Introduction
Epigallocatechin-3-gallate (EGCG), a major
biologically active polyphenol in green tea has long been known to
exhibit potential health benefits, including anti-oxidant,
anticancer and anti-inflammatory effects (1–3).
More recently there have been an increasing number of studies
demonstrating the potent anticancer effect of EGCG against various
cancer cell lines in vitro, including breast (4), pancreatic (5), colorectal (6) and gastric cancer cell lines (7).
Ovarian cancer is a major cause of mortality among
the gynecological malignancies globally. Despite significant
improvement in surgical technology and therapy regimens in previous
years, the molecular mechanisms underlying the disease progression
remain poorly understood (8,9). The
development of novel therapeutic agents targeting this potentially
fatal gynecological disease is important to improve the prognosis
of treatment. However, little is known of the effects of EGCG on
human ovarian cancer progression and the associated molecular
signaling mechanisms.
It is well demonstrated that a number of cellular,
extracellular and cytokine-associated components trigger multiple
downstream protein kinase pathways, thus exhibiting a role in the
regulation of cell proliferation and migration during cancer
development. Among these pathways, the mitogen-activated protein
kinases (MAPK) cascades are the most well-studied (10–12).
MAPK are proline-directed serine/threonine kinases that have been
classified into at least six subfamilies. As the most important
member of the MAPKs family, extracellular signal-regulated kinases
1 and 2 (ERK1/2) is required for cell mobility, proliferation and
migration (13,14). Another important member of the MAPK
family, p38, is essential in regulating a number of cellular
processes, including inflammation, cell differentiation, cell
growth and cell death (15–17).
However, whether these signaling pathways are involved in
EGCG-regulated cell proliferation and migration during ovarian
cancer growth remains unknown. In addition, the secretion of matrix
metalloproteinases (MMPs) is crucial in cancer cell metastasis and
is closely associated with the migration behavior of cancer
(18,19). The effects of EGCG on MMP
expression in ovarian cancer remain to be investigated.
In the current study, the effect of EGCG on the cell
proliferation and migration in OVCAR-3 cells was investigated, as
well as the signaling pathways involved in these actions.
Materials and methods
Cell line and cell culture
The OVCAR-3 human ovarian adenocarcinoma cell line
was obtained from the Cell Bank of Type Culture Collection of
Chinese Academy of Sciences (Shanghai, China) and maintained in
RPMI-1640 (Gibco-BRL, Life Technologies, Grand Island, NY, USA)
supplemented with 10% fetal bovine serum (FBS, HyClone, Logan, UT,
USA), 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mM
L-glutamine. All cells were cultured in a humidified atmosphere of
5% CO2 at 37°C. The cells used in this study were at
passages 23–26.
MTT assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl
tetrazolium bromide (MTT) viability assay was performed as
previously described with slight modifications (17). Briefly, cells were seeded into
96-well plates with 0.5×104 cells/well. Following 16 h
of attachment, different concentrations of EGCG (0–200 μM,
PeproTech, Rocky Hill, NJ, USA) were applied to the cells in
RPMI-1640 culture media and incubated for a further 48 h. The cells
were washed with phosphate-buffered saline (PBS) and 200 μl MTT
(0.5 mg/ml) was added to each well and further incubated for 4 h.
The MTT solution was carefully removed by aspiration and the
formazan product was dissolved in 150 ml dimethylsulfoxide.
Absorbance was measured at 570 nm on a microplate reader (BioTek
Instruments, Winooski, VT, USA). The same experiments were
performed for time-course (2, 4 and 6 days) treatment with 100 μM
EGCG. To determine the role of the p38 MAPK pathway in cell
proliferation, additional cells were subjected to the same assay in
the presence of 10 μM SB203580 (a specific p38 inhibitor, 1 h
pretreatment; CalBiochem, San Diego, CA, USA). Cell proliferation
studies were performed in three independent experiments.
