Introduction
Hypoxia is known to have a detrimental effect on
human and animal health. A low O2 concentration in the
human body can cause a variety of physiological changes, including
an increased heart rate, pulmonary ventilation and cerebral blood
flow. Different cell subpopulations exhibit different responses to
hypoxia, for instance, hemoglobin content increases with altitude.
However, the functional and physiological changes of other blood
components, inlcuding leukocytes, are not completely understood.
Neutrophils are suggested to be important in the host defense and
acute inflammatory response. Hypoxia is a well documented
inflammatory stimulus that contributes to tissue polymorphonuclear
leukocyte (PMN) accumulation (1).
It also represents an important regulator of inflammatory responses
since it can cause inhibition of neutrophil apoptosis mediated by
hypoxia-inducible factor (HIF)-1α-dependent NF-κB activity
(2,3). Similarly, neutrophil polarization is
an initiation signal for cell migration and a constituent of the
innate immune response to bacterial infection (4,5).
Despite these findings and other parallel clinical and animal
studies demonstrating bacterial killing, phagocytic activity and
wound healing under hypoxia (6–8), few
studies have examined the effect of hypoxia on neutrophil function
in vitro. It was suggested that extreme hypoxia (<30
mmHg) caused a small (15–25%) but significant reduction in
chemotactic migration (9) and that
hypoxia impaired the capacity of human peripheral blood neutrophils
to generate reactive oxygen species (ROS) and kill
Staphylococcus aureus. However, the hypoxic challenge did
not compromise their motility, migration, receptor regulation or
degranulation responses (10).
Ca2+ signals have been implicated in
numerous cellular functions, including cell movements and are a
critical regulator of cell migration and chemotaxis (11–13).
Ca2+ influx through the plasma membrane is regulated in
one of at least two ways; i) depletion of the intracellular
Ca2+ stores, termed store-operated Ca2+ entry
(SOCE) (14,15) and ii) receptor occupation, termed
receptor-operated Ca2+ entry (ROCE) (16). Previous studies have identified two
molecules, stromal interaction molecule 1 (STIM1) and Orai1 (also
named CRACM1), that are responsible for SOCE (17–19).
STIM1 acts as a Ca2+ sensor and Orai1 is an essential
pore-forming component of the SOCE channel (20,21).
Co-expression of Orai1 and STIM1 is sufficient to reconstitute the
store-operated Ca2+ channel function (22–24).
Store-operated Ca2+ influx controls a variety of
physiological and pathological processes (25–27),
including the migration and polarization of various cell types,
including neutrophils. In nonexcitable cells, store-operated
Ca2+ influx is the predominant Ca2+ entry
mechanism (15,28). Previous studies have indicated that
SOCE is involved in cell polarization, migration and metastasis by
regulating a variety of cytosolic Ca2+ signals (15,29,30),
and it may also be important in the N-formyl-Met-Leu-Phe
(fMLP)-induced cell polarization of the neutrophil-like HL-60 cells
(31). However, the role of SOCE
in neutrophil polarization under hypoxia is unclear and thus needs
to be elucidated. This question is addressed in the present study
using differentiated HL-60 (dHL-60) cells that have been
demonstrated to be a valid model system for the analysis of human
neutrophil polarization (32) and
easy for genetic manipulation.
Given the important role of SOCE in cell
polarization, we hypothesized that the effect of hypoxia on cell
polarization was mediated by SOCE. In the present study, STIM1 and
Orai1, essential components in SOCE, were used to study the effect
of hypoxia, and we also used plasmids to overexpress STIM1 and
Orai1 to further confirm the role of SOCE in the polarization of
dHL-60 cells under hypoxia.
Materials and methods
Materials
fMLP, dimethyl sulfoxide (DMSO), thapsigargin (TG)
and CaCl2 were purchased from Sigma-Aldrich (St. Louis,
MO, USA); Fluo-4 acetoxymethyl (AM) ester was obtained from
Invitrogen Life Technologies (Grand Island, NY, USA); monoclonal
rabbit anti-STIM1, -Orai1 and -HIF-1α antibodies were purchased
from Cell Signaling Technology, Inc. (Boston, MA, USA) and cell
lysis buffer for western blotting was obtained from KeyGen
(Nanjing, Jiangsu, China), respectively.
Cell culture
HL-60 cells, a promyelocytic leukemia cell line
provided by the China Center for Type Culture Collection (CCTCC;
Shanghai, China), were maintained in RPMI-1640 medium (Gibco-BRL,
Karlsruhe, Germany) supplemented with 10% fetal calf serum and 2 mM
of L-glutamine (Gibco-BRL) at 37ºC in a humidified atmosphere of
20% O2 and 5% CO2. The day prior to the
differentiation of HL-60 cells was designated as day 0. HL-60 cells
with a cell density of 106 cells/ml were induced to
differentiate into a neutrophil-like phenotype (dHL-60 cells) with
1.3% DMSO for 4–6 days (31),
which was used for subsequent experiments. Non-viable cells were
removed by centrifugation at 180 × g for 5 min at room temperature
and the cells were washed three times with 5 ml of
phosphate-buffered saline (PBS; 0.2 M of
Na2HPO4, 0.2 M of
NaH2PO4, pH 7.2±0.1). Then, the dHL-60 cells
incubated with DMSO for 4 days were transferred and cultured in a
hypoxic incubator (Forma Series-II; Thermo Fisher Scientific,
Rockford, IL, USA), which was flushed with a gas mixture consisting
of 3% O2, 5% CO2 and 92% nitrogen at 37ºC for
1–2 days.
