Introduction
Cisplatin (CP) or
cis-Diamminedichloroplatinum (II), is widely used as
an antitumor agent for the treatment of testis, bladder, lung and
ovarian cancer. However, severe side-effects such as acute kidney
injury (AKI), gastrointestinal toxicity, and ototoxicity limit its
use in the clinic. In particular, AKI is a major side-effect
(1–4). CP is toxic to the renal proximal
tubules (1,5,6). The
nephrotoxic potential of CP is multifactorial, with the major
factor being the induction of tubular cell apoptosis. The induction
of tubular cell apoptosis by CP has been intensively investigated
in the past decades. A number of apoptotic pathways have been
examined, including the intrinsic, extrinsic and endoplasmic
reticulum (ER) stress pathways (7).
ER is a key organelle of eukaryotic cells, where
lipid synthesis, protein folding (into tertiary and quaternary
structures) and protein maturation occur. The ER senses and
responds to homeostatic changes, with various stimuli, such as
ischemia, hypoxia, elevated protein synthesis and Ca+
overload-inducing ER stress (8).
The ER protein folding capacity is reduced under stress, leading to
an accumulation of unfolded proteins. A major response to ER stress
is the activation of the glucose-regulated protein 78 (GRP78)
through dissociation from its transmembrane receptor, which allows
subsequent regulation of the levels of accumulated unfolded
proteins (9). Slight and medium ER
stress can protect cells from death, but severe ER stress induces
caspase-12-dependent cell apoptosis (10).
Numerous compounds have been experimentally shown to
ameliorate cisplatin nephrotoxity, including vitamin E, melatonin,
furosemide, mannitol and erythropoietin. Grape seed
proanthocyanindin extract (GSPE) is derived from grape seeds. It is
shown to possess a variety of potent properties, such as
antioxidant, anti-inflammatory and antitumor activities, and to
mediate resistance to free radicals and protection from
cardiovascular diseases (11–13).
In this study, GSPE was used to treat the mouse model of CP-induced
nephropathy, in order to examine its potential protective effect(s)
and examine whether these effects are mediated by the inhibition of
ER stress-induced apoptosis occurring in tubular cells.
Materials and methods
Reagents
CP was purchased from Sigma-Aldrich (St. Louis, MO,
USA). Grape seed proanthocyanidin extract (purity > 96%, lot no.
G050412) was purchased from Tianjin Jianfeng Natural Product
R&D Co., Ltd. (Tianjin, China). Primary antibodies used in this
study were: rabbit anti-GRP78, rabbit anti-caspase-12 (Abcam Ltd.,
Hong Kong, China), rabbit anti-p-ERK, rabbit anti-ERK (Cell
Signaling Technology, Inc., Danvers, MA, USA) and mouse
anti-β-actin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
Anti-mouse and anti-rabbit secondary antibodies were purchased from
Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA). The
In Situ Cell Death Detection kit (Roche Diagnostics, Indianapolis,
IN, USA) was used for the terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay.
Animals
Seventy adult (6–8 weeks-old) male C57/BL6 mice,
weighing 20–25 g, were supplied by the Beijing Vital River
Laboratory Animal Technology Co., Ltd. (Beijing, China). Mice were
housed separately in metal cages with a 12 h dark/light cycle and
40–70% relative humidity, at a 18–22ºC temperature. Food and water
were available ad libitum. All experiments were conducted in
accordance with the NIH Guide for the Care and Use of Laboratory
Animals.
Animal treatment
Animals were randomly divided into four groups: i)
control group (N; n=10), which only received intraperitoneal (ip)
injection of vehicle solution (0.9% saline; 10 ml/kg); ii) CP group
(C; n=20), which only received an ip injection of 20.0 mg/kg CP
(dissolved in 0.9% saline to reach a concentration of 2.0 mg/ml);
iii) GSPE group (G; n=15), which received a single intragastric
(ig) administration of 500 mg/kg GSPE (dissolved in 0.9% saline to
reach 50 mg/ml); and iv) CP+GSPE group (C+G; n=20), which
successively received ig administration of 500 mg/kg GSPE at 30 min
before ip injection of CP, and ig administration of 500 mg/kg GSPE
after ip injection of CP 72 h.
The mice were sacrificed 120 h after the injection
of 0.9% saline or CP. Prior to sacrifice, the mice were weighed and
blood was collected from the endocanthion. From this sample, the
serum was separated by centrifugation (912 × g at 4ºC for 20 min)
and stored at −80ºC until assayed. Both kidneys were immediately
excised and weighed, then each kidney was cut in half by coronal
position. Two sections of each excised kidney were stored at −80ºC
for western blot analysis. The remaining sections were fixed in 4%
buffered paraformaldehyde at 4ºC and embedded in paraffin for
histopathologic observation, immunohistochemical study and TUNEL
assay.
