Introduction
Oral cancer ranks among the ten most frequently
diagnosed cancers worldwide and has one of the lowest five-year
survival rates (1,2). Mucoepidermoid carcinoma (MEC), a type
of oral cancer, is the most common malignant salivary gland tumor,
usually affecting the parotid and minor salivary glands of adults.
Due to the phenotypic and biological heterogeneity of MECs, several
studies have attempted to identify potential biomarkers and
therapeutic strategies to improve clinical assessment (3–6).
Naturally-occurring products, derived from plant
sources, are used as traditional medicines due to their potential
chemotherapeutic activity (7,8).
Smilax china L. is a small vine that grows in the southern
parts of China. It has a long history of indigenous use and has
been widely used in Chinese traditional medicine for treatment of
diverse diseases, particularly for acute and chronic pelvic
inflammation (9,10). Recently, several studies have
reported that Smilax china L.-derived compounds have
anticancer activities in various cancer cell lines, including
cervical, lung, gastric and breast cancers (11–13).
However, the apoptotic effect of Smilax china L. in MEC has
not been thoroughly investigated.
Mitogen-activated protein kinase (MAPK) cascades
have been reported to be central in integrating the signals from a
diverse group of extracellular stimuli to the nucleus, where
activation of specific transcription factors elicits cellular
responses by the regulation of gene expression (14). Three major MAPK pathways have been
identified in mammals, extracellular signal-regulated kinases
(ERKs), stress-activated protein kinases/c-Jun N-terminal kinases
(JNKs) and p38 MAPKs. Among them, the ERK1/2 pathway contributes to
survival signaling in various tumors and inhibition of the ERK1/2
signaling pathway may be more effective for inducing tumor cell
death than JNK and p38 (15). In
the present study, the antiproliferative effects of the methanol
extract of Smilax china L. (MESC), and the associated
signaling pathways in MC-3 human oral MEC cells, were
investigated.
Materials and methods
Reagents and antibodies
MESC was provided by Professor Ki-Han Kwon (Kwangju
University, Kwangju, Korea). Dulbecco’s modified Eagle’s medium
(DMEM), fetal bovine serum (FBS), 100X antibiotic solution, trypsin
and Dulbecco’s phosphate-buffered saline (PBS) were obtained from
WelGENE Inc. (Daegu, Korea). The actin antibody was purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Death
receptor 4 (DR4) antibody was obtained from R&D Systems Inc.
(Minneapolis, MN, USA). Antibodies against B-cell lymphoma 2
(Bcl-2) interacting mediator of cell death (Bim), truncated BH3
interacting-domain death agonist (t-Bid), Bcl-2 homologous
antagonist killer, Bcl-2-associated X protein, Bcl-2, B-cell
lymphoma-extra large, myeloid leukemia cell differentiation
protein, cleaved caspase-8, p-ERK, ERK, p-JNK, JNK, p-p38 and p38
were supplied by Cell Signaling Technology, Inc. (Danvers, MA,
USA). DAPI and cycloheximide (CHX) were acquired from Sigma-Aldrich
(St. Louis, MO, USA). PD98059 was provided by Calbiochem Technology
(San Diego, CA, USA).
Cell culture and drug treatment
MC-3 human MEC cells were provided by Professor Wu
Junzheng (Forth Military Medical University, Xi’an, China). MC-3
cells were cultured in DMEM supplemented with 10% FBS and 100 U/ml
each of penicillin and streptomycin in a humidified atmosphere
containing 5% CO2 at 37°C. An equal number of cells were
seeded and allowed to attach. When the cells reached 50%
confluency, they were treated with dimethylsulfoxide (DMSO) or MESC
(25, 50 and 100 μg/ml). MESC was dissolved in 0.1% DMSO (vehicle
control).
Cell viability assay
The MC-3 cells were treated with 25, 50 and 100
μg/ml MESC for 24 h and the number of viable cells was counted
using a hematocytometer (Neubauer Chamber, Thermo Fisher
Scientific, Waltham, MA, USA) with trypan blue (0.4%). Each
experiment was conducted in triplicate and the results were
expressed as the mean ± standard deviation for each treatment
group.
DAPI staining
Apoptotic cell death was determined morphologically
using the fluorescent nuclear dye, DAPI. MC-3 cells were harvested
by trypsinization and fixed in 100% ethanol overnight at −20°C. The
cells were re-suspended in PBS, deposited on poly-L-lysin-coated
slides and stained with DAPI. The cell morphology was observed
under a fluorescence microscope (Axio Imager.M2; Carl Zeiss Co.
Ltd., Seoul, Republic of Korea).