Cell electroporation
dHL-60 cells differentiated with DMSO for 4 days
were collected and washed twice with RPMI-1640 medium. Cells were
resuspended in ice-cold OPTI-MEMI. Plasmid DNA (2–5 μg/ml;
STIM1-mOrange, Orai1-mKO and pcDNA3.1) was added to a 400 μl
aliquot of dHL-60 cells at a cell density of 8×106
cells/ml (33,34). The mixture was then transferred to
an electroporation cuvette with a 4 mm electrode gap (Bio-Rad,
Hercules, CA, USA). Following 10 min incubation on ice, the mixture
was electroporated (295 V, 1180 μF, 500 Ω) in a Gene Pulser Xcell
Electroporation System (Bio-Rad) (31). Following electroporation, cells
were allowed to recover for 30 min on ice and then incubated in
RPMI-1640 medium with the presence of 10% fetal calf serum for 2
days. The transfection efficiency of STIM1 was ~80%. The cells were
processed for subsequent assays ~48 h following transfection.
Measurement of intracellular free
Ca2+ concentration ([Ca2+]i)
dHL-60 cells grown in normoxia for 4–6 days or
hypoxia for 1–2 days were suspended at 106 cells/ml in
Hanks’ balanced salt solution (HBSS; pH 7.4) and labeled with 2 μM
of Fluo-4 AM at 37ºC for 30 min in the dark. Cells were then washed
three times with HBSS on ice and then resuspended in
Ca2+-free buffer solution containing 0.3 mM of EGTA. The
green fluorescence of Fluo-4 was excited by a 10 mW multi-tune
argon laser at 488 nm and recorded through a 525 nm channel under
an inverted laser scanning confocal microscope (FV1000-IX71;
Olympus, Tokyo, Japan). For imaging with Fluo-4,
(Ca2+)i changes were defined as the ratio of
F to F0 (F/F0) following background
subtraction, where F was the change in fluorescence signal
intensity and F0 was the baseline calculated by
averaging three independent experiments prior to the application of
the stimulus.
Zigmond assay
HL-60 cells were induced to differentiate into
dHL-60 cells with DMSO for 4–6 days and then cultured in a hypoxic
environment for another 1–2 days. Cells were allowed to attach to
the cover slip (22×40 mm) at room temperature for 5 min before the
cover slip was inverted over the chamber (Neuro Probe,
Gaithersburg, MD, USA), as previously described (35). One channel of the chamber was
filled with HBSS (vehicle) and the other with 100 nM of fMLP.
Digital images of the cells were captured every 10 or 15 sec,
depending on the experiment, for a total of 30 min using an
inverted microscope with a ×20 objective (Olympus; IX-71). An
average of 100 cells were examined for each experiment and analysis
was performed for at least three independent experiments. The
percentage of cells that were polarized, i.e. with a directionally
oriented leading edge and trailing tail, was calculated as
described previously (36).
Western blotting
dHL-60 cells were washed with PBS at 37ºC and the
pellets obtained from centrifugation at 100 × g for 1 min were
suspended in lysis buffer at a cell density of 1.0×107
cells/ml and incubated on ice for 30 min. Following centrifugation
at 15,000 × g for 15 min at 4ºC, the supernatants were collected
and the protein preparations were subjected to a 10% sodium dodecyl
sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto a polyvinylidene difluoride membrane for western
blot analysis using antibodies against STIM1, Orai1 and HIF-1α.
Statistical analysis
Data are presented as the mean ± standard deviation
from three independent experiments and the Student’s t-test was
used for the comparison between two means and one-way analysis of
variance (ANOVA) for the comparison of more than two means using
SPSS software, version 13.0. P<0.05 was considered to indicate a
statistically significant difference.
Results
Polarization in dHL-60 cells under
normoxia and hypoxia
HL-60 cells differentiated for 4–6 days with 1.3%
DMSO can be used for polarization assays. In the present study,
HL-60 cells differentiated for 4 days and then exposed to hypoxia
(3% O2) for another 1–2 days were selected as the
polarization capacity was improved and the DMSO induced
differentiation of HL-60 cells to the neutrophil-like phenotype
(dHL-60 cells) was largely completed at day 4 (37). We compared the polarization between
N5 (5 day incubation under normoxic conditions) and N4+H1 (4 day
incubation under normoxic conditions and 1 day under hypoxic
conditions) and between N6 (6 day incubation under normoxic
conditions) and N4+H2 (4 day incubation under normoxic conditions
and 2 day under hypoxic conditions). No significant differences
between the N5 and the N4+H1 groups were identified. However, a
higher polarization was observed in the N4+H2 group than that in
the N6 group, suggesting that hypoxia can induce cell polarization
and the effect is associated with the stimulation time. It was also
observed that the percentage of cells polarized in the direction of
fMLP declined from N5 to N6, however increased from N4+H1 to N4+H2.