Assessments of renal function
Blood urea nitrogen (BUN) and serum creatinine (Scr)
levels were measured in a Cobas® 8000 modular analyser
(Roche Diagnostics) in Qilu Hospital, Shandong University. The
renal index (RI) was calculated as: both kindeys’ weight
(g)/animal’s weight (g) ×100.
Histopathologic observation
The pathologic changes in the kidney were examined
by periodic acid-Schiff (PAS) staining. One-fourth of the kidneys
was immersion-fixed in 10% buffered formalin and embedded in
paraffin to be further examined under a light microscope. Two 4-μm
thick sections were performed per animal at an interval of 100 μm
and were stained with PAS reagent. Tubular damage was scored as
follows: Each section was examined in 5 fields, and the average
percentage of the impaired renal tubules was then calculated. The
results of renal tubular damage were transformed into an index of
renal tubular necrosis, where no damage was assigned a 0 index;
<25% damage was assigned 1; 25–50% damage was assigned 2; 50–75%
damage was assigned 3 and >75% damage was assigned a 4
index.
Immunohistochemical study
For immunohistochemical analysis, tissue slices were
microwaved for 10–15 min in 0.01% sodium citrate buffer (pH 6.0) to
allow antigen retrieval. The tissue slices were cooled at room
temperature or in iced water, and then washed with PBS three times.
The tissue slices were immersed in 0.1% Triton X-100 for 15 min. To
block endogenous peroxidase activity, the tissue slices were
incubated with 3% hydrogen peroxide for 10 min in the dark. The
tissue slices were then incubated with 10% goat serum for 60 min at
37ºC, and with primary antibody at 4ºC overnight (anti-GRP78 1:200,
anti-p-ERK 1:50, anti-caspase-12 1:100), while the sections serving
as negative controls were incubated with PBS, instead of the
primary antibody. All the sections were incubated with secondary
antibodies for 60 min at 37ºC, and stained with
3,3′-diaminobenzidine (DAB) and hematoxylin. Semi-quantitative
analysis was performed on the colored sections using a computer
imaging analysis system (Leica QWin V3 image analysis software;
Leica Microsystems, Heidelberg, Germany). Briefly, 10 high-power
fields (×400) per section were randomly selected and two sections
per kidney were examined in each experiment. Specimens were scored
according to the intensity of the dye color and the percentage of
positively stained areas. Brown areas were considered as positive.
The intensity of the dye color was graded as 0 (no color); 1 (light
yellow); 2 (light brown) and 3 (brown), and the percentage of
positively stained areas was graded as 0 (<5%); 1 (5–25%); 2
(25–50%); 3 (51–75%) and 4 (>75%). The two grades were added to
give a final score of expression for each tested protein.
TUNEL assay
The TUNEL assay was conducted following the
manufacturer’s instructions. Sections were incubated with
proteinase K at 37ºC for 30 min, then with the mix of enzyme and
labeling solutions (1:9) at 37ºC for 60 min in the dark. The
sections serving as negative controls were incubated with labeling
solution only. Sections were then stained with
4′,6-diamidino-2-phenylindole (DAPI) for 10 min. The number of
TUNEL-positive nuclei was expressed as a percentage of total nuclei
per field. Ten fields per section and two sections per kidney were
examined in each experiment.
Protein sample preparation
The tissue samples were homogenized in TRIzol
(50–100 mg/ml TRIzol). Following the addition of chloroform (0.2
ml/ml TRIzol), the homogenates were centrifuged at 12,000 × g for
15 min at 4ºC. Supernatants were discarded, isopropanol (1.5 ml/ml
TRIzol) was added to the lower phase, followed by centrifugation at
12,000 × g for 15 min at 4ºC. The pellets were washed with 0.3 M
guanidine hydrochloride (2 ml/ml TRIzol) three times, then
dissolved in 1% SDS (100 μl SDS/ml TRIzol) at 50ºC for 30 min.
Protein concentrations were determined using the Pierce BCA Protein
Assay kit (Thermo Fisher Scientific Inc., Rockford, IL, USA).
Western blotting
Proteins (50 μg) were subjected to 10–12%
SDS-polyacrylamide gel electrophoresis, and gels were transferred
to cellulose acetate membranes. The membranes were blocked with 5%
skim milk at room temperature for 1 h, then incubated with primary
antibodies (anti-GRP78 1:250, anti-p-ERK 1:1,000, anti-ERK 1:1,000,
anti-caspase-12 1:500 and anti-β-actin 1:2,500) at 4ºC overnight.
After a 1-h incubation with secondary antibodies at room
temperature, the membranes were immersed in enhanced
chemiluminescence (ECL) reagent and exposed to an X-ray film.
Quantification of the luminosity of each protein band was performed
using Adobe Photoshop software (Adobe Photoshop 7.0, Adobe, San
Jose, CA, USA). GRP78, p-ERK and caspase-12 relative quantities
were expressed as a ratio of luminosity of the respective sample to
that of the N group.
Statistical analysis
Data are presented as means ± SD. Differences
between groups were evaluated using analysis of variance (ANOVA) or
Mann-Whitney U-tests. Differences were considered statistically
significant at P<0.05.
