Introduction
Fractured bones require a long cycle of healing
which results in patient suffering, prolonged hospitalization and
increasing economic costs for the individual and society. Bone
healing may be delayed or obstructed by various numerous factors,
including poor blood, loss of bone and soft tissue, metabolic
disease, alcohol drinking, osteoporosis and high intraosseous
pressure (1,2). Therefore, in the clinic, the
identification of methods to shorten the healing time and improve
the healing rate of bone defects and osteonecrosis is important for
bone healing.
Vacuum-associated closure (VAC) was first reported
by Raffl (3) in 1952, when is was
successfully applied to prevent infection and fluidity following
flap transplantation. In the clinic, VAC has been used as an
effective method to heal the soft tissue. However, its effects on
bone tissue have never been investigated. Under specific
circumstances, VAC may also promote rapid secondary wound healing
by improving the blood supply (4).
In soft tissue, VAC stretches cells, increases cell or tissue
proliferation, promotes blood flow and reduces bacteria count,
which helps to prevent cross infection, and the microenvironment of
hypoxia and subacidity caused by VAC promotes angiogenesis and the
influx of fibroblasts (5). The
aforementioned backgrounds and function of VAC in bone tissue are
investigated in the present study.
In a previous study, treatment of a compound
fracture and the accompanying serious soft tissue injury by VAC was
found to promote fracture union. However, the mechanism of VAC
effects must be explored. In addition, the effect of negative
pressure on bone healing must be investigated. Mesenchymal stem
cells (MSCs) are multipotent stem cells that differentiate into a
variety of cell types, including osteoblasts. The availability of
MSCs is widespread and the cells are easy to isolate and culture.
In modern bone repair clinics, MSCs have been employed as ideal
seeding cells for substitution therapy.
The aim of the present study was to investigate the
effects of negative pressure on the proliferation and
differentiation of MSCs, and the specific mechanisms involved in
these processes. Through this investigation, our understanding of
the precise mechanisms of MSCs in bone regeneration therapy may be
improved.
Materials and methods
MSC isolation and culture
Bone marrow tissue was collected from two patients
diagnosed with hip osteoarthritis in the Department of Orthopaedics
of The First Affiliated Hospital, Medical School of Xi’an Jiaotong
University (Xi’an, China). A 5-ml tissue sample was harvested by
aspiration from the femoral bone marrow cavity using heparin
syringes, following osteotomy, during hip replacement surgery. The
two patients had normal liver and kidney function and no metabolism
osteodystrophy or infectious diseases. Consent was obtained from
the patients prior to the sample selection in the study.
The collected bone marrow was put into centrifuge
tubes, along with 2 ml L-DMEM medium (Gibco-BRL, Carlsbad, CA, USA)
and centrifuged at 1,200 × g for 5 min to remove the upper and
medium fat droplets. The samples were then suspended in D-Hank’s
liquid, followed by separation on a Percoll gradient (Sigma, St.
Louis, MO, USA) via centrifugation at 1,200 × g for 10 min, then
the middle layer of white film (mononuclear cells) was drawn and
washed with D-Hank’s liquid. The cells were then placed in L-DMEM
medium in sterile culture bottles and cultured in an incubator at
37°C and 5% carbon dioxide. The third generation cells were
digested with trypsin and 2×105 cells were incubated
with antibodies against CD29, CD34, CD44, CD45 or HLA-DR, at room
temperature for 30 min and washed twice with PBS. Next, cells were
incubated with a secondary antibody labeled with FITC (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) for 15 min in the dark,
followed by suspension in PBS following washing. Cells were
examined via flow cytometry.
2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide
(XTT) analysis
MSCs were digested and harvested using 0.25% trypsin
(Wuhan Boshide Biotechnology, Wuhan, China) and 0.02% EDTA and were
plated in 96-well plates at a density of 1×104/ml. At 1,
3 and 5 days, 5 ml XTT (5 mg/ml in PBS) was added to each well and
the cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2. After 4-h incubation with XTT, the
cells were lysed using a solution of 20% SDS and 50% DMSO at pH 4.7
and the absorbance was measured at 570 nm with an EL-311SX
enzyme-linked immunosorbent assay reader (Bio-Tek Instruments,
Winooski, VT, USA)
Detection of apoptosis
Cell apoptosis was detected by flow cytometry
analysis that monitored annexin V-FITC binding and propidium iodide
uptake simultaneously, according to the manufacturer’s instructions
(Sigma). The samples were analyzed by fluorescence on a FACSan flow
cytometer (Beckman Coulter, Miami, FL, USA). The potential DNA
fragmentation was examined by the TUNEL apoptosis detection kit
(Chemicon, Temecula, CA, USA) following the manufacturer’s
instructions. Apoptotic bodies were stained by brilliant blue.