Western blot analysis results also demonstrated that HIF-1α
expression increased with hypoxia stimulation time, as shown in
Fig. 1.
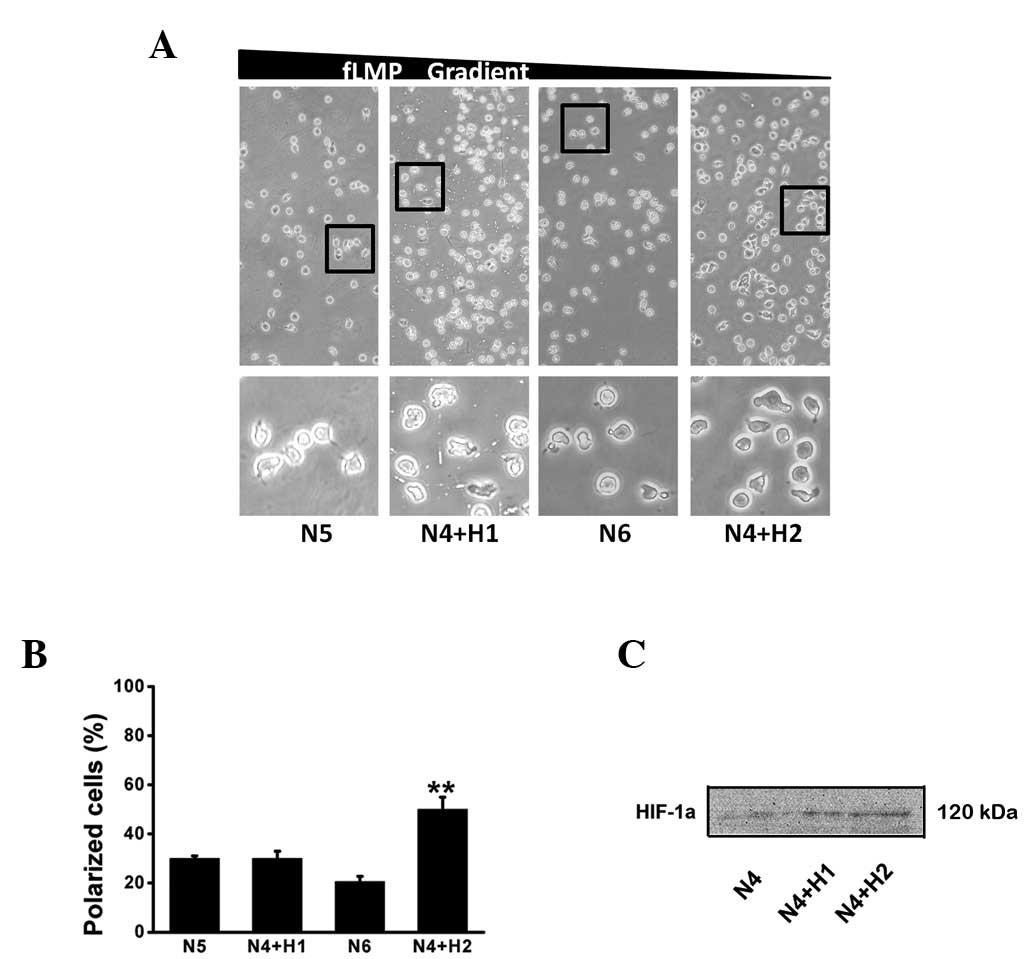 | Figure 1Effect of hypoxia on fMLP-induced
cell polarization toward an fMLP gradient. HL-60 cells were induced
to differentiate into dHL-60 cells with 1.3% DMSO for 4 days under
normoxic conditions and then exposed to hypoxia (3% O2)
for another 1–2 days (N4+H1 and N4+H2), or with 1.3% DMSO all the
time for 5 or 6 days under normoxic conditions (N5 and N6),
respectively. (A) dHL-60 cells were induced to polarize towards an
fMLP gradient in the Zigmond chamber for 15 min at 37ºC. Time-lapse
microscopy was used to record cell morphology on the bridge at a 30
sec interval and the direction of the fMLP gradient (0–100 nmol/l)
was indicated by the wedge. (B) The proportion of dHL-60 cells
polarized along the gradient direction was obtained from three
independent experiments. Bars represent the mean ± SD.
**P<0.01, as compared with N6. (C) HIF-1α levels
exposed to hypoxia for 1–2 days. fMLP, N-formyl-Met-Leu-Phe;
dHL-60, differentiated human neutrophil-like HL-60 cells; DMSO,
dimethyl sulfoxide; HIF-1α, hypoxia-inducible factor-1α; SD,
standard deviation; N5, 5 day incubation under normoxic conditions;
N4+H1, 4 day incubation under normoxic conditions and 1 day under
hypoxic conditions; N6, 6 day incubation under normoxic conditions;
N4+H2, 4 day incubation under normoxic conditions and 2 day under
hypoxic conditions. |
STIM1 and Orai1 expression under normoxia
and hypoxia
The dependence of cell polarization and chemotaxis
on SOCE proteins STIM1 and Orai1 has been described previously
(31,38). As shown in Fig. 2A, the expression of STIM1 and Orai1
was significantly reduced at day 2 of exposure to hypoxia, however
no apparent change was observed on the first day.