Migration assay
Cell migration was detected using a 24-well
transwell chamber with 8.0-μm pore polycarbonate filter inserts
(Costar, Cambridge, MA, USA). Cells (5×104 cells/well)
suspended in serum-free RPMI-1640 were overlaid in the upper
chamber. In each lower chamber, 800 μl RPMI-1640 with 10% FBS in
the presence of various concentrations of EGCG (0–100 μM) was
added. The inserts were incubated at 37°C in a humidified
atmosphere containing 5% CO2 for 16 h. Cells that had
migrated to the bottom of the inserts were stained with Calcein AM
(0.2 mg/ml; Molecular probes, Eugene, OR, USA) for 30 min, examined
and recorded under a microscope (ECLIPSE Ti; Nikon, Tokyo, Japan)
mounted with a CCD camera (Nikon). The numbers of migrated cells
were counted using the Metamorph image analysis program (Universal
Imaging Corporation, West Chester, PA, USA). Cell migration studies
were performed in four independent experiments.
Western blotting
Cells were treated with 100 μM EGCG for 0–120 min in
RPMI-1640 culture media and in 10 μM SB203580 for 60 min. To
determine changes in total and phosphorylated ERK1/2 and p38
protein levels, cells were washed twice with cold PBS, harvested
and lysed by sonication (Sonicator 300, Misonix, Inc., Farmingdale,
NY, USA) in buffer (4 mM sodium pyrophosphate; 50 mM HEPES, pH 7.5;
100 mM NaCl; 10 mM EDTA; 10 mM sodium fluoride; 2 mM sodium
orthovanadate [Na3VO4]; 1 mM PMSF; 1% Triton
X-100; 5 mg/ml leupeptin and 5 mg/ml of aprotinin). The protein
concentrations in the supernatants of the lysates were determined.
Proteins (15–20 μg/lane) were subjected to western blot analysis.
Proteins were separated on 10% SDS-PAGE gels and electroblotted
onto Immobilon-P membranes (Millipore, Bedford, MA, USA). Proteins
on the membranes were probed with an antibody against total or
phospho-specific ERK1/2 (1:2,000 dilution; rabbit polyclonal),
total (1:2,000 dilution; rabbit polyclonal) or phospho-specific p38
(1:1,000; dilution; rabbit polyclonal; all Cell Signaling
Technology, Inc., Danvers, MA, USA). Changes in total and
phosphorylated ERK1/2 and p38 protein levels were quantified. Data
on phosphorylated ERK1/2 and p38 were normalized to total ERK1/2
and p38. The same assay was performed on the expression of MMP-2/9
in response to EGCG treatment (0–100 μM). Western blotting studies
were run in at least three independent experiments.
Statistical analysis