Results
GSPE protects from CP-induced AKI
As shown in Table
I, 8 mice out of 10 survived in the N group, 15 mice out of 20
survived in the CP group, 13 out of 15 survived in the GSPE group,
and 16 out of 20 survived in the CP+GSPE group. The levels of BUN,
Scr and RI were significantly increased in the CP group compared to
the control group (P<0.05). Compared to the CP group, the levels
of BUN, Scr and RI were significantly decreased in the CP+GSPE
group (P<0.05). The levels of BUN, Scr and RI in the GSPE group
did not show significant differences compared to the control
group.
 | Table IAnimal groups and related biochemical
parameters. |
Table I
Animal groups and related biochemical
parameters.
| Group | No. | RI | BUN | Scr |
|---|
| N | 8 | 1.43±0.074 | 10.19±1.17 | 29.5±6.07 |
| C | 15 | 1.79±0.066a | 30.85±6.74a | 135.13±12.64a |
| G | 13 | 1.41±0.068b | 10.23±1.44b | 33.08±5.88b |
| C+G | 16 | 1.64±0.039a,b | 14.26±2.66a,b | 62.81±9.55a,b |
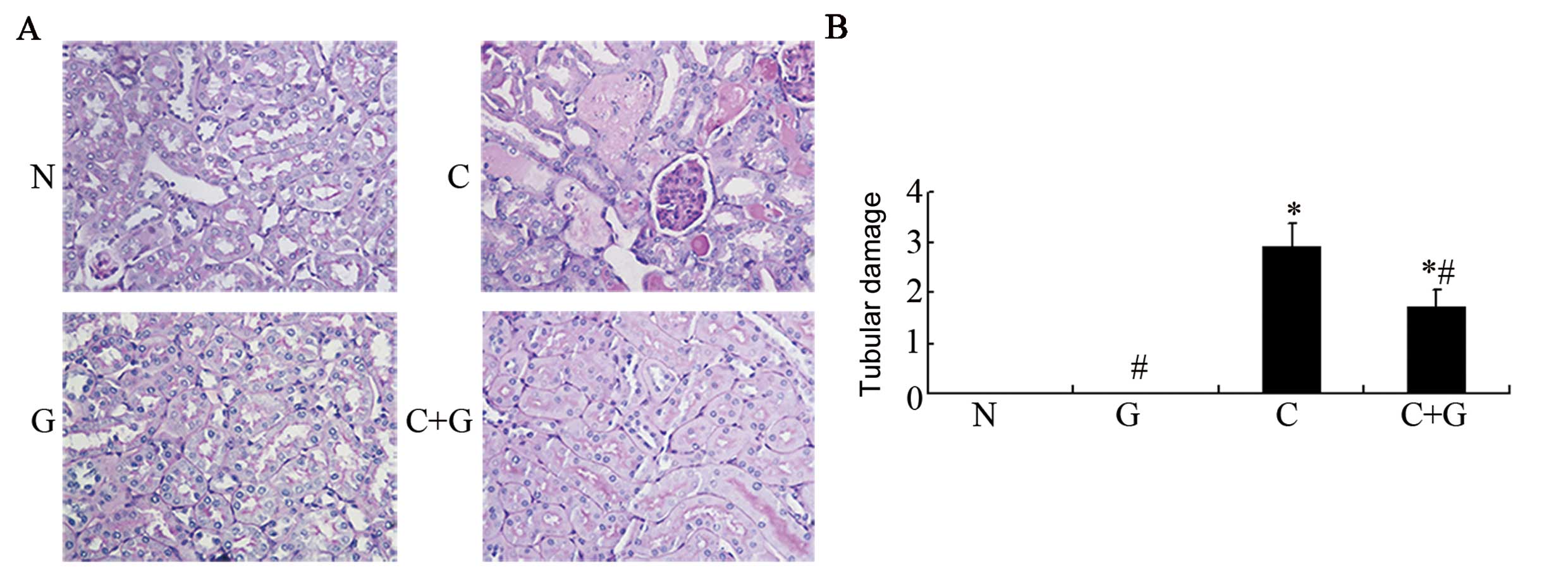
Results from histopathological examinations
following PAS staining are shown in Fig. 1A. We observed brush border damage,
renal tubular epithelial cell swelling, degeneration, necrosis,
tubular casts and cell vacuole degeneration in the proximal tubules
of kidney in the CP group, while in the control and GSPE groups,
the kidneys maintained normal structure. Compared to the CP group,
tubular damage was greatly improved in the CP+GSPE group. We
further calculated scores of tubular damage as shown in Fig. 1B. The CP group had an injury score
of 2.9, while the CP+GSPE group scored 1.7, and this difference was
significant (P<0.05), indicating that GSPE can block the renal
injury caused by CP.