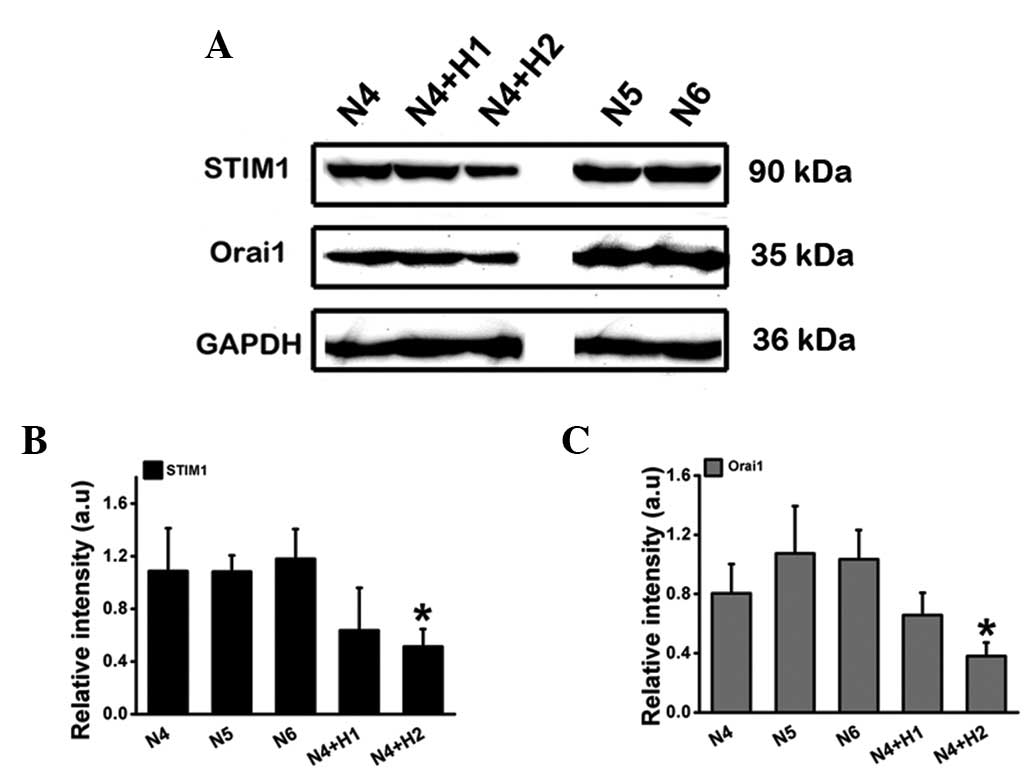 | Figure 2Effect of hypoxia on STIM1 and Orai1
expression level. Cell lysates were analyzed by SDS-PAGE and then
western blot analysis using antibodies against STIM1 and Orai1. (A)
STIM1 and Orai1 expression of dHL-60 cells stimulated by hypoxia
for 1–2 days. (B and C) The relative intensity of the expression of
STIM1 and Orai1. Data are expressed as the mean ± SD from three
independent experiments. *P<0.05, as compared with
N6. STIM1, stromal interaction molecule 1; SDS-PAGE, sodium dodecyl
sulfate polyacrylamide gel electrophoresis; SD, standard deviation;
dHL-60, differentiated human neutrophil-like HL-60 cells; N4, 4 day
incubation under normoxic conditions; N5, 5 day incubation under
normoxic conditions; N4+H1, 4 day incubation under normoxic
conditions and 1 day under hypoxic conditions; N6, 6 day incubation
under normoxic conditions; N4+H2, 4 day incubation under normoxic
conditions and 2 day under hypoxic conditions. |
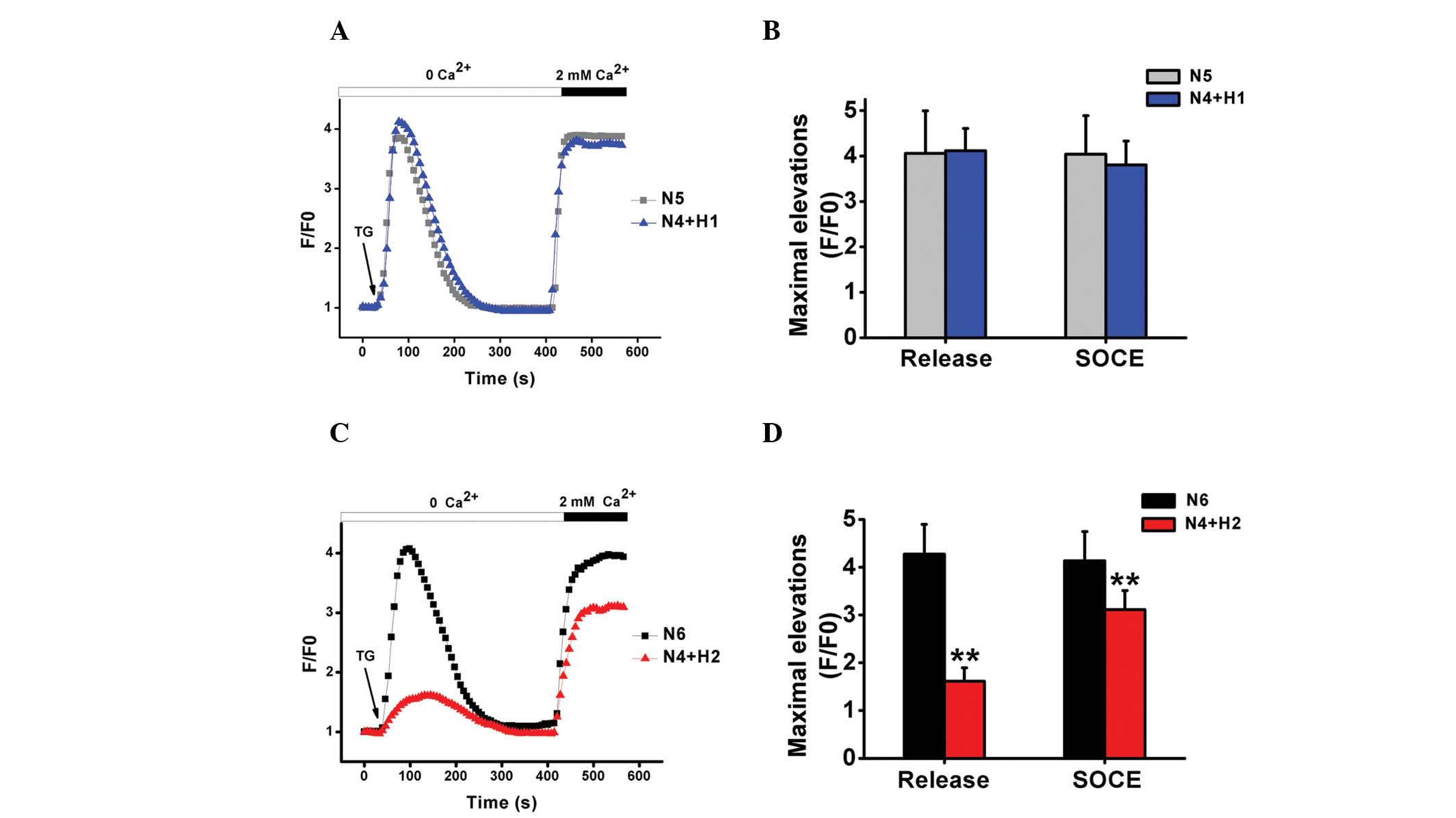
TG triggers SOCE-mediated Ca2+
entry in dHL-60 cells under normoxia and hypoxia
dHL-60 cells were treated with TG under
Ca2+-free conditions to investigate the mechanisms
underlying Ca2+ store-depletion and influx in dHL-60
cells under normoxic or hypoxic conditions. SOCE can be initiated
by Ca2+ store-depletion. No significant difference was
observed between N5 and N4+H1 following TG stimulation and
Ca2+ addition. However, hypoxia appeared to have a more
significant impact on TG-induced Ca2+ release in N4+H2
and partially inhibited subsequent Ca2+ influx by ~25%
following the addition of CaCl2, as shown in Fig. 3.
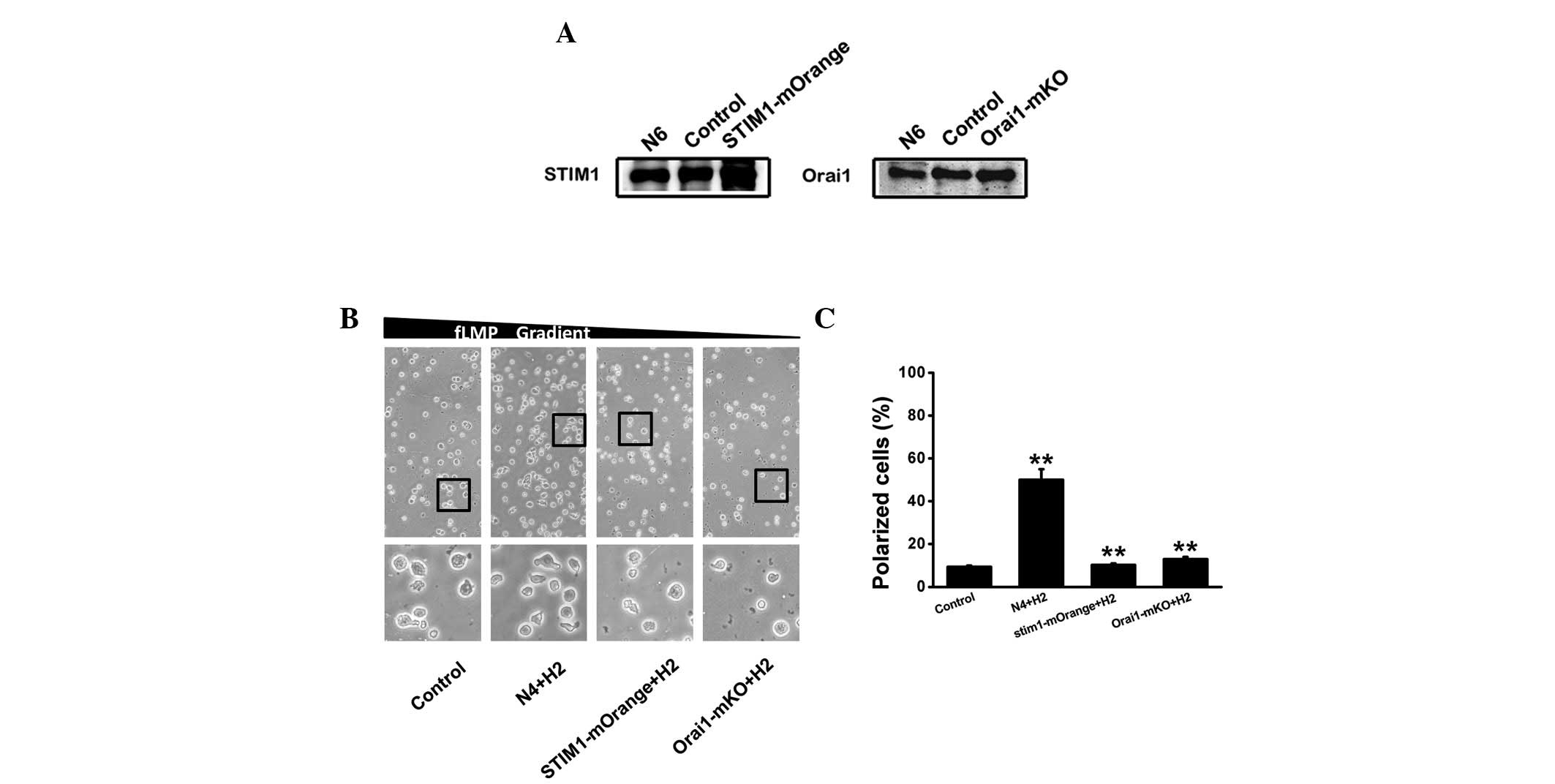
Polarization in dHL-60 cells following
STIM1 or Orail overexpression
In order to evaluate the effect of STIM1 and Orai1
on cell polarization in response to an fMLP gradient (0–100 nM)
under hypoxia, plasmid electroporation was used for the
overexpression of STIM1 and Orai1, as shown in Fig. 4A. The STIM1-mOrange plasmid
appeared to be more effective than Orai1-mKO and the expression was