Data were analyzed using one-way analysis of
variance (SigmaStat, Jandel Co., San Rafael, CA, USA). When an
F-test was significant, data were compared with their respective
control by the Bonferroni’s multiple comparison test or Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
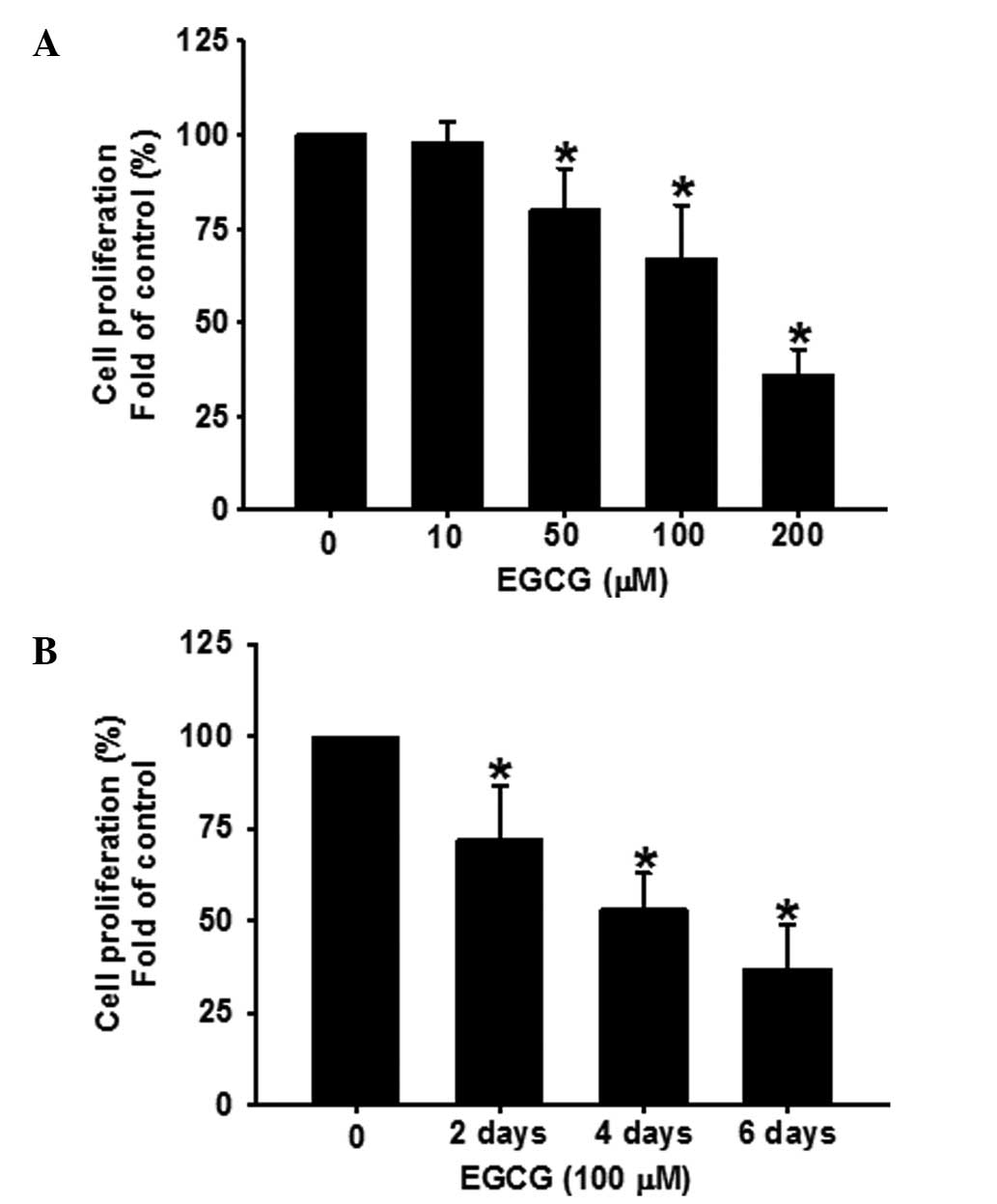
EGCG inhibits the growth of human ESCC
cells in a time- and dose-dependent manner
EGCG inhibits tumor growth in a number of cancer
types (4–7). Based on these studies, the effects of
EGCG on the proliferation of ESCC cells were determined. The doses
of EGCG used were comparable to those used in previous studies
(7). OVCAR-3 cells were treated
with EGCG (0–200 μM) for 48 h. As shown in Fig. 1A, EGCG dose-dependently (P<0.05)
inhibited the proliferation of OVCAR-3 cells. In addition, the cell
proliferation following treatment with EGCG for 2, 4 and 6 days,
respectively, was analyzed. As shown in Fig. 1B, EGCG time-dependently (P<0.05)
inhibited OVCAR-3 cell proliferation at a concentration of 100
μM.
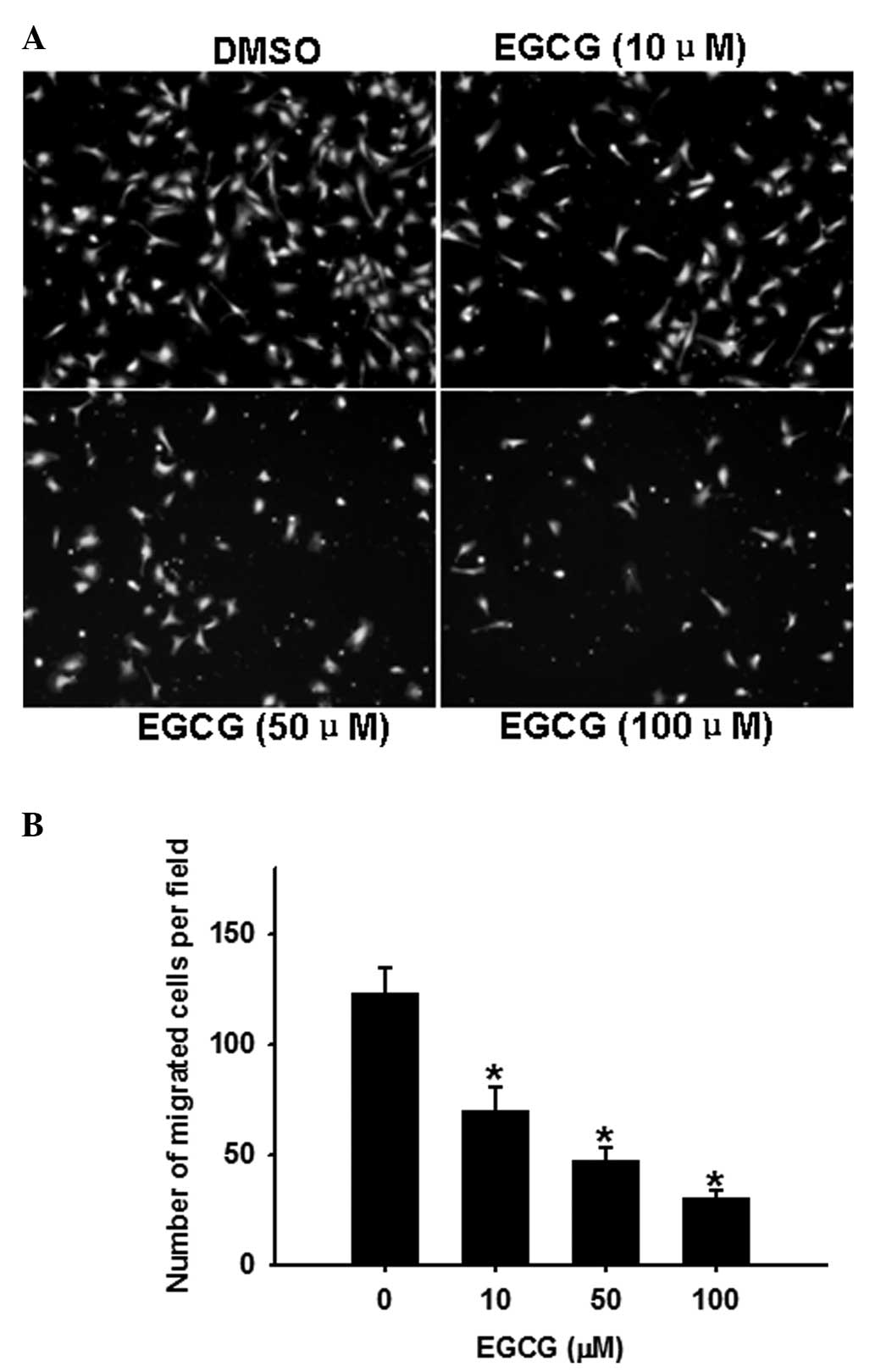
EGCG dose-dependently inhibits OVCAR-3
cell migration
The transwell chamber assay is a commonly used model
for analyzing the molecular mechanisms underlying cell migration.