GSPE inhibits apoptosis induced by
CP
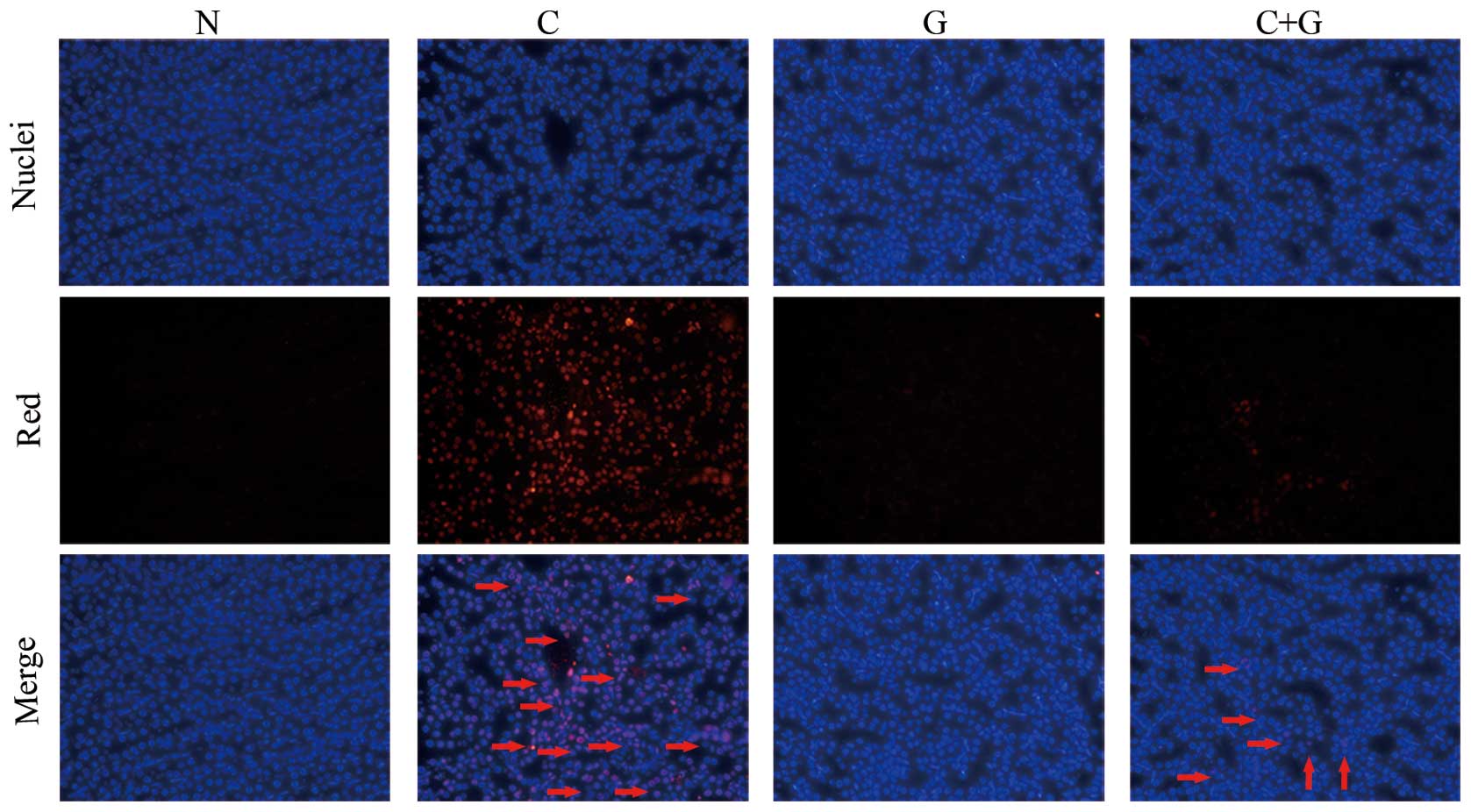
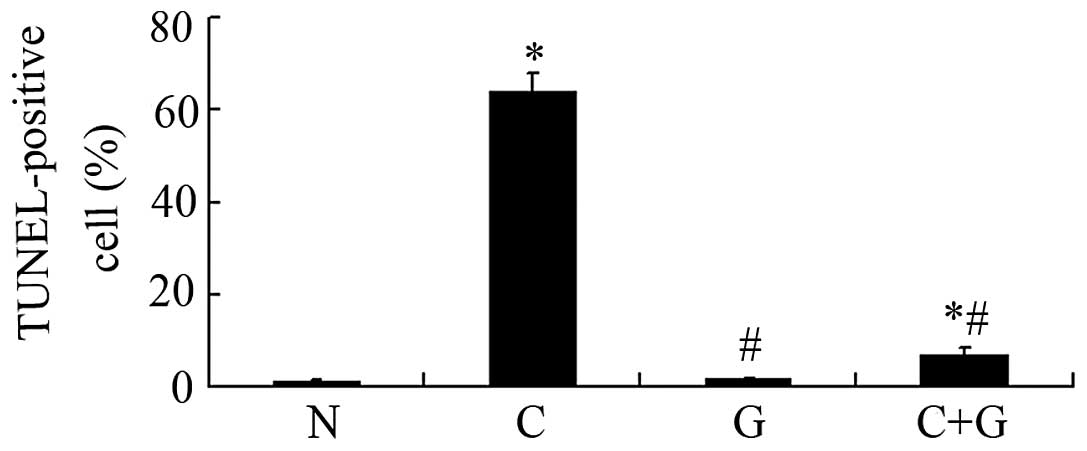
To assess whether GSPE protects proximal tubular
cell apoptosis induced by CP, the tissue sections were labeled with
an in situ TUNEL assay. As shown in Fig. 2, renal tubular epithelial cells
undergoing apoptosis were stained red. The CP group showed a high
number of TUNEL-positive cells compared to the control group
(P<0.05). The TUNEL-positive cells were significantly reduced in
the CP+GSPE group compared to the CP group (P<0.05). Limited
apoptosis was detected in the control and the GSPE group (Fig. 3).