enhanced by 80 and 20% compared with that treated with pcNDA3.1,
respectively. We investigated the effect of STIM1 and Orai1
overexpression on cell polarization in response to the fMLP
gradient. As shown in Fig. 4B,
STIM1 and Orai1 overexpression using plasmids resulted in a
substantial suppression of polarization in the direction of the
fMLP gradient at day 2 of hypoxia, with the percentage of polarized
cells in the presence of STIM1-mOrange, Orai1-mko and N4+H2 being
10, 13 and 50%, respectively.
Discussion
Despite considerable effort directed towards the
regulation of neutrophil functions in the past, there remain
numerous unknown factors concerning the responses of neutrophils to
hypoxia. In an in vivo experiment, Klokker et al
demonstrated that acute hypoxia induced marked alterations in the
immune system and natural killer cells were particularly sensitive
to the hypoxic stimulus (39).
Hypoxic hypoxia was revealed to increase the phagocytic activity of
human neutrophils directly with the hypoxia level (8). An in vitro study by Rotstein
et al suggested that extreme hypoxia (less than 30 mmHg)
caused a small (15–25%) but significant reduction in chemotactic
migration (9). These studies
suggest that an altered microenvironment may contribute to the
failure of host leukocytes to resolve infection. In humans,
hypoxemia (O2 saturation, 5–20%) significantly increased
the percentage of PMN positive cells for phagocytosis via
(Ca2+)i mobilization (40). There have been numerous other
studies demonstrating that Ca2+ influx via SOCE is
important in the polarization, migration and metastasis of
non-excitable cells following exposure to a variety of stimuli
(30,41). Hauser et al suggested that
prolonged elevations of (Ca2+)i due to
enhanced SOCE may alter the stimulus-response coupling to
chemotaxins and contribute to PMN dysfunction following injury
(42). However, the mechanisms by
which SOCE affects cell polarization under hypoxic conditions
remain unclear. In the present study, an attempt is made to study