Western blot analysis
Whole-cell lysates were extracted with lysis buffer
and quantified using the DC Protein Assay kit (Bio-Rad, Hercules,
CA, USA). Equal quantities of protein from each sample were mixed
with 5X loading buffer and heated at 95°C for 5 min. Equal
quantities of protein were separated by SDS-PAGE and transferred
onto a polyvinylidene fluoride membrane (Bio-Rad). The membranes
were blocked with 5% skimmed milk in Tris-buffered saline with
Tween 20 (TBST) buffer at room temperature for 2 h, washed with
TBST and maintained overnight at 4°C with primary antibody. The
membranes were then washed with TBST, followed by incubation with
horseradish peroxidase-conjugated secondary antibody (Santa Cruz
Biotechnology, Inc.) at room temperature for 2 h. Following an
additional wash with TBST, antibody-bound proteins were detected
using an Enhanced Chemiluminescence Western Blotting Luminol
Reagent (Santa Cruz Biotechnology, Inc.).
JC-1 assay
Alterations in mitochondrial membrane potential
(MMP) were measured using the Mitochondrial Membrane Potential
Detection kit (Stratagene, La Jolla, CA, USA). MC-3 cells were
treated with MESC and incubated with 1X JC-1 reagent for 15 min at
37°C in a 5% CO2 incubator. Cells were then washed with
PBS (3X). JC-1 fluorescence was measured using a microplate reader
(Plate CHAMELEON; Hidex, Turku, Finland).
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from the cells using an
easy-BLUE Total RNA Extraction kit (Intron, Seoul, Korea).
Subsequently, 1 μg RNA was used to synthesize the cDNA using the
Reverse Transcription system (Promega, Madison, WI, USA). The PCR
conditions of DR5 were as follows: 30 cycles; 1 min at 95°C, 1 min
at 57.8°C and 1 min 30 sec at 72°C. The PCR conditions of β-actin
were as follows: 28 cycles; 1 min at 95°C, 1 min at 60°C and 1 min
30 sec at 72°C. The primer sequences used were as follows: DR5
Sense: 5′-ATG AGA TCG TGA GTA TCT TGC AGC-3′ and antisense: 5′-TGA
CCC ACT TTA TCA GDA TCG TGT-3′ for DR5; and sense: 5′-GTG GGG CGC
CCC AGG CAC CA-3′ and antisense: 5′-CTC CTT AAT GTC ACG CAC GAT
TTC-3′ for β-actin. The PCR products were analyzed by 2% agarose
gel electrophoresis.
Statistical analysis
The data were assessed for statistical significance
using Student’s t-test. A value of P<0.05 compared with the
vehicle control was considered to indicate a statistically
significant difference.
Results
Effect of MESC on cell viability and
apoptosis in MC-3 cells
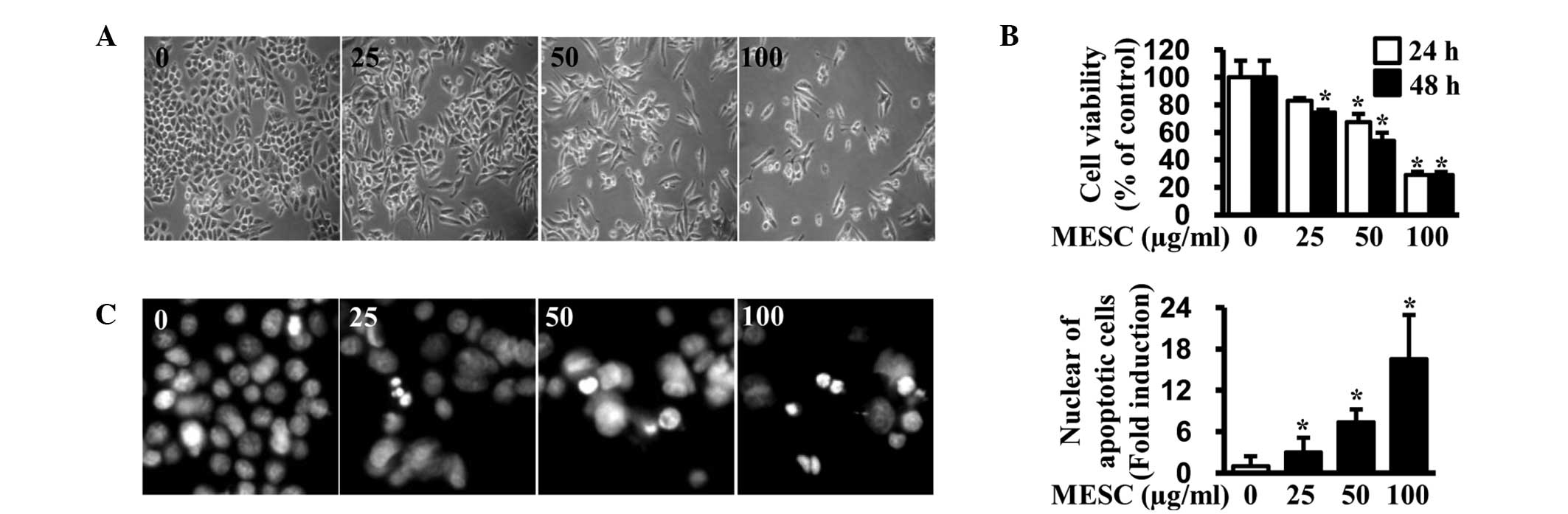
To investigate the growth-inhibitory effect of MESC
in MC-3 cells, an optic microscope was used and a trypan blue
exclusion assay was performed to observe morphological changes. The
results indicated that cells were detached and cell viability was
decreased by MESC in a dose-dependent manner (Fig. 1A and B). To examine whether the
antiproliferative effect of MESC was associated with apoptotic
events, DAPI staining was performed to evaluate nuclear
fragmentation and condensation (which is characteristic of cells
undergoing apoptosis). As shown in Fig. 1C, MESC increased the number of
condensed and fragmented nuclei compared with DMSO (vehicle
control). These observations suggest that MESC can induce apoptosis
to reduce the viability of MC-3 cells.