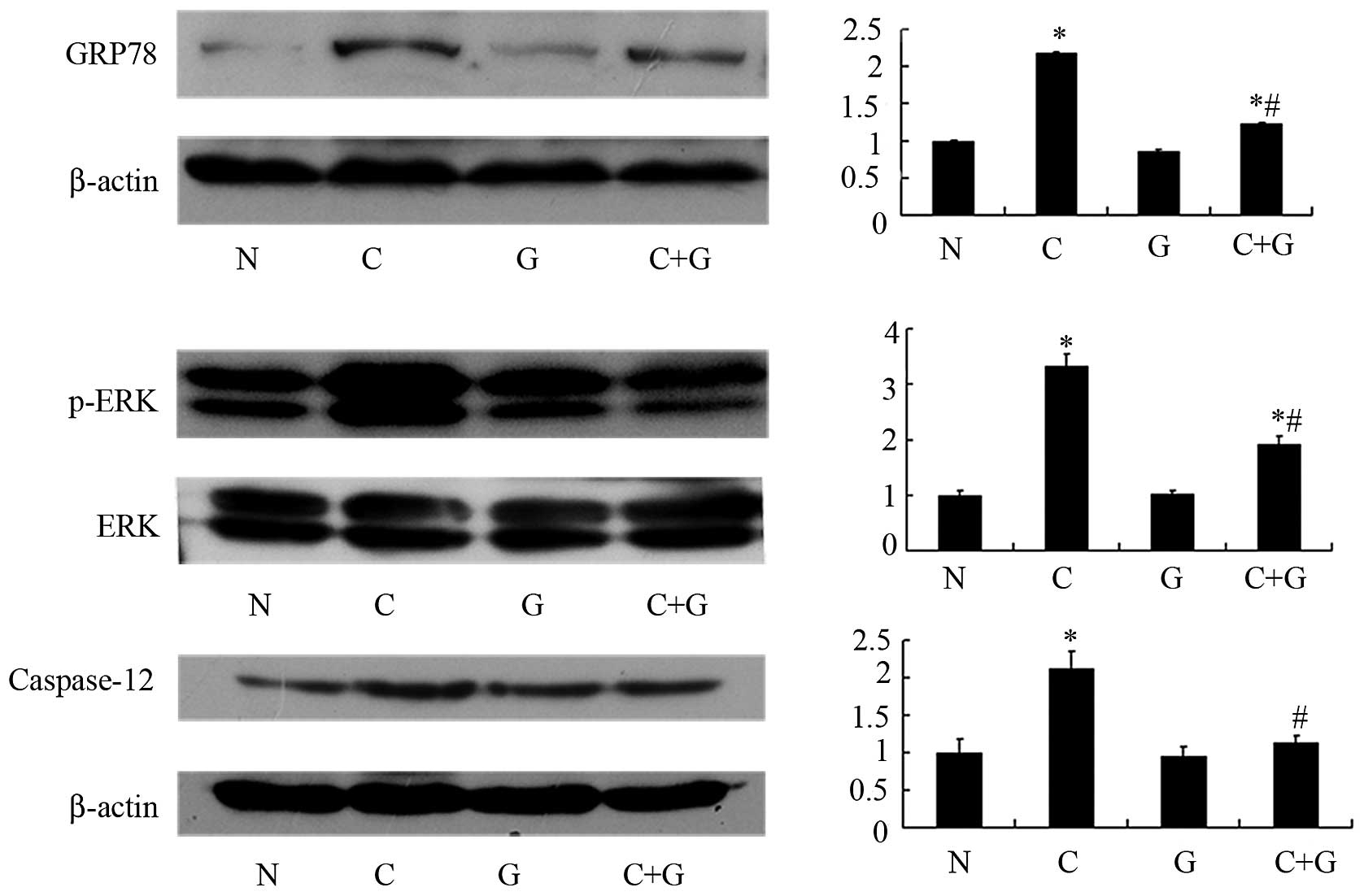
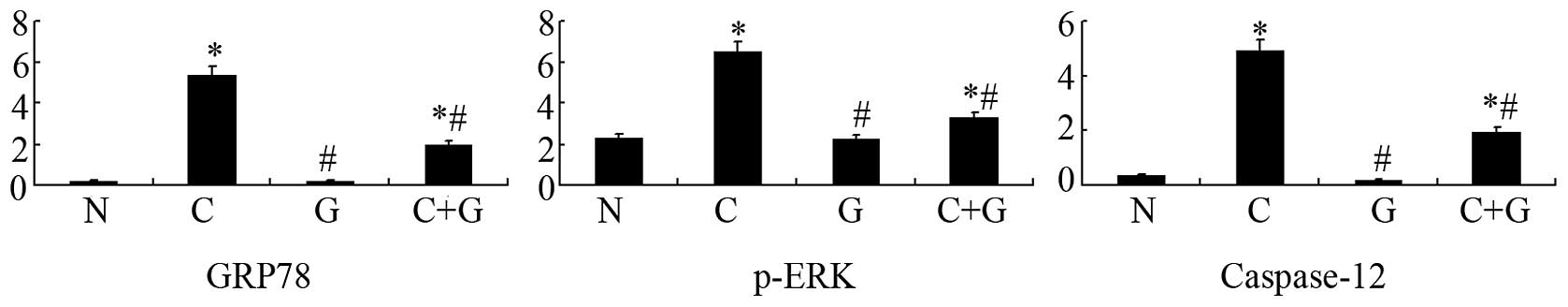
GSPE inhibits expression of GRP78, p-ERK
and caspase-12
Apoptosis occurred in the proximal tubular cells of
the CP group. In order to investigate whether GSPE protects from
CP-induced nephrotoxicity by attenuating ER stress-induced
apoptosis in proximal tubular cells, we examined the expression of
GRP78, p-ERK and caspase-12, which are important protein players in
ER stress-induced apoptosis. Expression of these proteins was
studied by western blotting and immunohistochemistry. The two
approaches showed that GRP78, p-ERK and caspase-12 are highly
expressed in the CP group, while their levels are significantly
reduced in the CP+GSPE group (P<0.05) (Figs. 4–6). These proteins showed limited
expression in the control and GSPE groups.
Discussion
CP is an antineoplastic drug widely used in the
clinic, with obvious curative effects, especially in the treatment
of solid-organ tumors; however, nephrotoxicity represents a major
dose-limiting side-effect of CP (1). The pathogenesis of CP-induced
nephrotoxicity is associated with several factors, including
inflammation, oxidative stress, DNA damage, mitochondrial
dysfunction and apoptosis (3,6,14,15).
Different pathways of apoptosis have been studied in this respect,
including the intrinsic and extrinsic pathways (7). It was recently suggested that ER
stress-mediated apoptosis is an important pathway in renal
apoptosis (16).
In this study, the CP group showed a significant
increase in the level of BUN, Cr and RI compared to the control
group. Histopathological examination showed that the CP group has
significant structural damage compared to the control group. The
number of TUNEL-positive cells was significantly increased in the
CP group compared to the N group. These results are consistent with
those from previous studies (17,18).
By contrast, the CP+GSPE group showed a significant decrease in the
levels of BUN, Cr and RI compared to the CP group. Histopathology