the effect of hypoxia on polarization in differentiated human
neutrophil-like HL-60 cells and the role of SOCE in this
process.
The impact of hypoxia on dHL-60 cells, a phenotype
analogous to neutrophils, was examined in the present study. The
results demonstrated that there was no significant difference in
the percentage of polarized dHL-60 cells between the N5 and N4+H1
groups, however, a higher cell polarization was observed in the
N4+H2 group compared with that in the N6 group, indicating that the
polarization of dHL-60 cells was increased in hypoxia. STIM1 and
Orai1 are important factors of SOCE that can modulate cell
polarization. Our experiments also demonstrated that STIM1 and
Orai1 were decreased in the N4+H2 group, which may be attributed to
the reduction in SOCE. To ascertain the effect of SOCE in this
response, (Ca2+)i was measured using
Fluo-4/AM imaging. The inhibitory effect of hypoxia on
Ca2+ influx was observed at day 2 of exposure to
hypoxia, suggesting that hypoxia did inhibit SOCE. However, it must
be noted that other possibilities which contribute to the
inhibition of Ca2+ influx cannot be completely ruled
out. SOCE is involved in TG-induced Ca2+ influx under
hypoxia. We also identified that hypoxia not only partially
inhibited subsequent Ca2+ influx, but also appeared to
have a more significant impact on TG-induced Ca2+
release in N4+H2. A plausible explanation for the observed
difference in Ca2+ release appears to be associated with
the altered signal response of endoplasmic reticulum
Ca2+ emptying following hypoxia or a difference in
related calmodulin receptor expression. Hypoxia may impact SOCE by
the components of SOCE, including STIM1 or Orai1, or it is possible
that other factors are involved which change SOCE. Our study
supports a complex signaling effect at work in mature dHL-60 cells
under hypoxia. Plasmid overexpression resulted in an enhancement of
SOCE and an inhibition of cell polarization, which further supports
the conclusion that SOCE is involved in the process and inhibits
the polarization of dHL-60 cells.