MESC activates caspase-8 and increases
t-Bid and Bim expression to damage MMP
It has been reported that a number of Bcl-2 protein
family members associate with the mitochondrial outer membrane,
affecting MMP and causing apoptotic cell death (16). Therefore, western blot analysis was
used to determine the protein expression levels of Bcl-2 family
members. The results indicated that MESC increased t-Bid and Bim
protein levels in MC-3 cells, whereas levels of other Bcl-2 family
members were unaltered (Fig. 2A and
B). A JC-1 assay was then performed to measure MMP and the
results indicated that MESC significantly damaged MMP in a
dose-dependent manner (Fig. 2C).
The involvement of caspase-8 in MESC-induced apoptosis was also
determined and data revealed that MESC induced cleavage of
caspase-8 in MC-3 cells (Fig. 2D).
Thus, the results suggest that MESC may induce apoptosis through
the activation of caspase-8 and increased levels of t-Bid and Bim,
thereby causing alterations in MMP.
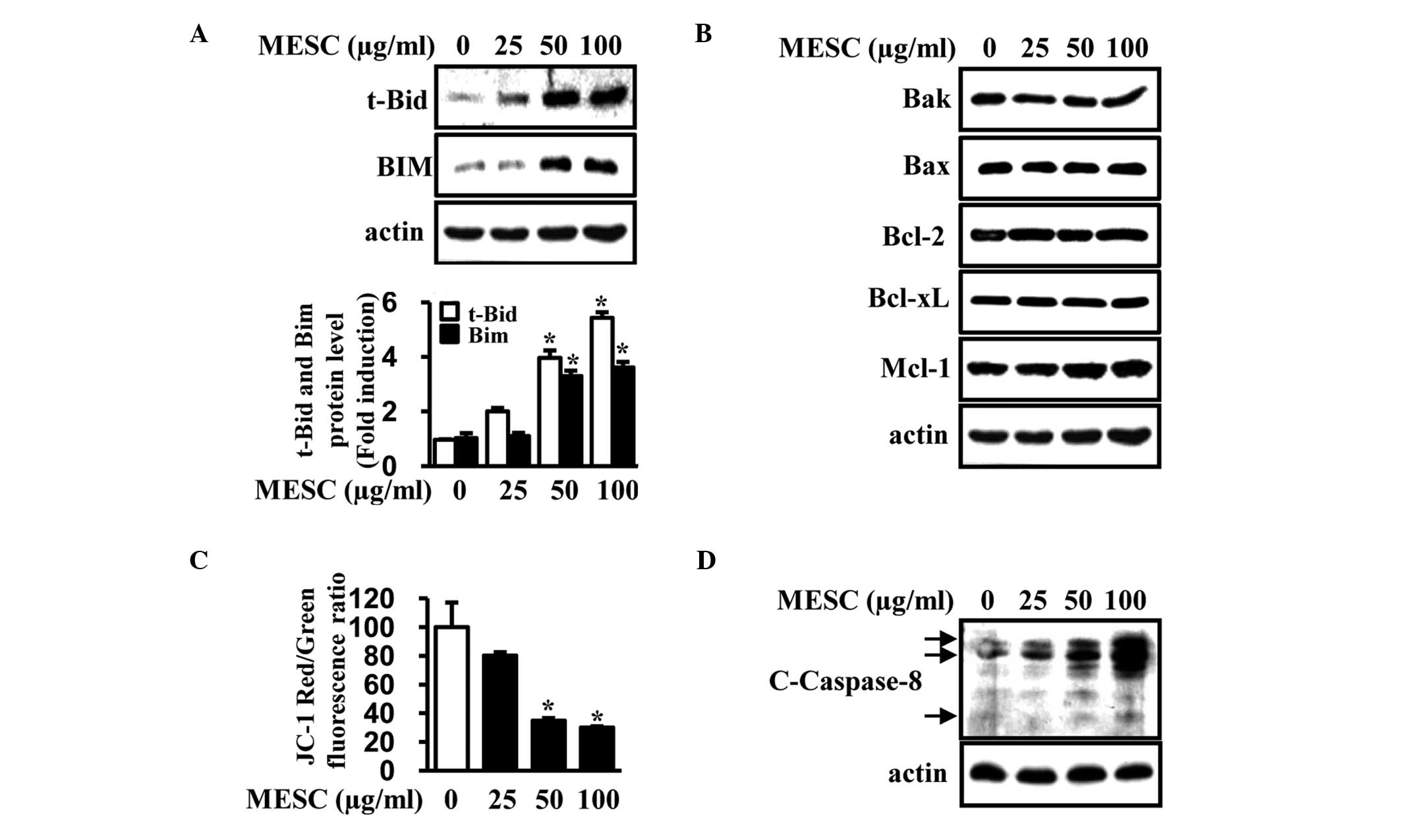 | Figure 2Induction of t-Bid, Bim and cleaved
caspase-8 to damage MMP by MESC. MC-3 cells were treated with
dimethylsulfoxide (vehicle control) or various doses of MESC (25,
50 and 100 μg/ml) for 24 h. (A and B) Expression of various members
of the Bcl-2 protein family (t-Bid, Bim, Bak, Bax, Bcl-2, Bcl-xl