also showed that the CP+GSPE group has very limited structural
damage. The number of TUNEL-positive cells was significantly
reduced in the CP+GSPE group compared to the CP group. This
indicates that GSPE can inhibit the apoptosis of renal tubular
epithelial cells induced by CP and may thus improve the renal
dysfunction. The mechanism by which GSPE protects from CP-induced
nephropathy involves its antioxidant properties (19,20).
It has been shown that oxidative stress proteins are downregulated
by GSPE in diabetic nephropathy (12,13).
GSPE is a highly efficient natural antioxidant extracted from
natural grape seeds. Its antioxidant activity is 50 times higher
than that of vitamin E and 20 times that of vitamin C. It has also
been shown that GSPE has powerful anti-inflammatory effects, which
can be used in the treatment and prevention of diseases (21–27).
Recent studies provided evidence that GSPE may serve as a useful
agent in the prevention of diseases such as atherosclerosis,
gastric ulcer, cataract, diabetes, and can also protect from
methylmercury-induced neurotoxicity (13,28).
In addition, GSPE is known to modulate apoptosis (29). A recent study found that the
apoptosis caused by the ER stress pathway plays an important role
in CP-induced nephropathy (18).
Therefore, CP can lead to apoptosis of the renal tubular epithelial
cells by inducing ER stress.
ER stress can be caused by a number of factors such
as ischemia, hyperglycemia, hypoxia and heat shock (10). GRP78 is an important molecular
chaperone, localized in the ER and extensively used as an indicator
of ER stress induction (16). The
phosphorylated-extracellular signal-regulated kinase (p-ERK) has an
early and crucial role in cell protection and survival during
stress, even mild (10). p-ERK is
a transmembrane ER protein involved in signal transduction. In the
inactive state, ERK and two additional ER-stress sensor proteins,
IRE-1 and ATF6, are associated with the ER chaperone Grp78/BiP.