Thus, it can be concluded that hypoxia alters PMN
functions, including polarization. This may be explained by the
downregulation of STIM1 and Orai1 expression, and SOCE. SOCE
inhibits the polarization of dHL-60 cells under hypoxic conditions,
which may be the mechanism by which the neutrophils adapt to
hypoxia. SOCE is also a key modulator for immune deficiency under
hypoxia, potentially as a therapy target. However, the mechanisms
responsible for the differential Ca2+ release regulation
by TG is not clearly understood. Neutrophils are important in the
immune response under hypoxic conditions and this underscores the
requirement for further study concerning the regulatory mechanisms
and gene expression involved in endoplasmic reticulum calcium
emptying.
Acknowledgements
This study was supported by the National Basic
Research Program of China (no. 2012CB518200), the Program of
International S&T Co-operation of China (no. 0S2012GR0195) and
the National Natural Science Foundation of China (no. 30393133 and
no. 81071611). We would like to thank Professor Tao Xu for
providing the plasmids (STIM1-mOrange and Orai1-mKo) for us.
References
|
1
|
Eltzschig HK, Thompson LF, Karhausen J, et
al: Endogenous adenosine produced during hypoxia attenuates
neutrophil accumulation: coordination by extracellular nucleotide
metabolism. Blood. 104:3986–3992. 2004. View Article : Google Scholar
|
|
2
|
Thompson AA, Binham J, Plant T, Whyte MK
and Walmsley SR: Hypoxia, the HIF pathway and neutrophilic
inflammatory responses. Biol Chem. 394:471–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walmsley SR, Print C, Farahi N, et al:
Hypoxia-induced neutrophil survival is mediated by
HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 201:105–115.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Onsum M and Rao CV: A mathematical model
for neutrophil gradient sensing and polarization. PLoS Comput Biol.
3:e362007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
O’Donnell NG, McSharry CP, Wilkinson PC
and Asbury AJ: Comparison of the inhibitory effect of propofol,
thiopentone and midazolam on neutrophil polarization in vitro in
the presence or absence of human serum albumin. Br J Anaesth.
69:70–74. 1992.PubMed/NCBI
|
|
6
|
Allen DB, Maguire JJ, Mahdavian M, et al:
Wound hypoxia and acidosis limit neutrophil bacterial killing
mechanisms. Arch Surg. 132:991–996. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jönsson K, Hunt TK and Mathes SJ: Oxygen
as an isolated variable influences resistance to infection. Ann
Surg. 208:783–787. 1988.PubMed/NCBI
|
|
8
|
Krupina TN, Korotaev MM, Pukhova IaI,
Tsyganova NI and Likhacheva NP: Comparative evaluation of studies
of the action of different levels of hypoxia on the human
immunobiological status. Kosm Biol Aviakosm Med. 11:38–43. 1977.(In
Russian).
|
|
9
|
Rotstein OD, Fiegel VD, Simmons RL and
Knighton DR: The deleterious effect of reduced pH and hypoxia on
neutrophil migration in vitro. J Surg Res. 45:298–303. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGovern NN, Cowburn AS, Porter L, et al:
Hypoxia selectively inhibits respiratory burst activity and killing
of Staphylococcus aureus in human neutrophils. J Immunol.
186:453–463. 2011. View Article : Google Scholar
|
|
11
|
Marks PW and Maxfield FR: Transient
increases in cytosolic free calcium appear to be required for the
migration of adherent human neutrophils. J Cell Biol. 110:43–52.
1990.
|
|
12
|
Meshulam T, Proto P, Diamond RD and
Melnick DA: Calcium modulation and chemotactic response: divergent
stimulation of neutrophil chemotaxis and cytosolic calcium response
by the chemotactic peptide receptor. J Immunol. 137:1954–1960.