and Mcl-1) was detected by western blot analysis. Protein levels
were normalized to actin. The expression of t-Bid and Bim proteins
were confirmed in triplicate with columnar graphs expressing the
mean ± SD. (C) MMP was measured using the JC-1 assay kit and data
are presented as the mean ± SD of triplicate experiments. (D)
Cleaved caspase-8 was detected using western blot analysis in MC-3
cells. *P<0.05, vs. the vehicle control group. MMP,
mitochrondrial membrane potential; MESC, methanol extract of
Smilax china L.; Bcl-2, B-cell lymphoma 2; t-Bid, truncated
BH3 interacting-domain death agonist; Bim, B-cell lymphoma 2
(Bcl-2) interacting mediator of cell death; Bak, Bcl-2 homologous
antagonist killer; Bax, Bcl-2-associated X protein; Bcl-x1, B-cell
lymphoma-extra large; Mcl-1, induced myeloid leukemia cell
differentiation protein; SD, standard deviation. |
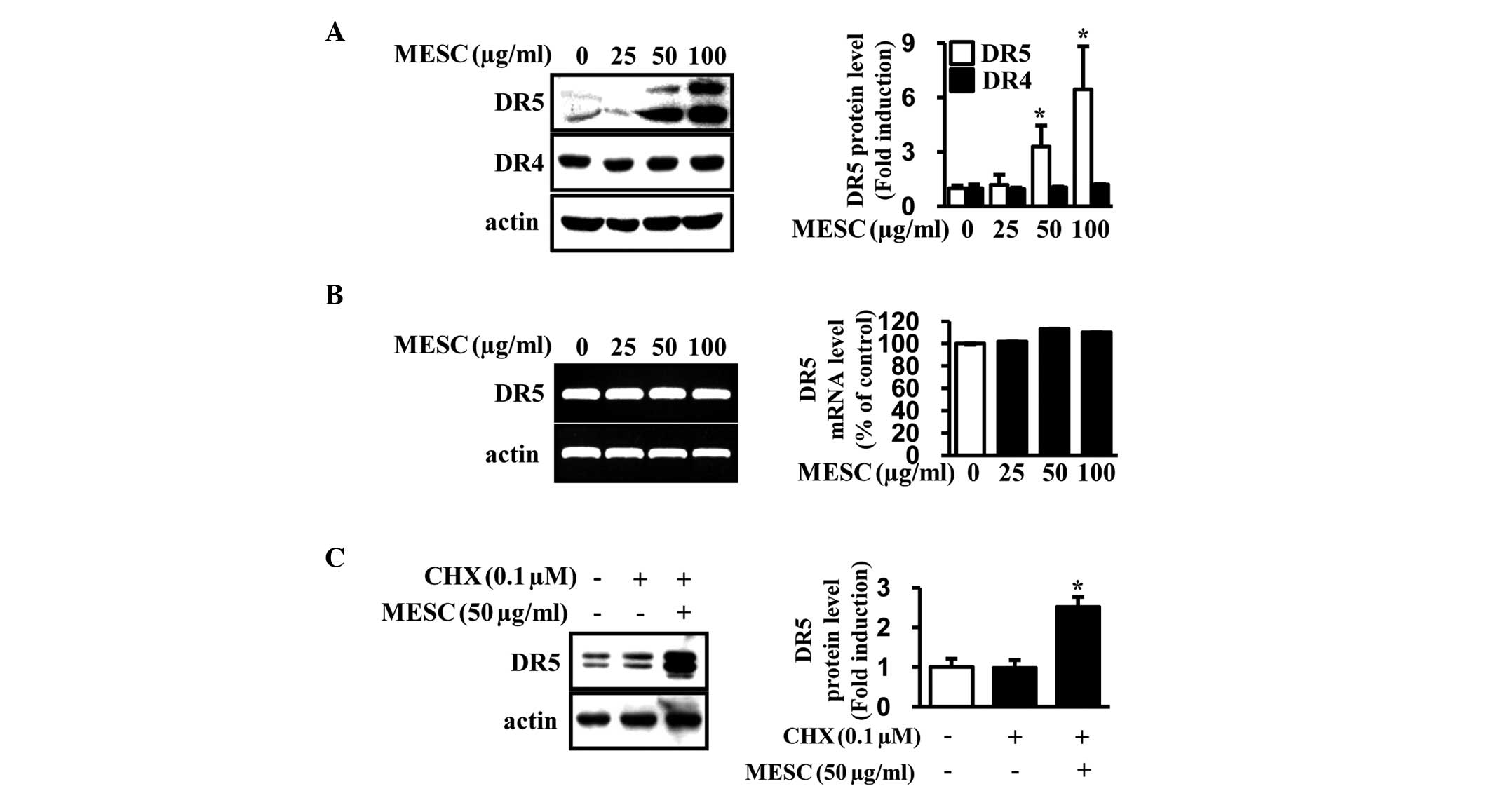
MESC increases DR5 through the
enhancement of protein stability
To further investigate whether MESC-induced
apoptosis is involved in extrinsic pathways associated with DRs,
the protein levels of DR4 and 5 were detected. As shown in Fig. 3A, MESC increased the expression of
DR5 in a dose-dependent manner compared with the vehicle control
but did not affect DR4 expression. The expression levels of DR5
