proliferation and differentiation of the tested cells
(14).
The treatment of dexamethasone on the preosteoblast
clearly showed an increased level of ALP activity. This is similar
to previous reports which showed that treatment with
10−8 to 10−7 M dexamethasone presented a
significant induction in the ALP activity of human bone marrow
cells, leading to the osteoblast differentiation of bone marrow
cells (4,16). The current study showed that the
addition of 20 ng/ml FGF-2 to 100 nM dexamethasone produced an
increase in ALP activity in comparison with that of the 100 nM
dexamethasone group, however, this was not observed to be
statistically significant.
Since BMP pathways are involved in osteoblast
differentiation, factors associated with BMP signaling were
evaluated (17). The present study
clearly showed that the expression of BMPRIA and BMPRII was
affected with the treatment of dexamethasone and FGF-2. Previous
reports have shown that BMPRIA and BMPRII were induced by BMP-2 and
it was hypothesized that the induction of BMPRIA and BMPRII may be
sufficient for the induction of bone formation (14,18).
The current study has demonstrated that the combination of 100 nM
dexamethasone and 20 ng/ml FGF-2 produced the highest expression of
BMPRIA and BMPRII, leading to the highest value of pSmad1/5/8
expression.
The results, in terms of the effect of the
combination of dexamethasone and FGF-2 on osteoblastic
differentiation may be controversial, due to the different systems,
maturation stages of the cells studied, heterogenicity of the
osteoblast population, culture conditions and species differences
(2,19).
Within the limits of this study, dexamethasone
significantly enhanced osteoblast differentiation, however, the
combined delivery of dexamethasone and FGF-2 did not produce
synergistic effects on osteoblast differentiation under the current
experimental conditions. Additional studies are required to
evaluate divergent conditions to select the optimal dosage and
selective timing for the delivery of the agents.
Acknowledgements
This study was supported by Seoul St. Mary’s
Clinical Medicine Research Program 2013 through the Catholic
University of Korea.
References
|
1
|
Naik PN, Chimatadar SA and Nandibewoor ST:
Interaction between a potent corticosteroid drug - dexamethasone
with bovine serum albumin and human serum albumin: a fluorescence
quenching and fourier transformation infrared spectroscopy study. J
Photochem Photobiol B. 100:147–159. 2010. View Article : Google Scholar
|
|
2
|
Ishida Y and Heersche JN:
Glucocorticoid-induced osteoporosis: both in vivo and in vitro
concentrations of glucocorticoids higher than physiological levels
attenuate osteoblast differentiation. J Bone Miner Res.
13:1822–1826. 1998. View Article : Google Scholar
|
|
3
|
Atmani H, Chappard D and Basle MF:
Proliferation and differentiation of osteoblasts and adipocytes in
rat bone marrow stromal cell cultures: effects of dexamethasone and
calcitriol. J Cell Biochem. 89:364–372. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coelho MJ and Fernandes MH: Human bone
cell cultures in biocompatibility testing. Part II: effect of
ascorbic acid, beta-glycerophosphate and dexamethasone on
osteoblastic differentiation. Biomaterials. 21:1095–1102. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hakki SS, Nohutcu RM, Hakki EE, Berry JE,
Akkaya MS and Somerman MJ: Dexamethasone and basic-fibroblast
growth factor regulate markers of mineralization in cementoblasts
in vitro. J Periodontol. 76:1550–1558. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mansukhani A, Bellosta P, Sahni M and
Basilico C: Signaling by fibroblast growth factors (FGF) and
fibroblast growth factor receptor 2 (FGFR2)-activating mutations
blocks mineralization and induces apoptosis in osteoblasts. J Cell
Biol. 149:1297–1308. 2000. View Article : Google Scholar
|
|
7
|
Xiao G, Jiang D, Gopalakrishnan R and
Franceschi RT: Fibroblast growth factor 2 induction of the
osteocalcin gene requires MAPK activity and phosphorylation of the
osteoblast transcription factor, Cbfa1/Runx2. J Biol Chem.
277:36181–36187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sobue T, Naganawa T, Xiao L, et al:
Over-expression of fibroblast growth factor-2 causes defective bone
mineralization and osteopenia in transgenic mice. J Cell Biochem.
95:83–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kotev-Emeth S, Pitaru S, Pri-Chen S and
Savion N: Establishment of a rat long-term culture expressing the
osteogenic phenotype: dependence on dexamethasone and FGF-2.
Connect Tissue Res. 43:606–612. 2002. View Article : Google Scholar
|
|
10
|
Lee SY, Lim J, Khang G, et al: Enhanced ex
vivo expansion of human adipose tissue-derived mesenchymal stromal
cells by fibroblast growth factor-2 and dexamethasone. Tissue Eng
Part A. 15:2491–2499. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park JB: Low dose of doxycyline promotes
early differentiation of preosteoblasts by partially regulating the
expression of estrogen receptors. J Surg Res. 178:737–742. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JB: The effects of dexamethasone,
ascorbic acid, and β-glycerophosphate on osteoblastic
differentiation by regulating estrogen receptor and osteopontin
expression. J Surg Res. 173:99–104. 2012.
|
|
13
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances differentiation of
osteoblastic cells by regulating the expressions of
phospho-Smad1/5/8. Exp Ther Med. 4:303–306. 2012.PubMed/NCBI
|
|
14
|
Park JB: Effects of fibroblast growth
factor 2 on osteoblastic proliferation and differentiation by
regulating bone morphogenetic protein receptor expression. J
Craniofac Surg. 22:1880–1882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JB: Effects of doxycycline,
minocycline, and tetracycline on cell proliferation,
differentiation, and protein expression in osteoprecursor cells. J
Craniofac Surg. 22:1839–1842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Beloti MM and Rosa AL: Osteoblast
differentiation of human bone marrow cells under continuous and
discontinuous treatment with dexamethasone. Braz Dent J.
16:156–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fakhry A, Ratisoontorn C, Vedhachalam C,
et al: Effects of FGF-2/−9 in calvarial bone cell cultures:
differentiation stage-dependent mitogenic effect, inverse
regulation of BMP-2 and noggin, and enhancement of osteogenic
potential. Bone. 36:254–266. 2005.
|
|
18
|
Nakamura Y, Wakitani S, Nakayama J,
Wakabayashi S, Horiuchi H and Takaoka K: Temporal and spatial
expression profiles of BMP receptors and noggin during
BMP-2-induced ectopic bone formation. J Bone Miner Res.
18:1854–1862. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim CH, Cheng SL and Kim GS: Effects of
dexamethasone on proliferation, activity, and cytokine secretion of
normal human bone marrow stromal cells: possible mechanisms of
glucocorticoid-induced bone loss. J Endocrinol. 162:371–379. 1999.
View Article : Google Scholar
|