Introduction
Breast cancer is one of most common types of
malignancy that occurs in females around the world. In the United
States, in 2013, ~232,340 females were diagnosed with breast cancer
and ~39,620 breast cancer-associated mortalities were estimated
(1). In addition, breast cancer is
the most common cause of cancer-associated mortality in females in
China. Accurate prognosis and effective treatments against breast
cancer require a more in depth understanding of the cellular and
molecular mechanisms involved in breast cancer development and
progression.
Caveolin 1, a 21–24 kDa membrane protein, is a major
structural component of caveolae, which are identifiable plasma
membrane invaginations. It has been suggested that caveolin 1
functions as a scaffold protein for signal transduction,
transformation, endocytosis, cholesterol homeostasis, the cell
cycle, cell migration and invasion (2–4).
Emerging evidence has demonstrated that caveolin 1
serves as a tumor suppressor protein and knockdown of caveolin 1
activates anchorage-independent growth of transformed cells
(5). However, caveolin 1 has also
been demonstrated to have a tumor promoting role in prostate
cancer, renal cancer and esophageal squamous cell carcinoma
(6–8), suggesting that whether caveolin 1
acts as tumor suppressor or facilitator depends upon specific tumor
types.
The potential function of caveolin 1 in the
development and progression of breast cancer remains unclear. In
human breast cancer MCF-7 cells, the overexpression of caveolin 1
is associated with the suppression of cell growth and inhibition of
migration and invasion (9).
However, there is evidence that caveolin 1 also acts as a tumor
promoter in breast cancer. It has been reported that the depletion
of caveolin 1 decreased migration, polarization and focal adhesion
in MDA-MB-231 cancer cells (basal-like phenotype) (10). This is consistent with another
study, which demonstrated that caveolin 1 is highly associated with
the breast cancer basal-like phenotype (11). In the present study, human breast
cancer BT474 cells were used to analyze the role of caveolin 1 in
BT474 cells. It was hypothesized that caveolin 1 may serve as a
tumor promoter in BT474 cells, leading to tumor growth, migration
and invasion.
Materials and methods
Cell lines and reagents
The human breast cancer BT474 cell line was
purchased from American Type Cell Culture (Manassas, VA, USA). The
cells were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS; HyClone Laboratories, South Logan, UT, USA) and
1% penicillin/streptomycin (Beyotime Biotech, Nanjing, China) in
the presence of 5% CO2 and at 37°C. Antibodies
[anti-caveolin 1, anti-p-extracellular signal-regulated kinase 1/2
(ERK1/2) and anti-ERK1/2] were purchased from Cell Signaling
Technology, Inc. (Beverly, MA, USA). Anti-matrix metalloproteinase
1 (MMP-1), anti-MMP-2 and E-cadherin were purchased from Epitomics,
Inc. (Burlingame, CA, USA). Anti-MMP-9, anti-cyclin D1 and
anti-GAPDH were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Anti-c-Fos and anti-β-catenin were purchased
from Abcam (Cambridge, MA, USA).
Small interfering RNA (siRNA)
The target siRNA against human caveolin 1
(si-h-Cav-1) was designed and constructed by Guangzhou Ribio
Biotech Co., Ltd (Guangzhou, China). The sequence was as follows:
si-h-Cav-1, forward 5′-GCA UCAACUUGCAGAAAGAdTdT-3′ and reverse
3′-dTdTCGUA GUUGAACGUCUUUCU-5′.
Prior to transfection the medium was replaced with
penicillin/streptomycin-free RPMI-1640 complete medium. BT474 cells
were then transfected with si-h-Cav-1 or negative control siRNA
(siCon) using Lipofectamine™ 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA).
Western blot analysis
The cells were seeded in six-well plates and were
allowed to grow to 60–80% confluence. The concentration of the
total protein was determined using the bicinchoninic acid protein
assay kit (Beyotime Biotech). SDS-PAGE (Beyotime Biotech) was used
to separate the total protein, prior to transfer onto PVDF
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% milk in 0.1% Tris-buffered saline with
Tween® 20 (BioSharp, Seoul, South Korea) (TBST) for 1 h
at 37°C, prior to being incubated with primary antibody overnight
at 4°C followed by three washes in 0.1% TBST for 5 min. The
membranes were then incubated in horseradish peroxidase-conjugated
anti-rabbit secondary antibody (1:5,000; Boster Biological
Technology, Co., Ltd., Wuhan, Hubei, China) for 1 h at 37°C,
following washing in 0.1% TBST for 5 min three times. An enhanced
chemiluminescence Substrate Reagent kit (Thermo Fisher Scientific,
Waltham, MA, USA) was added and the band intensity of the blot was
quantified using a gel imager (Bio-Rad, Hercules, CA, USA). GAPDH
was used as an internal standard.