When the levels of unfolded and/or misfolded proteins increase, ERK
and ER-stress sensors dissociate from Grp78/BiP, and activate
downstream molecules (30). The
extracellular signal-regulated kinase (ERK) has been shown to
mediate CP-induced toxicity in renal proximal tubule cells
(31). Caspase-12 plays a key role
in ER stress-induced apoptosis (32). Caspase-12 is a marker of apoptosis
and specifically localizes in the ER. It was previously
demonstrated that caspase-12-mediated apoptosis is specific to ER,
and that caspase-12 cannot be activated when apoptosis occurs via
membrane or mitochondrial signals (33). Thus, GRP78 and p-ERK are protein
markers of ER stress and caspase-12 is a marker of ER
stress-induced apoptosis.
To determine the mechanism by which GSPE protects
tubular cells from CP-induced apoptosis, the protein levels of
GRP78, p-ERK and caspase-12 were measured. GRP78, p-ERK and
caspase-12 were highly expressed in CP-treated mice that showed
important structural alterations in the kidney, indicating that
CP-induced nephropathy involves ER stress-induced apoptosis. GSPE
can attenuate ER stress-induced apoptosis, as evidenced in the
present study by the significant reduction in the levels of GRP78,
p-ERK and caspase-12, observed following administration of the
extract. The present results suggest that exposure to CP activates
ER stress-regulated survival and apoptotic signaling pathways in
renal tubular cells. Moreover, we present evidence that the
caspase-12-dependent apoptotic pathway may be involved in
CP-induced nephropathy. The reduction in the expression of GRP78,
p-ERK and caspase-12 caused by GSPE demonstrates that GSPE can
attenuate ER stress-induced apoptosis via the caspase-12-dependent
pathway.
In conclusion, this study showed that GSPE can
protect from CP-induced AKI. The underlying mechanism involves the
inhibition of ER stress-mediated apoptosis via the
caspase-12-dependent pathway. These results suggest that GSPE can
be applied to treat CP-induced nephropathy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81200529), the Natural
Science Foundation of Shandong Province (grant no. ZR2012HQ001) and
the Independent Innovation Foundation of Shandong University
(IIFSDU; grant no. 2012TS167).
Abbreviations:
|
CP
|
cisplatin
|
|
GSPE
|
grape seed proanthocyanidin
extract
|
|
ER
|
endoplasmic reticulum
|
|
GRP78
|
glucose-regulated protein 78
|
|
BUN
|
blood urea nitrogen
|
|
Scr
|
serum creatinine
|
|
RI
|
renal index
|
|
AKI
|
acute kidney injury
|
References
|
1
|
Miller RP, Tadagavadi RK, Ramesh G and
Reeves WB: Mechanisms of cisplatin nephrotoxicity. Toxins.
2:2490–2518. 2010. View Article : Google Scholar
|
|
2
|
Arany I and Safirstein RL: Cisplatin
nephrotoxicity. Semin Nephrol. 23:460–464. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao X, Panichpisal K, Kurtzman N and
Nugent K: Cisplatin nephrotoxicity: a review. Am J Med Sci.
334:115–124. 2007. View Article : Google Scholar
|
|
4
|
Cohen SM and Lippard SJ: Cisplatin: from
DNA damage to cancer chemotherapy. Prog Nucleic Acid Res Mol Biol.
67:93–130. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanigan MH and Devarajan P: Cisplatin
nephrotoxicity: molecular mechanisms. Cancer Ther. 1:47–61.
2003.PubMed/NCBI
|
|
6
|
Brady HR, Kone BC, Stromski ME, Zeidel ML,
Giebisch G and Gullans SR: Mitochondrial injury: an early event in
cisplatin toxicity to renal proximal tubules. Am J Physiol.
258:1181–1187. 1990.PubMed/NCBI
|
|
7
|
Pabla N and Dong Z: Cisplatin
nephrotoxicity: mechanisms and renoprotective strategies. Kidney
Int. 73:994–1007. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu GH, Sun YY, Li ZH, et al: Apoptosis
induced by endoplasmic reticulum stress involved in diabetic kidney
disease. Biochem Biophys Res Commun. 370:651–656. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schroder M and Kaufman RJ: The mammalian
unfolded protein response. Annu Rev Biochem. 74:739–789. 2005.
View Article : Google Scholar
|
|
10
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng M, Gao HQ, Xu L, Li BY, Zhang H and
Li XH: Cardioprotective effects of grape seed proanthocyanidins
extracts in streptozocin induced diabetic rats. J Cardiovasc
Pharmacol. 50:503–509. 2007.PubMed/NCBI
|
|
12
|
Li BY, Cheng M, Gao HQ, et al:
Back-regulation of six oxidative stress proteins with grape seed
proanthocyanidin extracts in rat diabetic nephropathy. J Cell
Biochem. 104:668–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li X, Xiao Y, Gao H, et al: Grape seed
proanthocyanidins ameliorate diabetic nephropathy via modulation of
levels of AGE, RAGE and CTGF. Nephron Exp Nephrol. 111:31–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leibbrandt ME, Wolfgang GH, Metz AL,
Ozobia AA and Haskins JR: Critical subcellular targets of cisplatin
and related platinum analogs in rat renal proximal tubule cells.