mRNA were also analyzed (by RT-PCR) and the results revealed that
DR5 mRNA levels were unchanged (Fig.
3B). To further examine how MESC increases DR5 protein levels,
the effects of MESC on protein turnover were investigated using
CHX, a protein synthesis inhibitor. The results demonstrated that
co-treatment of MESC and CHX significantly increased DR5 protein
levels, suggesting that MESC significantly improved DR5 protein
stability in MC-3 cells (Fig.
3C).
MESC inactivates the ERK1/2 pathway to
induce apoptosis in MC-3 cells
To further investigate whether MESC-induced
apoptosis is associated with the MAPK pathway through DRs, the
phosphorylation of ERK, JNK, and p38 was analyzed. The results
indicated that MESC interrupted the activation of ERK in MC-3 cells
but did not induce any changes in the phosphorylation or total
expression of JNK and p38 (Fig. 4A and
B). To further confirm whether inactivation of ERK was required
for the induction of DR5 and apoptosis in MC-3 cells, the ERK
inhibitor (PD98059) was used. The results demonstrated that
co-treatment with MESC and the ERK inhibitor significantly
increased the expression of DR5 in MC-3 cells and that the ERK
inhibitor potentiated MESC-induced apoptosis in MC-3 cells
(Fig. 4C and D). These results
suggest that ERK may be associated with MESC-induced DR5 to induce
apoptosis in MC-3 cells.