Quantitative PCR (qPCR)
PCR was performed according to methods described
previously (3) using the following
primer sequences: Osteoprotegrin (OPG) forward,
5′-AATCAACTCAAAAATGTGGAATAGATGT-3′ and reverse,
5′-GCGTAAACTTTGTAGGAACAGCAA-3′; Osteoprotegrin ligand (OPGL)
forward, 5′-GCGTAAACT TTGTAGGAACAGCAA-3′ and reverse, 5′-AACCAT
GAGCCATCCACCAT-3′; osteocalcin forward, 5′-GGCAGTAAGGTGGTGAATAG-3′
and reverse, 5′-TCCTGGAAGCCAATGTG-3′; and β-actin forward,
5′-AGGCACCAGGGCGTGAT-3′ and reverse,
5′-TCGTCCCAGTTGGTGACGAT-3′.
Alkaline phosphatase (ALP) activity
Following plating at a density of 5×103
cells/cm2 in 6-well plates, cells were treated for 2
weeks, followed by solubilization of cellular proteins with 1%
Triton-X in 0.9% NaCl and centrifugation at 12,000 × g for 10 min.
The supernatants were assayed for ALP activity (Pointe Scientific,
Detroit, MI, USA). One unit was defined as the activity that
produces 1 nmol p-nitrophenol in 30 min. The protein
concentrations were determined with a Bio-Rad protein assay kit
(Bio-Rad, Hercules, CA, USA) and ALP activity was normalized to the
cellular protein contents.
Type I collagen and vascular endothelial
growth factor (VEGF) detection
When the MSCs were treated under VAC conditions for
2 weeks, cells in slices were fixed for 20 min in 4%
paraformaldehyde fluid. The primary antibodies were mouse
anti-human type I collagen and VEGF monoclonal antibodies (Wuhan
Bioshide Biotechnology). The cells were washed with PBS, blocked
with goat serum and incubated with the primary antibody at a
dilution of 1:100 at 4°C overnight. Following washing with PBS, the
cells were incubated with the secondary antibody (Santa Cruz
Biotechnology, Inc.) at 37°C for 30 min. Next, the ABC regents were
incubated at room temperature for 20 min, washed with PBS and
stained using DAB.
Western blot analysis of endoplasmic
reticulum (ER) stress
In order to investigate the mechanism of MSC
apoptosis, several ER stress-associated factors were detected using
this assay, including p-PRKR-like ER kinase (PERK),
inositol-requiring enzyme 1 (IRE-1) and activating transcription
factor 6 (ATF6). These proteins are the main factors of the
unfolded protein response (UPR) pathway. The downstream factors,
which may trigger cell apoptosis, were also detected. A western
blot analysis method was employed to analyze the ER stress
factors.
The MSC lysates were separated by 15% SDS-PAGE and
electrotransferred onto nitrocellulose membranes. Following
blocking with 5% skimmed milk in phosphate buffered saline Tween-20
overnight at 4°C, the membranes were incubated with goat pAb
anti-human CHOP (1:2,000), mouse mAb anti-human p-Perk (1:3,000),
mouse mAb anti-human IRE1 (1:2,000), mAb anti-human β-actin (1:600)
(all Santa Cruz Biotechnology, Inc.), pAb anti-human caspase 3
(1:1,000) (Santa Cruz Biotechnology, Inc.), mAb anti-full length
(1:1,000) and spliced XBP1 (Stressgen, Farmingdale, NY, USA), mAb
anti-full length (1:2,000) and cleaved ATF6, anti-eIF2-α (1:2,000)
(both Santa Cruz Biotechnology, Inc.) for 2 h at room temperature.
Next, membranes were incubated with horseradish
peroxidase-conjugated anti-mouse (1:5,000), anti-rabbit or
anti-goat IgG (1:2,000) (all Santa Cruz Biotechnology, Inc.). The
reactive signals were visualized by an ECL kit (PE Applied
Biosystems, Foster City, CA, USA).
Statistical analysis
Quantitative analysis of immunoblot images was
performed using computer-assisted software Image Total Tech
(Pharmacia, New York, NY, USA). Briefly, the image of the
immunoblot was scanned with Typhoon (Pharmacia), digitalized and
saved as TIF format. The values of each target blot were evaluated.
Data are presented as the mean ± standard deviation. A statistical
analysis was performed using a t-test. P<0.05 was used to
indicate a statistically significant difference.