In this assay (Fig. 2), EGCG
(0–100 μM) dose-dependently inhibited (P<0.05) OVCAR-3 cell
migration.
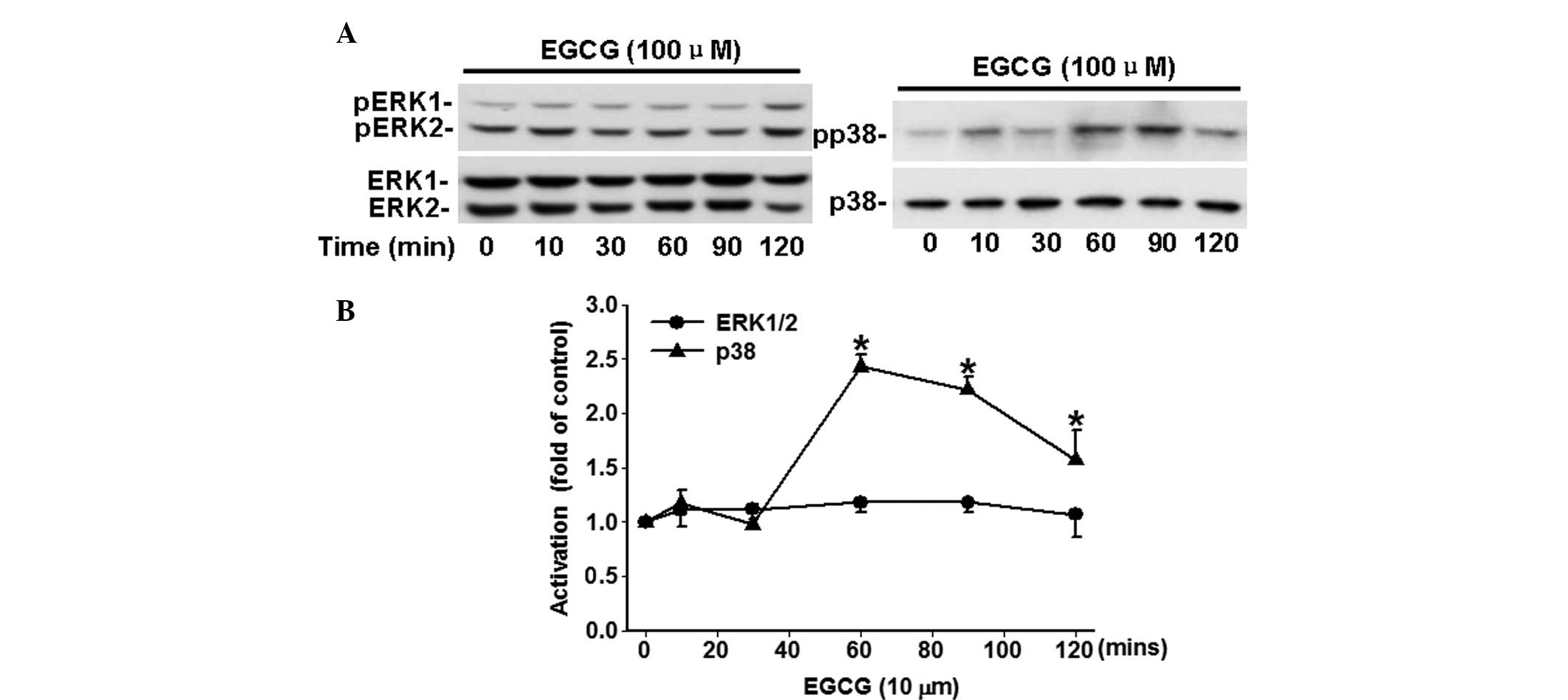
EGCG time-dependently inhibits the
phosphorylation of p38, but not ERK1/2
The potential effects of EGCG on the activation of
ERK1/2 and p38, which closely associated with carcinoma cell
proliferation and migration, were analyzed. As shown in Fig. 3, EGCG time-dependently (P<0.05)
increased the phosphorylation of p38 in comparison with the time 0.
However, a significant change in the phosphorylation of ERK1/2 was
not observed following treatment with 100 μM of EGCG.
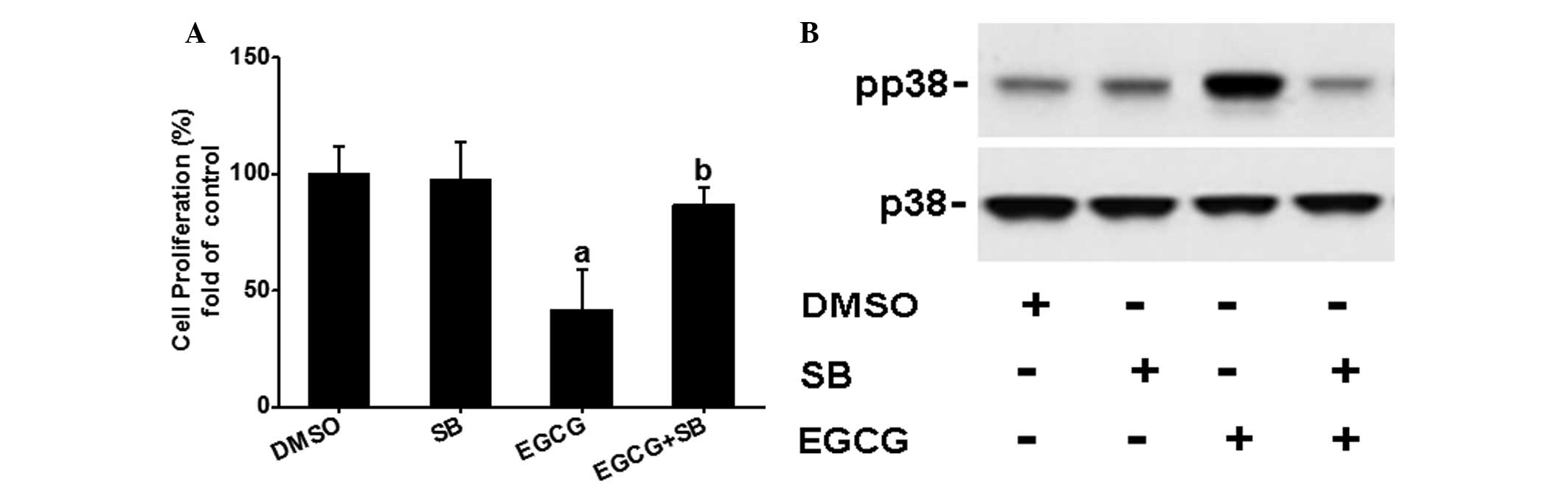
SB203580 partially blocks EGCG-inhibited
OVCAR-3 cell proliferation
To further determine whether the p38 signaling
pathway is involved in the EGCG-inhibited OVCAR-3 cell
proliferation, the effects of SB203580 on EGCG regulated cell
proliferation were examined. As shown in Fig. 4A and B, SB203580 completely
diminished EGCG-induced p38 activation, but partially blocked EGCG
inhibited (P<0.05) OVCAR-3 cell proliferation, suggesting that
the p38 signaling pathway may be partially involved in the
EGCG-mediated inhibitory effects.