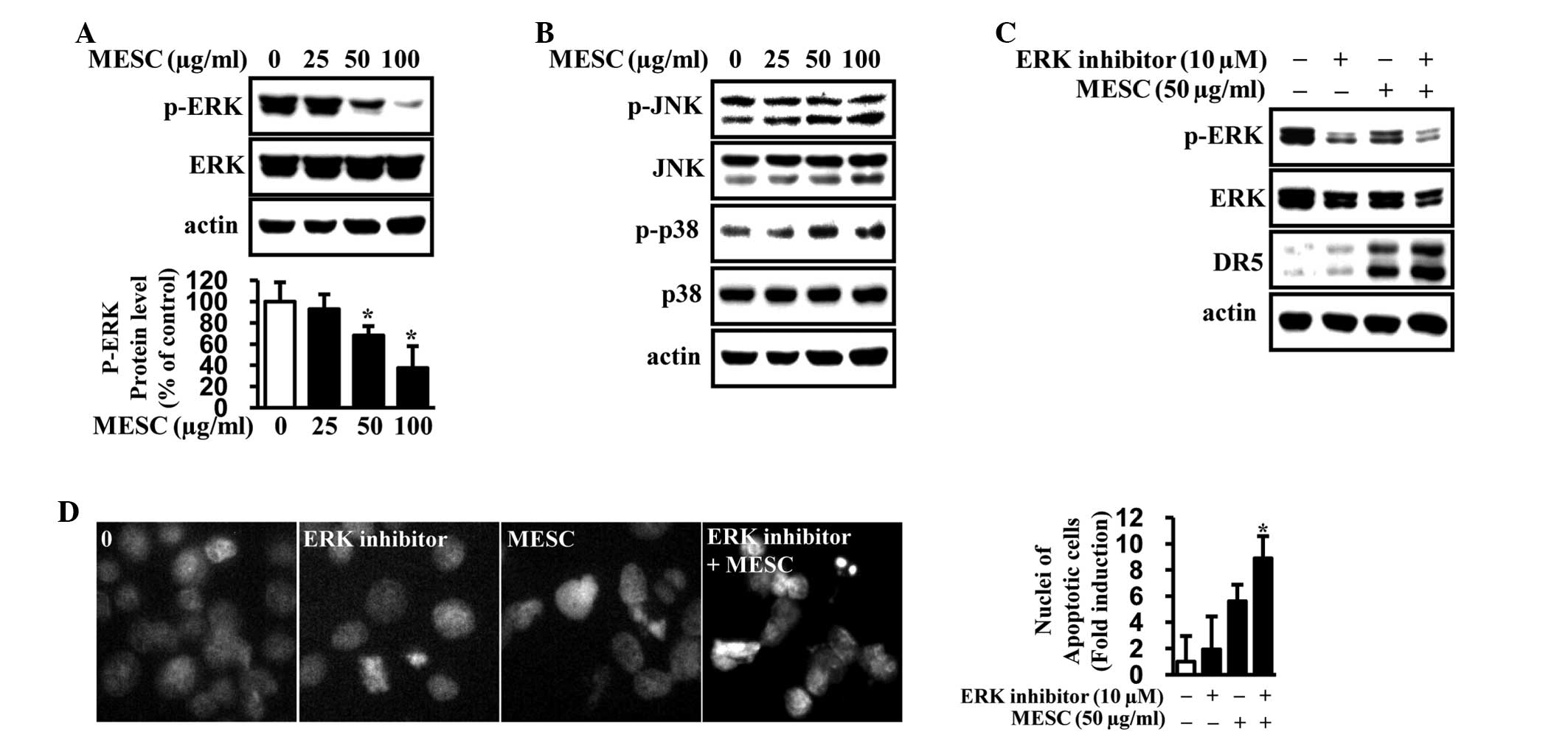 | Figure 4The association between ERK1/2
signaling and MESC-induced apoptosis in MC-3 cells. MC-3 cells were
treated with dimethylsulfoxide (vehicle control) or various doses
of MESC (25, 50 and 100 μg/ml) for 24 h. (A and B) Proteins were
extracted to analyze p-ERK, ERK, p-JNK, JNK, p-p38 and p38
expression by western blot analysis. Protein levels were normalized
to actin. The expression of p-ERK proteins was confirmed in
triplicate with columnar graphs representing the mean ± SD.
*P<0.05, vs. the vehicle control group. (C) Following
treatment of the cells with 10 μM ERK inhibitor in the absence or
presence of 50 μg/ml MESC for 24 h, p-ERK, ERK and DR5 expression
was analyzed by western blot analysis. (D) Fluorescence microscopy
images of the DAPI-stanined MC-3 cells. The apoptotic cells were
counted and the data are presented as the mean ± SD of triplicate
experiments; *P<0.05, vs. the MESC treatment group.
ERK, extracellular signal-regulated kinase; MESC, methanol extract
of Smilax china L.; JNK, c-Jun N-terminal kinase; SD,
standard deviation; DR, death receptor. |
Discussion
Naturally-occurring products derived from plants are
an abundant source of bioactive constituents that have been
important in pharmacology to date. Smilax china L., a common
Chinese medicine, has been extensively used for the clinical
treatment of syphilis, acute bacillary dysentery, chronic nephritis
and cancer. A previous study reported that Smilax china L.
exhibited a significant antitumor effect through cell cycle arrest
and apoptotic induction in HeLa human cervix carcinoma cells
(11). In addition, G1/S cycle
arrest and induction of apoptosis have been shown to act as
molecular mechanisms of antiproliferative activitiy associated with
Smilax china L. (12).
However, the anticancer activity of Smilax china L. in MEC
remains poorly understood. Thus, the present study aimed to explain
the ability of Smilax china L. to induce apoptosis and a
number of molecular mechanisms. In the present study, MESC was
found to decrease cell viability and induce apoptosis in the MC-3
human MEC cell line. Apoptosis is an important mechanism by which
the majority of current anticancer agents induce cancer cell death
(17). The specific apoptotic
pathway activated by an anticancer drug is important not only for
predicting its efficacy but also for gaining insight for improved
design of therapeutic trials. Therefore, results of the present
study suggest that MESC may represent a drug candidate by inducing
apoptosis in MC-3 cells.