1986.
|
|
13
|
Pettit EJ and Fay FS: Cytosolic free
calcium and the cytoskeleton in the control of leukocyte
chemotaxis. Physiol Rev. 78:949–967. 1998.PubMed/NCBI
|
|
14
|
Parekh AB and Putney JW Jr: Store-operated
calcium channels. Physiol Rev. 85:757–810. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lewis RS: The molecular choreography of a
store-operated calcium channel. Nature. 446:284–287. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salmon MD and Ahluwalia J: Pharmacology of
receptor operated calcium entry in human neutrophils. Int
Immunopharmacol. 11:145–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feske S, Gwack Y, Prakriya M, et al: A
mutation in Orai1 causes immune deficiency by abrogating CRAC
channel function. Nature. 441:179–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roos J, DiGregorio PJ, Yeromin AV, et al:
STIM1, an essential and conserved component of store-operated Ca2+
channel function. J Cell Biol. 169:435–445. 2005.PubMed/NCBI
|
|
19
|
Vig M, Peinelt C, Beck A, et al: CRACM1 is
a plasma membrane protein essential for store-operated
Ca2+ entry. Science. 312:1220–1223. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prakriya M, Feske S, Gwack Y, Srikanth S,
Rao A and Hogan PG: Orai1 is an essential pore subunit of the CRAC
channel. Nature. 443:230–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yeromin AV, Zhang SL, Jiang W, Yu Y,
Safrina O and Cahalan MD: Molecular identification of the CRAC
channel by altered ion selectivity in a mutant of Orai. Nature.
443:226–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mercer JC, Dehaven WI, Smyth JT, et al:
Large store-operated calcium selective currents due to
co-expression of Orai1 or Orai2 with the intracellular calcium
sensor, Stim1. J Biol Chem. 281:24979–24990. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Peinelt C, Vig M, Koomoa DL, et al:
Amplification of CRAC current by STIM1 and CRACM1 (Orai1). Nat Cell
Biol. 8:771–773. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soboloff J, Spassova MA, Tang XD,
Hewavitharana T, Xu W and Gill DL: Orai1 and STIM reconstitute
store-operated calcium channel function. J Biol Chem.
281:20661–20665. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dolmetsch RE, Xu K and Lewis RS: Calcium
oscillations increase the efficiency and specificity of gene
expression. Nature. 392:933–936. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewis RS: Calcium signaling mechanisms in
T lymphocytes. Annu Rev Immunol. 19:497–521. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yoo AS, Cheng I, Chung S, et al:
Presenilin-mediated modulation of capacitative calcium entry.
Neuron. 27:561–572. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parekh AB and Penner R: Store depletion
and calcium influx. Physiol Rev. 77:901–930. 1997.PubMed/NCBI
|
|
29
|
Yang S, Zhang JJ and Huang XY: Orai1 and
STIM1 are critical for breast tumor cell migration and metastasis.
Cancer Cell. 15:124–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schaff UY, Dixit N, Procyk E, Yamayoshi I,
Tse T and Simon SI: Orai1 regulates intracellular calcium, arrest,
and shape polarization during neutrophil recruitment in shear flow.
Blood. 115:657–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zou W, Meng X, Cai C, et al:
Store-operated Ca2+ entry (SOCE) plays a role in the
polarization of neutrophil-like HL-60 cells by regulating the
activation of Akt, Src, and Rho family GTPases. Cell Physiol
Biochem. 30:221–237. 2012.
|
|
32
|
Hauert AB, Martinelli S, Marone C and
Niggli V: Differentiated HL-60 cells are a valid model system for
the analysis of human neutrophil migration and chemotaxis. Int J
Biochem Cell Biol. 34:838–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Z, Lu J, Xu P, Xie X, Chen L and Xu T:
Mapping the interacting domains of STIM1 and Orai1 in Ca2+
release-activated Ca2+ channel activation. J Biol Chem.
282:29448–29456. 2007.PubMed/NCBI
|
|
34
|
Ji W, Xu P, Li Z, et al: Functional
stoichiometry of the unitary calcium-release-activated calcium
channel. Proc Natl Acad Sci USA. 105:13668–13673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zigmond SH: Ability of polymorphonuclear
leukocytes to orient in gradients of chemotactic factors. J Cell
Biol. 75:606–616. 1977. View Article : Google Scholar
|
|
36
|
Heit B, Liu L, Colarusso P, Puri KD and
Kubes P: PI3K accelerates, but is not required for, neutrophil
chemotaxis to fMLP. J Cell Sci. 121:205–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zou W, Chu X, Cai C, et al: AKT-mediated
regulation of polarization in differentiated human neutrophil-like
HL-60 cells. Inflamm Res. 61:853–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cai C, Tang S, Wang X, et al: Requirement
for both receptor-operated and store-operated calcium entry in
N-formyl-methionine-leucine-phenylalanine-induced neutrophil
polarization. Biochem Biophys Res Commun. 430:816–821. 2013.
View Article : Google Scholar
|
|
39
|
Klokker M, Kharazmi A, Galbo H, Bygbjerg I
and Pedersen BK: Influence of in vivo hypobaric hypoxia on function
of lymphocytes, neutrocytes, natural killer cells, and cytokines. J
Appl Physiol. 74:1100–1106. 1993.PubMed/NCBI
|
|
40
|
Simms HH and D’Amico R: Regulation of
whole blood polymorphonuclear leukocyte phagocytosis following
hypoxemia and hypoxemia/reoxygenation. Shock. 1:10–18. 1994.
View Article : Google Scholar
|
|
41
|
Lee C, Xu DZ, Feketeova E, et al:
Store-operated calcium channel inhibition attenuates neutrophil
function and postshock acute lung injury. J Trauma. 59:56–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hauser CJ, Fekete Z, Livingston DH, Adams
J, Garced M and Deitch EA: Major trauma enhances store-operated
calcium influx in human neutrophils. J Trauma. 48:592–597. 2000.
View Article : Google Scholar : PubMed/NCBI
|