Immunofluorescence analysis
The cells were seeded in six-well plates at 50–60%
confluence, prior to being treated for 48 h with si-h-Cav-1 or
siCon, respectively. The cells were washed with cold
phosphate-buffered saline (PBS) twice and then fixed in 4%
paraformaldehyde at room temperature for 10 min. The cells were
subsequently washed twice in PBS and the slides were blocked with
1% bovine serum albumin (BSA; Amresco, Solon, OH, USA) in 0.1% PBS
with Tween 20 with 0.3 M glycine for 30 min. The slides were then
incubated with anti-caveolin 1 antibody for 2 h at room
temperature. Fluorescein isothiocyanate-conjugated goat-anti-rabbit
immunoglobulin G was used to detect the primary antibody for 1 h at
room temperature in the dark and DAPI (0.5 μg/ml; Sigma-Aldrich,
St. Louis, MO, USA) was used to label nuclei for 3 min at room
temperature in the dark. Image capture and processing were
performed using an Olympus IX71 fluorescence microscope (Olympus,
Tokyo, Japan).
Cell proliferation assay and chemotherapy
sensitivity assay
To assess cell proliferation and chemotherapy
sensitivity to doxorubicin (Dox; Sigma-Aldrich), the Cell Counting
kit-8 (CCK-8) assay (Dojindo Lab., Kumamoto, Japan) was used in
accordance with the manufacturer’s instructions. The cells were
seeded in 96-well plates at a density of 5×103
cells/well. The culture medium was removed and 100 μl diluted CCK-8
(1:9; diluted in RPMI-1640 medium) was added to each well, and the
cells were incubated at 37°C for 1.5 h. The optical density was
then detected at a wavelength of 450 nm using a microplate reader
(Thermo Fisher Scientific).
Colony formation assay
For the colony formation assay, following
transfection with si-h-Cav-1 or siCon, the cells were seeded in six
plates (3×102/well). The cells were cultured for 9 days
prior to being stained with crystal violet, and then images of the
cells were captured and analyzed for colony formation.
Transwell migration and invasion
assays
The upper transwell chamber (8 μm pore size; Corning
Inc., Union City, CA, USA), coated (Sigma-Aldrich; invasion assay)
or not coated with ECM gel (migration assay), was covered by
5×104 cells in 200 μl medium containing 0.1% BSA. The
lower chamber was then filled with 200 μl RPMI-1640 medium
containing 30% FBS (HyClone Laboratories). The cells were then
cultured at 37°C and 5% CO2 for 22 h, prior to being
fixed with 70% ethanol and stained with 0.1% crystal violet. The
number of cells were counted in multiple random fields using the
Olympus IX71 fluorescence microscope (Olympus).
Flow cytometric analysis
For the cell cycle assay, cells (1–2×105)
were collected and washed twice with PBS and centrifuged at 1,500 ×
g for 5 min. The cells were then resuspended and fixed in 70%
ethanol in PBS overnight at −20°C. Fixed cells were washed twice
with PBS and centrifuged at 1,500 × g for 5 min prior to being
resuspended in 500 μl propidium iodide (50 μg/ml; BioSharp, Seoul,
South Korea) with RNase A (50 μg/ml; Amresco). The cells were then
incubated at 4°C for 30 min in the dark and subsequently analyzed
using flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analysis of all the data were performed
using the SPSS 11.0 software (SPSS, Inc., Chicago, IL, USA). The
results are presented as the mean ± standard deviation. Student’s
t-test was used to evaluate significant differences and P<0.05
was considered to indicate a statistically significant
difference.