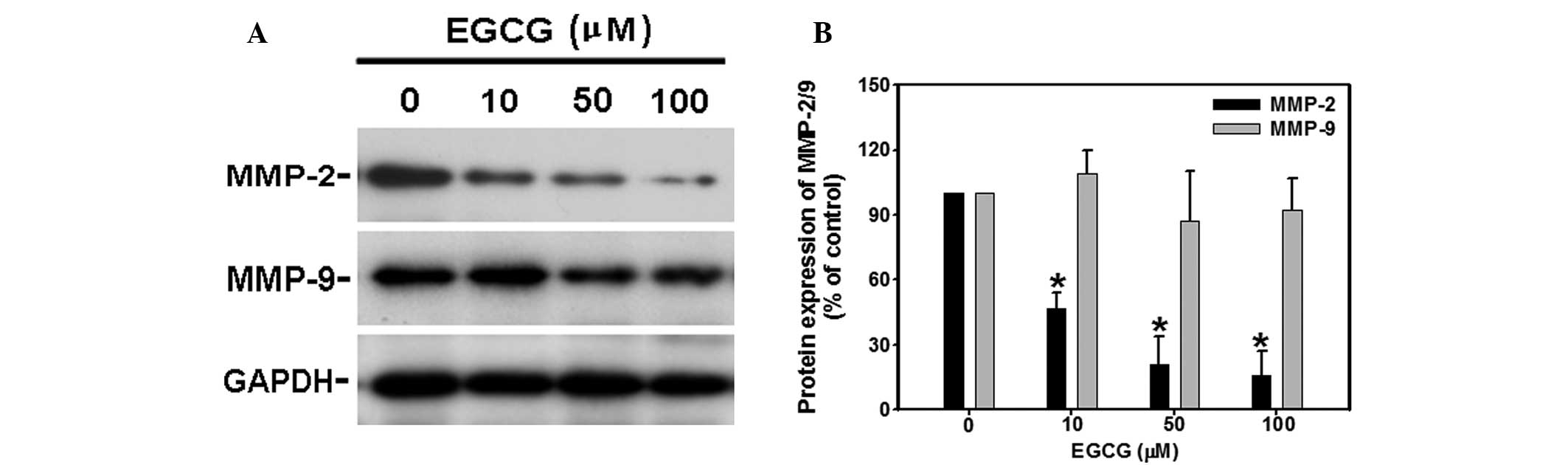
EGCG dose-dependently inhibits the
protein expression of MMP2, but not MMP9
Given MMP involvement in the cancer migration
process, the effects of EGCG on MMP expression were further
investigated by western blotting. As shown in Fig. 5A, following 48 h treatment, EGCG
significantly (P<0.05) decreased protein expression of MMP-2 at
10, 50 and 100 μM, respectively. However, EGCG did not alter MMP9
and GAPDH protein levels at any time point of the EGCG treatments
observed (Fig. 5B).
Discussion
EGCG is one of the most widely characterized
polyphenols in green tea and has been extensively investigated for
its chemopreventive effects on tumor formation and development in
several types of cancer (4–7). In
the present study, significant evidence is provided that EGCG is
capable of inhibiting proliferation and migration of human ovarian
carcinoma cells, partially through the regulation of the activation
of p38 kinase and reduction of MMP2 expression, suggesting that
EGCG may possess anticancer potential in human ovarian cancer.