Results
Proliferation and apoptosis of negative
pressure-treated MSCs
Using the flow cytometry analysis, cells were
observed to be homogenous and, as is appropriate for MSCs,
expressed CD29, CD34, CD44, CD45 and HLA-DR (data not shown). The
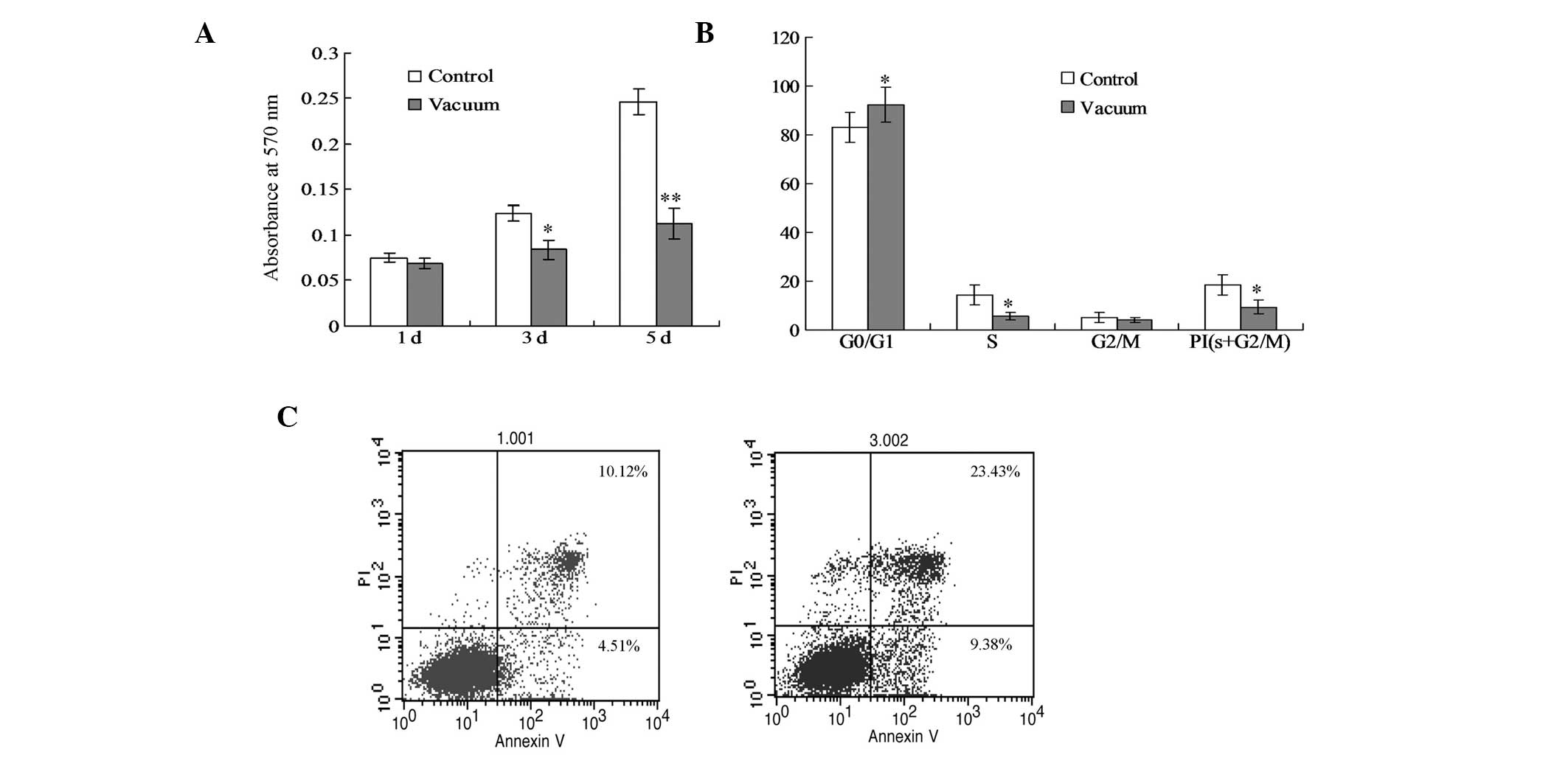
XTT analysis results indicated that, in the experimental group, the
proliferation of MSCs was reduced significantly compared with the
control group (Fig. 1A;
P<0.05). Flow cytometry detection revealed that the apoptosis
rate of the negative pressure-treated group (vacuum) was
significantly higher compared with that of the control group
(Fig. 1B). Furthermore, the
percentage of cells in the S phase of the cellular cycle in the
negative pressure-treated group was decreased significantly
compared with the control group (Fig.
1C).