Kidney Int. 48:761–770. 1995. View Article : Google Scholar
|
|
15
|
Kharbanda S, Ren R, Pandey P, et al:
Activation of the c-Abl tyrosine kinase in the stress response to
DNA-damaging agents. Nature. 376:785–788. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lakshmanan AP, Thandavarayan RA,
Palaniyandi SS, et al: Modulation of AT-1R/CHOP-JNK-Caspase12
pathway by olmesartan treatment attenuates ER stress-induced renal
apoptosis in streptozotocin-induced diabetic mice. Eur J Pharm Sci.
44:627–634. 2011.PubMed/NCBI
|
|
17
|
Wei Q, Dong G, Franklin J and Dong Z: The
pathological role of Bax in cisplatin nephrotoxicity. Kidney Int.
72:53–62. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kong DY, Li Z, Gao CL, et al:
Erythropoietin protects against cisplatin-induced nephrotoxicity by
attenuating endoplasmic reticulum stress-induced apoptosis. J
Nephrol. 26:219–227. 2013. View Article : Google Scholar
|
|
19
|
Cetin A, Arslanbas U, Saraymen B, Canoz O,
Ozturk A and Sagdic O: Effects of grape seed extract and origanum
onites essential oil on cisplatin-induced hepatotoxicity in rats.
UHOD. 21:133–140. 2011. View Article : Google Scholar
|
|
20
|
Sayed AA: Proanthocyanidin protects
against cisplatin-induced nephrotoxicity. Phytother Res.
23:1738–1741. 2009. View
Article : Google Scholar
|
|
21
|
Chacon MR, Arola L and Guitierrez C:
Grape-seed procyanidins modulate inflammation on human
differentiated adipocytes in vitro. Cytokine. 47:137–142.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho ML, Heo YJ, Park MK, et al: Grape seed
proanthocyanidin extract (GSPE) attenuates collagen induced
arthritis. Immunol Lett. 124:102–110. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson VL, Brodsky N and Bhandari V:
Effect of antioxidants on apoptosis and cytokine release in fetal
rat Type II pneumocytes exposed to hyperoxia and nitric oxide.
Cytokine. 28:10–16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma L, Gao HQ, Li BY, Ma YB, You BA and
Zhang FL: Grape seed proanthocyanidin extracts inhibit vascular
cell adhesion molecule expression induced by advanced glycation end
products through activation of peroxisome proliferators-activated
receptor gamma. J Cardiovasc Pharmacol. 49:293–298. 2007.
View Article : Google Scholar
|
|
25
|
Terra X, Montagut G, Bustos M, et al:
Grape-seed procyanidins prevent low-grade inflammation by
modulating cytokine expression in rats fed a high-fat diet. J Nutr
Biochem. 20:210–218. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sen CK and Bagchi D: Regulation of
inducible adhesion molecule expression in human endothelial cells
by grape seed proanthocyanidin extract. Mol Cell Biochem. 216:1–7.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim H, Kim JY, Song HS, Park KU, Mun KC
and Ha E: Grape seed proanthocyanidin extract inhibits
interleukin-17-induced interleukin-6 production via MAPK pathway in
human pulmonary epithelial cells. Naunyn Schmiedebergs Arch
Pharmacol. 383:555–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang HB, Xu ZF, Liu W, et al: Effect of
grape seed proanthocyanidin extracts on methylmercury-induced
neurotoxicity in rats. Biol Trace Elem Res. 147:156–164. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cedó L, Auvi AC, Pallares V, et al: Grape
seed procyanidin extract modulates proliferation and apoptosis of
pancreatic beta-cells. Food Chemistry. 138:524–530. 2013.PubMed/NCBI
|
|
30
|
van der Kallen CJ, van Greevenbroek MM,
Stehouwer CD and Schalkwijk CG: Endoplasmic reticulum
stress-induced apoptosis in the development of diabetes: is there a
role for adipose tissue and liver? Apoptosis. 14:1424–1434.
2009.PubMed/NCBI
|
|
31
|
Clark JS, Faisal A, Baliga R, Nagamine Y
and Arany I: Cisplatin induces apoptosis through the ERK-p66shc
pathway in renal proximal tubule cells. Cancer Lett. 297:165–170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Szegezdi E, Fitzgerald U and Samali A:
Caspase-12 and ER-stress-mediated apoptosis: the story so far. Ann
NY Acad Sci. 1010:186–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakagawa T, Zhu H, Morishima N, et al:
Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and
cytotoxicity by amyloid-β. Nature. 403:98–103. 2000.PubMed/NCBI
|