An increasing number of studies have indicated that
EGCG is capable of inhibiting the growth of various types of cancer
cells, including lung (4), colon
(6) and gastric (7) cancer cells in culture. This
inhibitory effect was also demonstrated in the ovarian cancer cells
in the current study. The current data showed that EGCG
significantly inhibited ovarian cancer cell growth in a dose- and
time-dependent manner. However, little is known of the signaling
pathway involved in this inhibitory effect on ovarian cancer
growth.
Depending on the cell type, various signaling
kinases have been shown to be involved in cell proliferation during
tumor development. It is well established that the MAPK family are
actively involved in the regulation of cellular functions,
particularly of cell proliferation (20,21).
A previous study by Shankar et al (5) showed that EGCG significantly reduced
ERK activity and enhanced p38 and JNK activities in a human
pancreatic tumor xenograft model. In accordance with this report,
the current study showed that EGCG significantly activated p38 in a
time-dependent manner. SB203580, the specific inhibitor of p38,
completely inhibited the EGCG-induced increased phosphorylation of
p38. However, significant changes in the activity of ERK1/2 in
response to EGCG were not observed in the present data, which may
be attributable to the different cell types.
Cancer metastasis is a highly coordinated multistep
process involving cell invasion, cell-cell and cell-matrix adhesion
and remodeling of the extracellular matrix (22,23).
The current study indicated that EGCG dose-dependently inhibits
OVCAR-3 cell migration. However, the molecular mechanisms
underlying this effect remain unknown. It is well observed that a
number of proteinases are involved in the degradation of the ECM by
cancer cells, including MMPs, serine proteinase, and in particular,
members of the uPA-plasmin system (24). The secretion of MMPs is crucial in
cancer cell metastasis and is involved in cancer cell migration and
adhesion. Among human MMPs, MMP-2/9 are abundantly expressed in
various malignant tumors, including ovarian cancer (25–27).
The present data demonstrated that EGCG significantly decreased the
expression of MMP-2, but not MMP-9, which is also supported by
previous studies where EGCG reduced MMP-2 expression in human lung
and breast cancer cells (28,29).
In conclusion, this preliminary investigation has
shown that the anticancer effect of EGCG in an ovarian cancer cell
model may be mediated via the activation of p38 MAPK signaling
pathway as well as the decreased expression of MMP-2. These
findings reveal EGCG as a potential novel therapeutic anticancer
therapy.
Acknowledgements
This study was supported by a grant from the Youth
Innovation Foundation of the First Affiliated Hospital of Zhengzhou
University.
References
|
1
|
Katiyar SK and Elmets CA: Green tea
polyphenolic antioxidants and skin photoprotection (Review). Int J
Oncol. 18:1307–1313. 2001.PubMed/NCBI
|
|
2
|
Singh BN, Shankar S and Srivastava RK:
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms,
perspectives and clinical applications. Biochem Pharmacol.
82:1807–1821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mukhtar H and Ahmad N: Green tea in
chemoprevention of cancer. Toxicol Sci. 52(2 Suppl): S111–S117.
1999. View Article : Google Scholar
|
|
4
|
Hsu YC and Liou YM: The anti-cancer
effects of (-)-epigallocatechin-3-gallate on the signaling pathways
associated with membrane receptors in MCF-7 cells. J Cell Physiol.
226:2721–2730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shankar S, Ganapathy S, Hingorani SR and
Srivastava RK: EGCG inhibits growth, invasion, angiogenesis and
metastasis of pancreatic cancer. Front Biosci. 13:440–452. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sukhthankar M, Alberti S and Baek SJ:
(-)-Epigallocatechin-3-gallate (EGCG) post-transcriptionally and
post-translationally suppresses the cell proliferative protein
TROP2 in human colorectal cancer cells. Anticancer Res.
30:2497–2503. 2010.PubMed/NCBI
|
|
7
|
Onoda C, Kuribayashi K, Nirasawa S, Tsuji
N, Tanaka M, Kobayashi D and Watanabe N:
(-)-Epigallocatechin-3-gallate induces apoptosis in gastric cancer
cell lines by down-regulating survivin expression. Int J Oncol.