MSCs differentiation
When the MSCs were treated with negative pressure
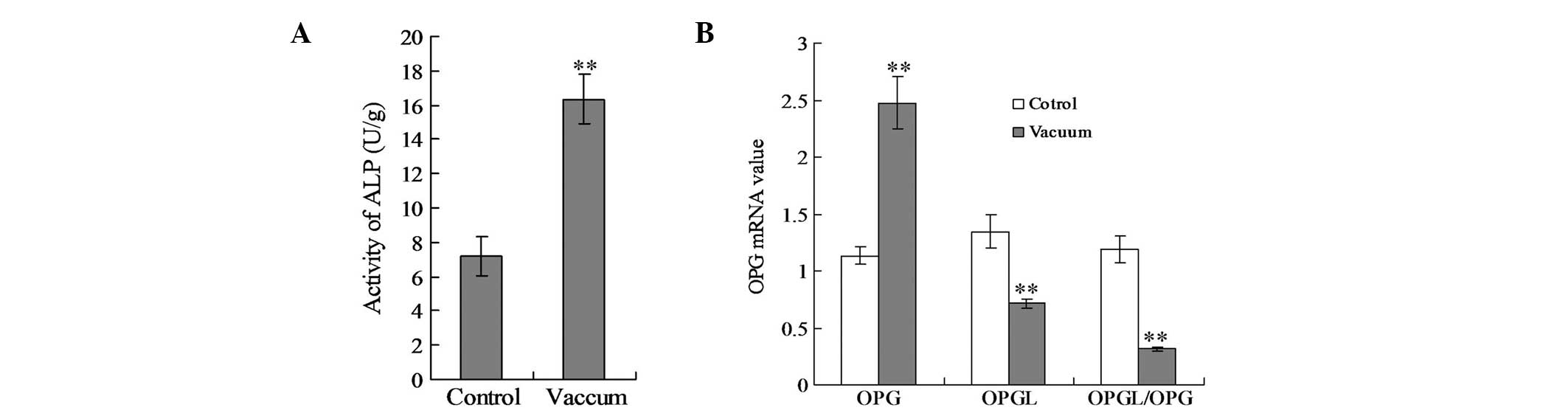
for 2 weeks, the activity of ALP in the vacuum group (16.34±1.47
U/g) was significantly higher compared with that of the control
group (7.19±1.18 U/g) (Fig. 2A;
P<0.01).
The qPCR results illustrated that the OPG mRNA
transcription of the control group was significantly increased
compared with the vacuum group (Fig.
2B; P<0.01). By contrast, OPGL mRNA transcription was
significantly decreased (P<0.01). The ratio of OPGL to OPG mRNA
transcription reduced significantly in the vacuum group (Fig. 2B; P<0.01).
Immunohistochemical staining of type I
collagen and VEGF
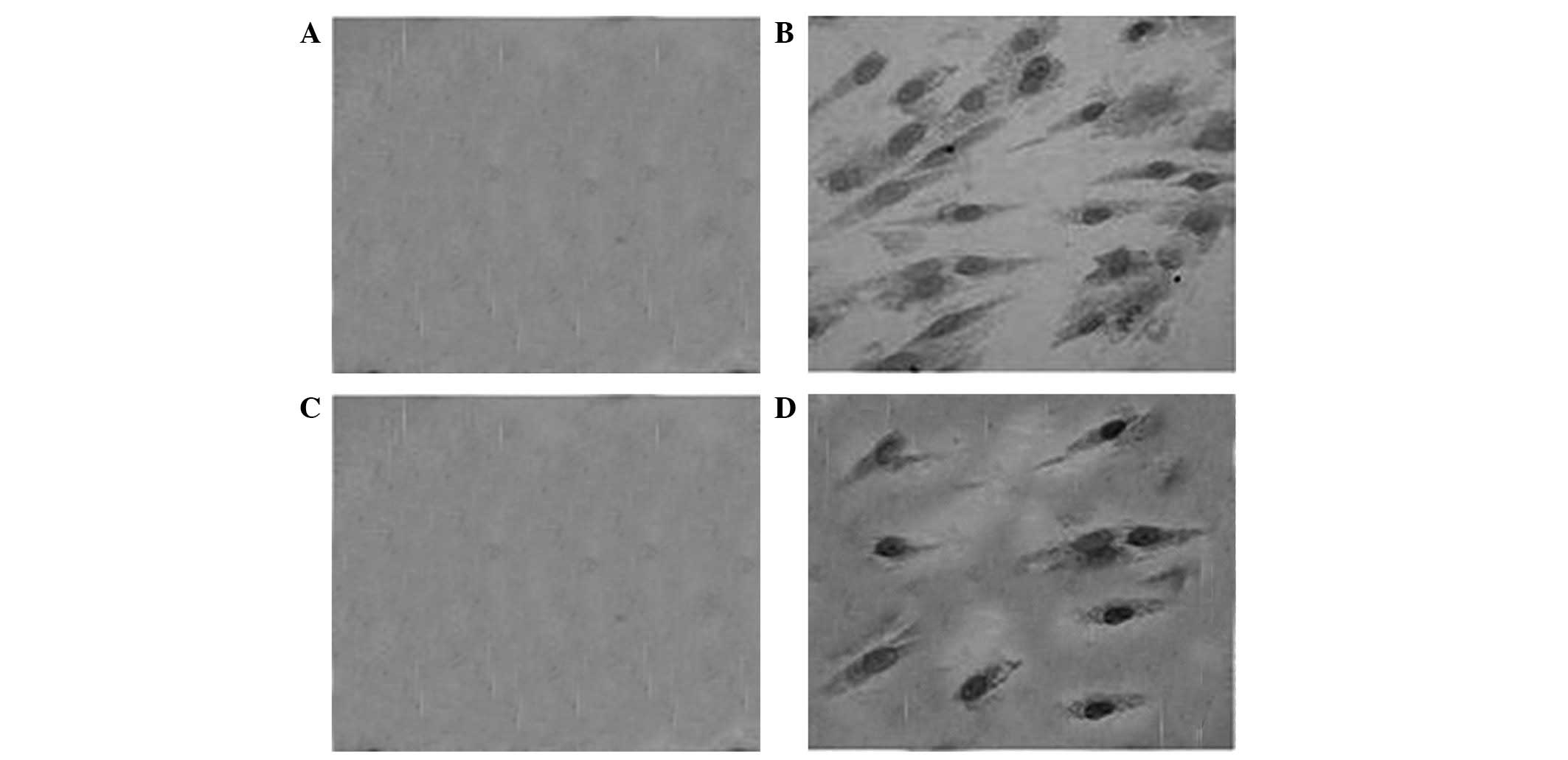
The immunohistochemical staining results indicated
that type I collagen was not expressed in the control group
(Fig. 3A), but was positively