Apoptosis is tightly regulated by anti-apoptotic and
pro-apoptotic molecules, including proteins of the Bcl-2 family
(18,19). Therefore, the present study
investigated the contribution of Bcl-2 protein family members to
MESC-induced apoptosis. Results revealed that MESC resulted in an
increase in t-Bid and Bim protein expression, whereas the
expression of other family members was not altered. t-Bid and Bim
are associated with the mitochondrial outer membrane and are able
to disrupt the MMP, resulting in apoptosis in cancer cells. A JC-1
assay was performed to detect the MMP in the present study. The
results indicated that MESC significantly damaged MMP, suggesting
that the ability of MESC to impair MMP may be associated with
MESC-induced apoptosis.
Initiation of apoptosis can be broadly separated
into two major pathways, the DR pathway and the mitochondria
pathway (20). The DR pathway is
mediated by interaction of DRs with their cognate ligands, leading
to recruitment of the Fas-associated death domain protein and
activation of caspase-8. A number of studies have demonstrated the
upregulation of DRs by naturally-occurring chemicals to initiate
apoptosis (21–23). In the present study, MESC was found
to activate caspase-8, indicating that MESC-induced apoptosis may
be associated with the extrinsic pathway. Thus, the expression
patterns of DR4 and 5 were analyzed and the results demonstrated
that MESC upregulated DR5 protein levels in a dose-dependent
manner, while DR5 mRNA levels were unchanged. To gain insight into
the potential mechanism of MESC regulation of DR5 protein
expression, the protein synthesis inhibitor, CHX, was used. Results
revealed that co-treatment of CHX and MESC significantly enhanced
MESC induction of DR5 protein expression compared with CHX
treatment only, suggesting that MESC significantly improves DR5
protein stability in MC-3 cells.
MAPK is important in regulating cell proliferation
and cell survival in response to growth stimulation and stress
(24). Modulation of the MEK/ERK
pathway is associated with specific cellular responses, including
regulation of the cell cycle, cell proliferation and apoptosis
(25,26). Recently, our group also reported
that phenethyl isothiocyanate, from cruciferous vegetables,
regulates ERK1/2 or p38, modifying DRs for apoptosis in a number of
cancer cell types (27,28). These observations suggest that
natural products may affect the MAPK pathway to activate the
extrinsic apoptotic pathway. In the present study, MESC was
similarly shown to significantly inactivate the ERK pathway whilst
the ERK inhibitor (PD980589) potentiated MESC-induced apoptosis
through the induction of DR5. These data are consistent with
previous reports that ERK may be the upstream target of DR5 protein
(29,30).
In conclusion, the present study demonstrates that
MESC decreases cell viability and induces apoptosis in MC-3 human
oral MEC cells. These observations are accompanied by the increase
of DR5 through enhanced protein stability, to induce t-Bid and Bim.
In addition, MESC inactivates ERK signaling to induce apoptotic
cell death. Therefore, this study markedly suggests that Smilax
china L. may be a potential chemotherapeutic agent against MEC
cells and that ERK inhibition may act as an effective molecule for
Smilax china L.-induced apoptosis.
Acknowledgements
This study was supported by a grant from the Basic
Science Research Program through the National Research Foundation
of Korea funded by the Ministry of Education, Science and
Technology (grant no. 2012003731) and research funds of Chonbuk
National University (Jeonju, South Korea) in 2013.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson NW, Warnakulasuriya S, Gupta PC,
et al: Global oral health inequalities in incidence and outcomes
for oral cancer: causes and solutions. Adv Dent Res. 23:237–246.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frankenthaler RA, el-Naggar AK, Ordonez
NG, Miller TS and Batsakis JG: High correlation with survival of
proliferating cell nuclear antigen expression in mucoepidermoid
carcinoma of the parotid gland. Otolaryngol Head Neck Surg.
111:460–466. 1994.PubMed/NCBI
|
|
4
|
Hicks MJ, el-Naggar AK, Byers RM, Flaitz
CM, Luna MA and Batsakis JG: Prognostic factors in mucoepidermoid
carcinomas of major salivary glands: a clinicopathologic and flow
cytometric study. Eur J Cancer B Oral Oncol. 30B:329–334. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Press MF, Pike MC, Hung G, et al:
Amplification and overexpression of HER-2/neu in carcinomas of the
salivary gland: correlation with poor prognosis. Cancer Res.
54:5675–5682. 1994.PubMed/NCBI
|
|
6
|
Handra-Luca A, Ruhin B, Lesty C and Fouret
P: P27, SKP2, and extra-cellular signal-related kinase signalling
in human salivary gland mucoepidermoid carcinoma. Oral Oncol.