38:1403–1408. 2011.PubMed/NCBI
|
|
8
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar
|
|
9
|
Urban N and Drescher C: Potential and
limitations in early diagnosis of ovarian cancer. Adv Exp Med Biol.
622:3–14. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mizutani K, Ito H, Iwamoto I, et al:
Essential roles of ERK-mediated phosphorylation of vinexin in cell
spreading, migration and anchorage-independent growth. Oncogene.
26:7122–7131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ray RM, Vaidya RJ and Johnson LR: MEK/ERK
regulates adherens junctions and migration through Rac1. Cell Motil
Cytoskeleton. 64:143–156. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang YH, Yang IJ and Shin HM: Herbal
formula HMC05 prevents human aortic smooth muscle cell migration
and proliferation by inhibiting the ERK1/2 MAPK signaling cascade.
J Nat Med. 66:177–184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gayer CP, Craig DH, Flanigan TL, Reed TD,
Cress DE and Basson MD: ERK regulates strain-induced migration and
proliferation from different subcellular locations. J Cell Biochem.
109:711–725. 2010.PubMed/NCBI
|
|
15
|
Tangkijvanich P, Santiskulvong C, Melton
AC, Rozengurt E and Yee HF Jr: p38 MAP kinase mediates
platelet-derived growth factor-stimulated migration of hepatic
myofibroblasts. J Cell Physiol. 191:351–361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li T, Feng Z, Jia S, Wang W, Du Z, Chen N
and Chen Z: Daintain/AIF-1 promotes breast cancer cell migration by
up-regulated TNF-α via activate p38 MAPK signaling pathway. Breast
Cancer Res Treat. 131:891–898. 2012.PubMed/NCBI
|
|
17
|
Xue A, Xue M, Jackson C and Smith RC:
Suppression of urokinase plasminogen activator receptor inhibits
proliferation and migration of pancreatic adenocarcinoma cells via
regulation of ERK/p38 signaling. Int J Biochem Cell Biol.
41:1731–1738. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Choi BD, Jeong SJ, Wang G, Park JJ, Lim
DS, Kim BH, Cho YI, Kim CS and Jeong MJ: Secretory leukocyte
protease inhibitor is associated with MMP-2 and MMP-9 to promote
migration and invasion in SNU638 gastric cancer cells. Int J Mol
Med. 28:527–534. 2011.PubMed/NCBI
|
|
19
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: an overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hilger RA, Scheulen ME and Strumberg D:
The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie.
25:511–518. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geest CR and Coffer PJ: MAPK signaling
pathways in the regulation of hematopoiesis. J Leukoc Biol.
86:237–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brooks SA, Lomax-Browne HJ, Carter TM, et
al: Molecular interactions in cancer cell metastasis. Acta
Histochem. 112:3–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hwang ES and Park KK: Magnolol suppresses
metastasis via inhibition of invasion, migration, and matrix
metalloproteinase-2/-9 activities in PC-3 human prostate carcinoma
cells. Biosci Biotechnol Biochem. 74:961–967. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun LC, Luo J, Mackey LV, Fuselier JA and
Coy DH: A conjugate of camptothecin and a somatostatin analog
against prostate cancer cell invasion via a possible signaling
pathway involving PI3K/Akt, alphaVbeta3/alphaVbeta5 and MMP-2/-9.
Cancer Lett. 246:157–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmalfeldt B, Prechtel D, Härting K,
Späthe K, Rutke S, Konik E, Fridman R, Berger U, Schmitt M, Kuhn W
and Lengyel E: Increased expression of matrix metalloproteinases
(MMP)-2, MMP-9, and the urokinase-type plasminogen activator is
associated with progression from benign to advanced ovarian cancer.
Clin Cancer Res. 7:2396–2404. 2001.PubMed/NCBI
|
|
28
|
Deng YT and Lin JK: EGCG inhibits the
invasion of highly invasive CL1-5 lung cancer cells through
suppressing MMP-2 expression via JNK signaling and induces G2/M
arrest. J Agric Food Chem. 59:13318–13327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sen T, Moulik S, Dutta A, Choudhury PR,
Banerji A, Das S, Roy M and Chatterjee A: Multifunctional effect of
epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A
(MMP-2) in human breast cancer cell line MCF-7. Life Sci.
84:194–204. 2009. View Article : Google Scholar : PubMed/NCBI
|