expressed in the vacuum group as a large number of yellow brown
particles in the cellular hyaloplasm in those cells (Fig. 3B). In addition, the expression of
VEGF in the vacuum group was stronger compared with the control
group (Fig. 3A and B).
UPR pathway is involved in the inhibition
of MSC proliferation
To explore the mechanisms of inhibition of
proliferation in the negative pressure-treated MSCs, the main UPR
factors, p-Perk, IRE-1 and ATF6, were detected by western blot
analysis. As shown in Fig. 4A, the
treatment of negative pressure triggered the phosphorylation of
Perk (p-Perk), the cleavage of ATF6 protein and the activation of
IRE-1. The protein expression of p-Perk, cleaved ATF6 and IRE-1 was
significantly enhanced by the negative pressure treatment compared
with the control group (Fig. 4A;
P<0.05).
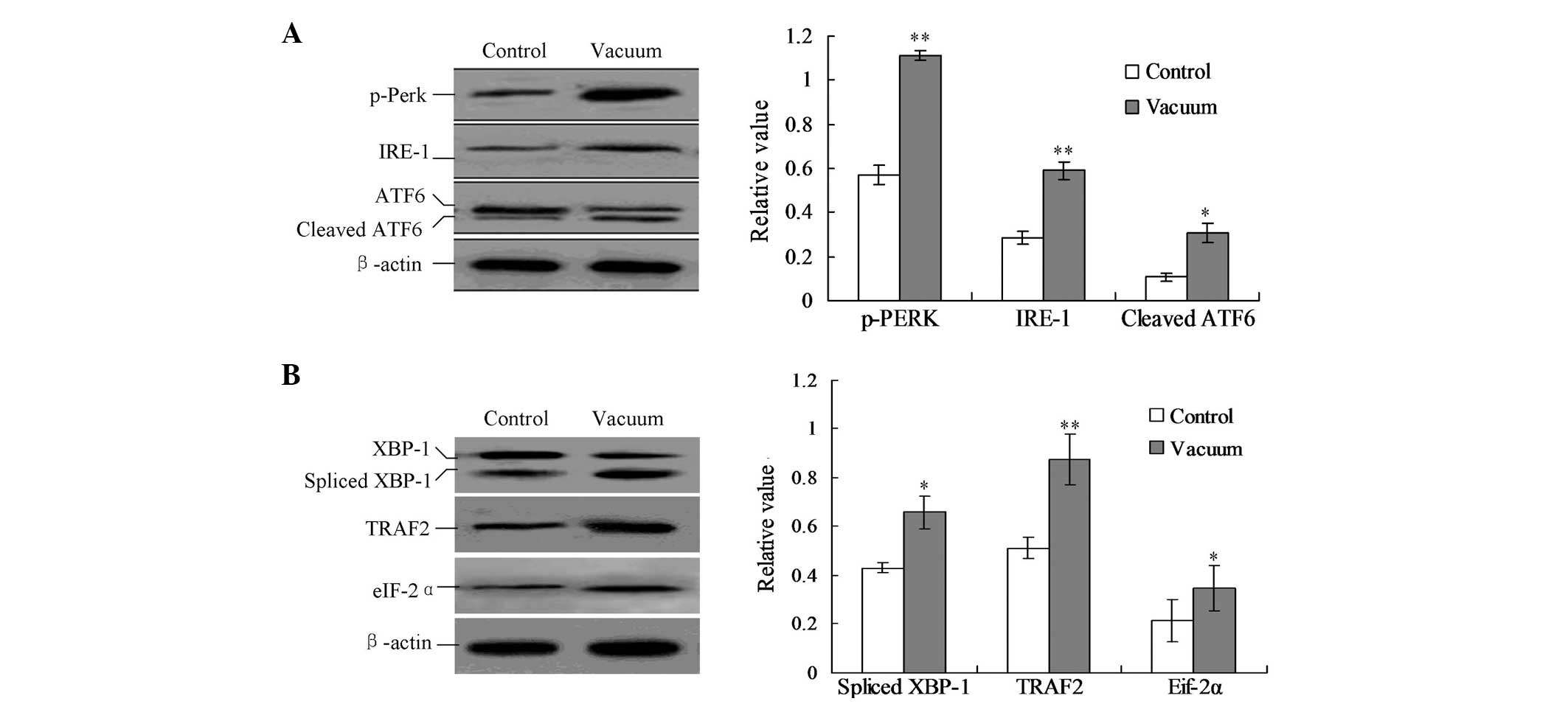 | Figure 4Detection of the UPR pathway proteins.