42:1005–1010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abelson PH: Medicine from plants. Science.
247:5131990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Efferth T, Kahl S, Paulus K, et al:
Phytochemistry and pharmacogenomics of natural products derived
from traditional Chinese medicine and Chinese materia medica with
activity against tumor cells. Mol Cancer Ther. 7:152–161. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khan I, Nisar M, Ebad F, et al:
Anti-inflammatory activities of Sieboldogenin from Smilax
china Linn: experimental and computational studies. J
Ethnopharmacol. 121:175–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shu XS, Gao ZH and Yang XL:
Anti-inflammatory and anti-nociceptive activities of Smilax
china L. aqueous extract. J Ethnopharmacol. 103:327–332. 2006.
View Article : Google Scholar
|
|
11
|
Xu W, Liu J, Li C, Wu HZ and Liu YW:
Kaempferol-7-O-beta-D-glucoside (KG) isolated from Smilax
china L. rhizome induces G2/M phase arrest and apoptosis on
HeLa cells in a p53-independent manner. Cancer Lett. 264:229–240.
2008.PubMed/NCBI
|
|
12
|
Li YL, Gan GP, Zhang HZ, et al: A
flavonoid glycoside isolated from Smilax china L. rhizome in
vitro anticancer effects on human cancer cell lines. J
Ethnopharmacol. 113:115–124. 2007.
|
|
13
|
Wu LS, Wang XJ, Wang H, Yang HW, Jia AQ
and Ding Q: Cytotoxic polyphenols against breast tumor cell in
Smilax china L. J Ethnopharmacol. 130:460–464. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Samanta AK, Huang HJ, Bast RC Jr and Liao
WS: Overexpression of MEKK3 confers resistance to apoptosis through
activation of NFkappaB. J Biol Chem. 279:7576–7583. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Zhu H, Xu CJ and Yuan J: Cleavage of
BID by caspase 8 mediates the mitochondrial damage in the Fas
pathway of apoptosis. Cell. 94:491–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim R, Emi M, Tanabe K, Uchida Y and
Arihiro K: The role of apoptotic or nonapoptotic cell death in
determining cellular response to anticancer treatment. Eur J Surg
Oncol. 32:269–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Crompton M: Bax, Bid and the
permeabilization of the mitochondrial outer membrane in apoptosis.
Curr Opin Cell Biol. 12:414–419. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fischer U and Schulze-Osthoff K: New
approaches and therapeutics targeting apoptosis in disease.
Pharmacol Rev. 57:187–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kok SH, Yeh CC, Chen ML and Kuo MY:
Esculetin enhances TRAIL-induced apoptosis through DR5 upregulation
in human oral cancer SAS cells. Oral Oncol. 45:1067–1072. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JY, Kim EH, Kim SU, Kwon TK and Choi
KS: Capsaicin sensitizes malignant glioma cells to TRAIL-mediated
apoptosis via DR5 upregulation and survivin downregulation.
Carcinogenesis. 31:367–375. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yadav VR, Prasad S, Kannappan R, et al:
Cyclodextrin-complexed curcumin exhibits anti-inflammatory and
antiproliferative activities superior to those of curcumin through
higher cellular uptake. Biochem Pharmacol. 80:1021–1032. 2010.
View Article : Google Scholar
|
|
24
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chang F, Steelman LS, Shelton JG, et al:
Regulation of cell cycle progression and apoptosis by the
Ras/Raf/MEK/ERK pathway (Review). Int J Oncol. 22:469–480.
2003.PubMed/NCBI
|
|
26
|
Agell N, Bachs O, Rocamora N and
Villalonga P: Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+),
and calmodulin. Cell Signal. 14:649–654. 2002.
|
|
27
|
Huong LD, Shin JA, Choi ES, et al:
β-Phenethyl isothiocyanate induces death receptor 5 to induce
apoptosis in human oral cancer cells via p38. Oral Dis. 18:513–519.
2012.
|
|
28
|
Huong le D, Shim JH, Choi KH, et al:
Effect of β-phenylethyl isothiocyanate from cruciferous vegetables
on growth inhibition and apoptosis of cervical cancer cells through
the induction of death receptors 4 and 5. J Agric Food Chem.
59:8124–8131. 2011.
|
|
29
|
Sung B, Ravindran J, Prasad S, Pandey MK
and Aggarwal BB: Gossypol induces death receptor-5 through
activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer
cells to TRAIL. J Biol Chem. 285:35418–35427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belyanskaya LL, Ziogas A,
Hopkins-Donaldson S, et al: TRAIL-induced survival and
proliferation of SCLC cells is mediated by ERK and dependent on
TRAIL-R2/DR5 expression in the absence of caspase-8. Lung Cancer.
60:355–365. 2008. View Article : Google Scholar : PubMed/NCBI
|