Results
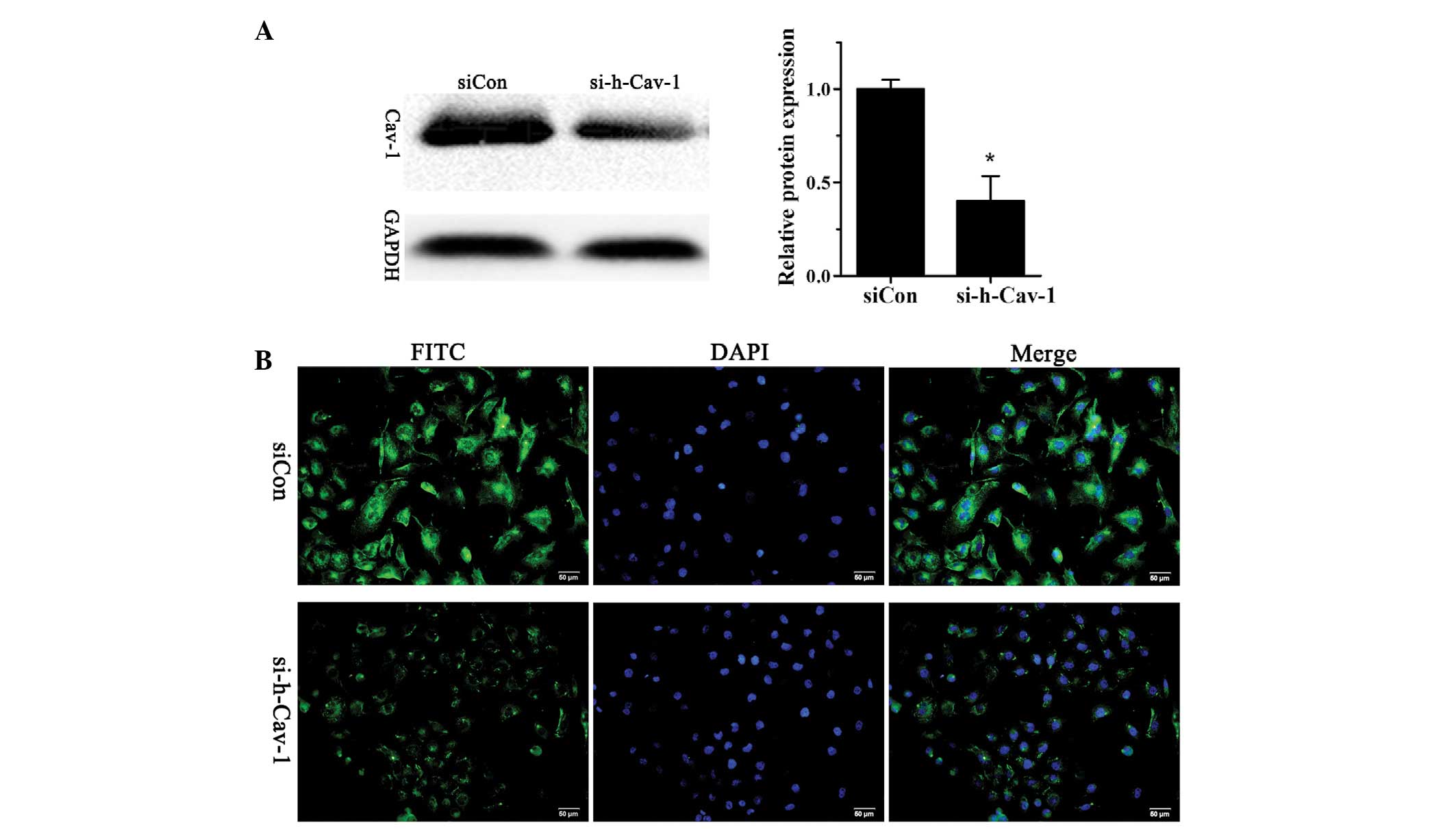
Stable knockdown of caveolin 1 in human
breast cancer BT474 cells
In order to investigate the role of caveolin 1 in
human breast cancer, BT474 cells were transfected with si-h-Cav-1
to knockdown caveolin 1 expression or siCon as a control. The
successful knockdown of caveolin 1 was confirmed using western
blotting (Fig. 1A) and
immunofluorescence analysis (Fig.
1B).