(A) Detection of IRE1, p-Perk and cleaved ATF6. (B) Detection of
the downstream TRAF2 protein, eIF-2α protein and spliced XBP1
protein. Protein expression was calculated by the gray numerical
value of each specific product versus that of β-actin. The average
data of each preparation are evaluated based on three independent
reactions and represented as the mean ± standard deviation.
*P<0.05 and **P<0.01, vs. control
group. UPR, unfolded protein response; ATF6, activating
transcription factor 6; TRAF2, TNF receptor-associated factor 2;
eIF-2α, eukaryotic translation initiation factor 2α; XBP1, X-box
binding protein 1; p-PERK, p-PRKR-like ER kinase; IRE-1,
inositol-requiring enzyme 1. |
In addition, the downstream ER stress-associated
proteins of the UPR pathway were detected, including the spliced
X-box binding protein 1 (XBP1), TNF receptor-associated factor 2
(TRAF2) and eukaryotic initiation translation factor 2α (eIF-2α)
proteins. In the negative pressure treatment group, the levels of
spliced XBP1, TRAF2 and eIF-2α proteins were significantly
increased compared with those of the control group (Fig. 4B; P<0.05).
Discussion
In the clinic, the unique features of the negative
pressure therapy method have been hypothesized to contribute to an
optimized wound environment, including edema reduction, stimulation
of angiogenesis and local blood flow, interstitial fluid flow and
exudate management. The method also affects wound perfusion, growth
factor, cytokine expression and cellular activity. These roles lead
to enhanced granulation tissue formation and improved wound healing
parameters (6–8). Therefore, the treatment should be
focused on inducing osteogenesis through the effects of mechanical
stimulation and growth factors in order to increase the early blood
supply and restart bone healing (9). However, its effects on bone tissues
and functions in bone repair are poorly understood. In the present
study, the effects of negative pressure on osteogenesis in human
MSCs and the specific mechanisms were investigated.
For our clinical practice, VAC cured soft tissue
defects when applied at a pressure of −50 kPa for 30 min at a
frequency of 2 times per day. In the compound injuries, bone
healing was also improved when treated with negative pressure. We
hypothesized that the negative pressure treatment may promote bone
healing through mechanical stimulation, by increasing blood supply,
recruitment of osteogenitor cells and inhibiting the proliferation
of the osteogenitor cells (MSCs). MSCs are usually used as the
seeding cells for gene and cell therapy of skeletal diseases, owing
to their potential for differentiation into osteogenitor cells
(10,11). The oxygen concentration in
vitro culture is ~20%, whereas in vivo oxygen
concentration may decrease to only 0.4%–4.7% (12). Therefore, in the present study, the
intermittent vacuum incubator (with 2% oxygen tension) was selected
to incubate the MSCs with only a few cell cultures, including DMEM.
Previous studies have indicated that the disorders correlated to
bone repair often appear in older patients or those in which the
ability of cells to proliferate has decreased (13,14).
Therefore, the target cells (MSCs) from the older patients were
selected, and the effects of apoptosis on the proliferation of MSCs
were investigated.
The present study also illustrated that the
proliferation of MSCs was inhibited under the low-intensity and
intermittent negative pressure. This inhibition may be attributed
to the temporal hypoxia caused by negative pressure and the
apoptosis caused by specific stress stimuli for the MSCs. A number
of factors may affect the differentiation and proliferation of the
MSCs, including mechanical stimulation and apoptotic responses. In
the present study, the MSCs in the vacuum group exhibited
significantly higher levels of apoptosis compared with the control
group (P<0.05). The apoptosis inhibited the proliferation of the
MSCs and triggered differentiation into osteoblasts in a subsequent
step (15,16). Our previous study also indicated
that negative pressure may lead to upregulation of HIF-1, which
contributes to the maintenance of oxygen homeostasis in
physiological or pathological conditions. HIF-1 plays a significant
role in the regulation of acute and intensive hypoxia (17,18).
Towler (19) demonstrated that
HIF-1 signaling in the development and differentiation of
osteoblasts was central to the coupling of angiogenesis and long
bone development in mice, and thus strategies promoting HIF-1
signaling in osteoblasts are likely to augment bone formation and
accelerate fracture repair. Therefore, the treatment of negative
pressure was hypothesized to be capable of triggering
differentiation into osteoblasts.
In order to investigate the effects of the negative
pressure on the differentiation of MSCs, ALP activity, type I
collagen and VEGF expression were also detected. The results
demonstrate that the three factors were enhanced significantly
compared with the untreated control group, and promoted and induced
differentiation into osteoblasts. This effect of negative pressure
may be attributed to mechanical stimulation and cellular hypoxia.
Mechanical stimulation plays a basic role in the cell development.
Various mechanical stimuli have been revealed to act through
numerous signaling pathways that result in changes in gene
expression and proliferation of osteoblasts. Previous studies
(20,21) have indicated that mechanical
stimulation promotes the osteogenic differentiation of human bone
marrow stromal cells. Wiesmann et al (22) also reported that the collagen type
I and osteonectin expression levels were significantly enhanced by
the mechanical stimulation. Compared with other studies, the
shorter 30-min intermittent negative pressure treatment time at a
frequency of two times per day was hypothesized to increase the
expression of type I collagen and VEGF, which are mediated by HIF-1
and promote bone formation.
The present study demonstrated that intermittent
negative pressure decreased OPG and OPGL mRNA expression in MSCs.
OPG plays a key role in the physiological regulation of
osteoclastic bone resorption and acts by binding to OPGL. Negative
pressure decreased the ratio of OPG to OPGL in vitro and may
therefore correlate with osteogenesis and osteoclastogenesis, which
may associate with temporal hypoxia. A pronounced decrease in
OPG/OPGL caused by mechanical stimuli results in an increase in
bone formation and an inhibition of bone absorption (23,24).
The results of the present study revealed a concomitant change of
OPG and OPGL mRNA expression in response to a variety of mechanical
stimuli. However, these observations are inconsistent with a study
by Rubin et al which demonstrated that the change was
attributed to decreased mRNA expression of OPGL only.
To investigate the specific mechanism of cell
proliferation of negative pressure-treated MSCs, ER
stress-associated proteins (UPR pathway) (25,26),
including p-Perk, IRE1 and cleaved ATF6 levels, were detected.
Three proteins were demonstrated to be activated when treated with
negative pressure. Therefore, the UPR pathway may be involved in ER
stress-associated apoptosis. Furthermore, the downstream factors,
including spliced XBP1, TRAF2 and eIF-2α proteins were also
detected. The activation of Perk may phosphorylate eIF-2α proteins
and the results markedly indicated the emergence of an ER stress
(or UPR pathway) following treatment with negative pressure in the
MSCs. The observation that treatment of negative pressure triggered
ER stress of MSCs is of great importance. Therefore we hypothesize
that, when treated with negative pressure, the proliferation of the
MSCs may be blocked by the ER stress-triggered apoptosis and induce
differentiation of MSCs.
In conclusion, treatment with low-intensity and
intermittent negative pressure may inhibit proliferation of MSCs
and trigger cellular apoptosis, further enhancing osteogenesis
activity and inducing differentiation of osteoblasts. The negative
pressure may improve bone formation and differentiation by
decreasing the ratio of OPGL/OPG mRNA, inducing type I collagen and
VEGF expression and ER stress-trigged apoptosis. Therefore, this
method may be promising for the treatment of bone regeneration
disorders.
References
|
1
|
Kerachian MA, Harvey EJ, Cournoyer D, Chow
TY and Séquin C: Avascular necrosis of the femoral head: vascular
hypotheses. Endothelium. 13:237–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rodriguez-Merchan C and Forriol F:
Nonunion: general principles and experimental data. Clin Orthop
Relat Res. 419:4–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raffl AB: The use of negative pressure
under skin flaps after redical mastectomy. Ann Surg. 136:10481952.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deva AK, Buckland GH, Fisher E, et al:
Topical negative pressure in wound management. Med J Aust.
173:128–131. 2000.
|
|
5
|
Banwell PE and Musgrave M: Topical
negative pressure therapy: mechanisms and indications. Int Wound J.
1:95–106. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrie D, Potter M and Banwell P: The
management of lower extremity wounds using topical negative
pressure. Int J Low Extrem Wounds. 2:198–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JW, Lee MS, Lee CH, Kim HY, Chae SU,
Kwak HB and Oh J: Effect of interferon-γ on the fusion of
mononuclear osteoclasts into bone-resorbing osteoclasts. BMB Rep.
45:281–286. 2012.
|
|
8
|
Potter MJ, Banwell P, Baldwin C, Clayton
E, Irvine L, Linge C, et al: In vitro optimization of topical
negative pressure regimens for antiogenesis into synthetic dermal
replacements. Burns. 34:164–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brownlow HC, Reed A and Simpson AH: The
vascularity of atrophic non-unions. Injury. 33:145–150. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li RD, Deng ZL, Hu N, Liang X, Liu B, Luo
J, et al: Biphasic effects of TGFβ1 on BMP9-induced osteogenic
differention of mesenchymal stem cells. BMB Rep. 45:509–514.
2012.
|
|
11
|
Cao L, Liu G, Gan Y, Fan Q, Yang F, Zhang
X, et al: The use of autologous enriched bone marrow MSCs to
enhance osteoporotic bone defect repair in long-term estrogen
deficient goats. Biomaterials. 33:5076–5084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsai CC, Yew TL, Yang DC, Huang WH and
Hung SC: Benefits of hypoxic culture on bone marrow multipotent
stromal cell. Am J Blood Res. 2:148–159. 2012.PubMed/NCBI
|
|
13
|
Chen CH, Wang SS, Wei EI, Chu TY and Hsieh
PC: Hyaluronan enhances bone marrow cell therapy for myocardial
repair after infarction. Mol Ther. 21:670–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu JY, Wang YH, Wang GJ, Ho ML, Wang CZ,
Yeh ML and Chen CH: Low-power GaAIAs laser irradiation promotes the
proliferation and osteogenic differentiation of stem cells via IGF1
and BMP2. PLoS One. 7:e440272012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Y, Ouyang Y, Chang Y, Luo C, Xu J,
Zhang C and Huang W: Evaluation of the proliferation and
differentiation behaviors of mesenchymal stem cells with partially
converted borate glass containing different amounts of strontium in
vitro. Mol Med Rep. 7:1129–1136. 2013.
|
|
16
|
Rampichová M, Chvojka J, Buzgo M, Prosecká
E, Mikeš P, Vysloužilová L, et al: Elastic three-dimensional poly
(ɛ-caprolactone) nanofibre scaffold enhances migration,
proliferation and osteogenic differentiation of mesenchymal stem
cells. Cell Prolif. 46:23–37. 2013.
|
|
17
|
Wu C, Rankin EB and Giaccia AJ: Blood and
bones: osteoblastic HIF signaling regulates erythropoiesis. Cell
Cycle. 11:2221–2222. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou H, Chen ZB, Tian HQ, Xu X, Wang YQ,
Xing GQ and Cheng L: Effects of hypoxia-inducible factor 1α on bone
conduction impairment in otitis media with effusion. Acta
Otolaryngol. 132:938–943. 2012.
|
|
19
|
Towler DA: Vascular biology and bone
formation: hints from HIF. J Clin Invest. 117:1477–1480. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jagodzinski M, Drescher M, Zeichen J, et
al: Effects of cyclic longitudinal mechanical strain and
dexamethasone on osteogenic differentiation of human bone marrow
stromal cells. Eur Cell Mater. 7:35–41. 2004.
|
|
21
|
Zhang P, Wu Y, Dai Q, Fang B and Jiang L:
p38-MAPK signaling pathway is not involved in osteogenic
differentiation during early response of mesenchymal stem cells to
continuous mechanical strain. Mol Cell Biochem. 378:19–28. 2013.
View Article : Google Scholar
|
|
22
|
Wiesmann A, Bühring HJ, Mentrup C and
Wiesmann HP: Decreased CD90 expression in human mesenchymal stem
cells by applying mechanical stimulation. Head Face Med. 2:82006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YG, Yang Z, Zhang H, et al: Effect
of negative pressure on human bone marrow mesenchymal stem cells in
vitro. Connect Tissue Res. 51:14–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim CH, You L, Yellowley CE and Jacobs CR:
Oscillatory fluid flow-induced shear stress decreases
osteoclastogenesis through RANKL and OPG signaling. Bone.
39:1043–1047. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Joung KH and Cho SC: Stress responses of
neonates related to maternal characteristics. Yonsei Med J.
52:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Momoi T, Fujita E, Senoo H and Momoi M:
Genetic factors and epigenetic factors for autism: endoplasmic
reticulum stress and impaired synaptic function. Cell Biol Int.
34:13–19. 2009.PubMed/NCBI
|