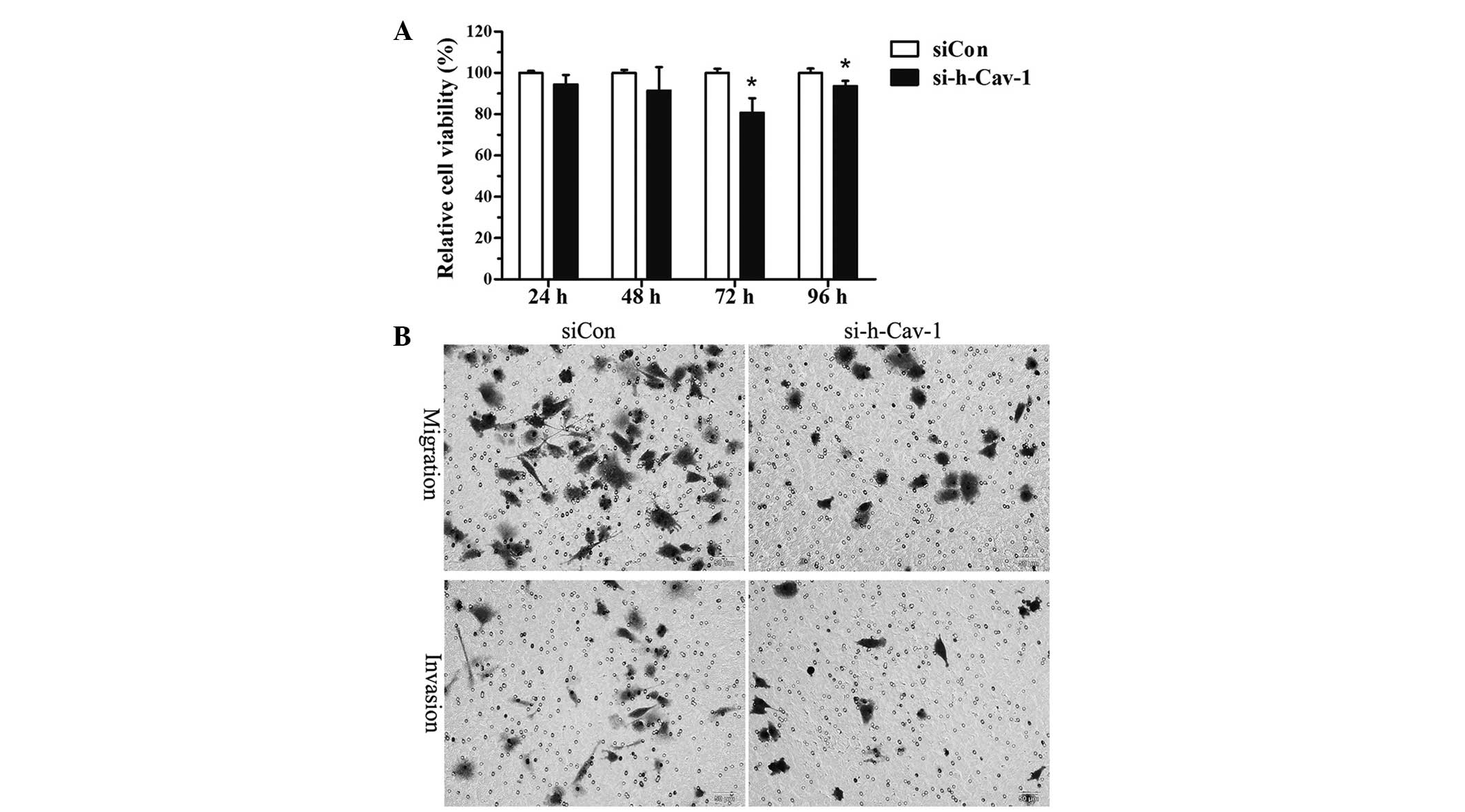
Effect of caveolin 1 knockdown on cell
growth, migration and invasion in BT474 cells
To examine whether caveolin 1 knockdown affects the
cell growth of BT474 cells, BT474 cells were transfected with
si-h-Cav-1 or siCon in 96-well plates. Cell proliferation was
evaluated using the CCK-8 kit at different time points (24, 48, 72
and 96 h). The results demonstrated that cell growth significantly
decreased 72 and 96 h after caveolin 1 knockdown in BT474 cells
compared with cells transfected with siCon (Fig. 2A). Transwell assays were performed
to detect the effect of caveolin 1 knockdown on the migration and
invasion of BT474 cells. The results demonstrated that caveolin 1
knockdown in BT474 cells attenuated their metastatic ability
(Fig. 2B).
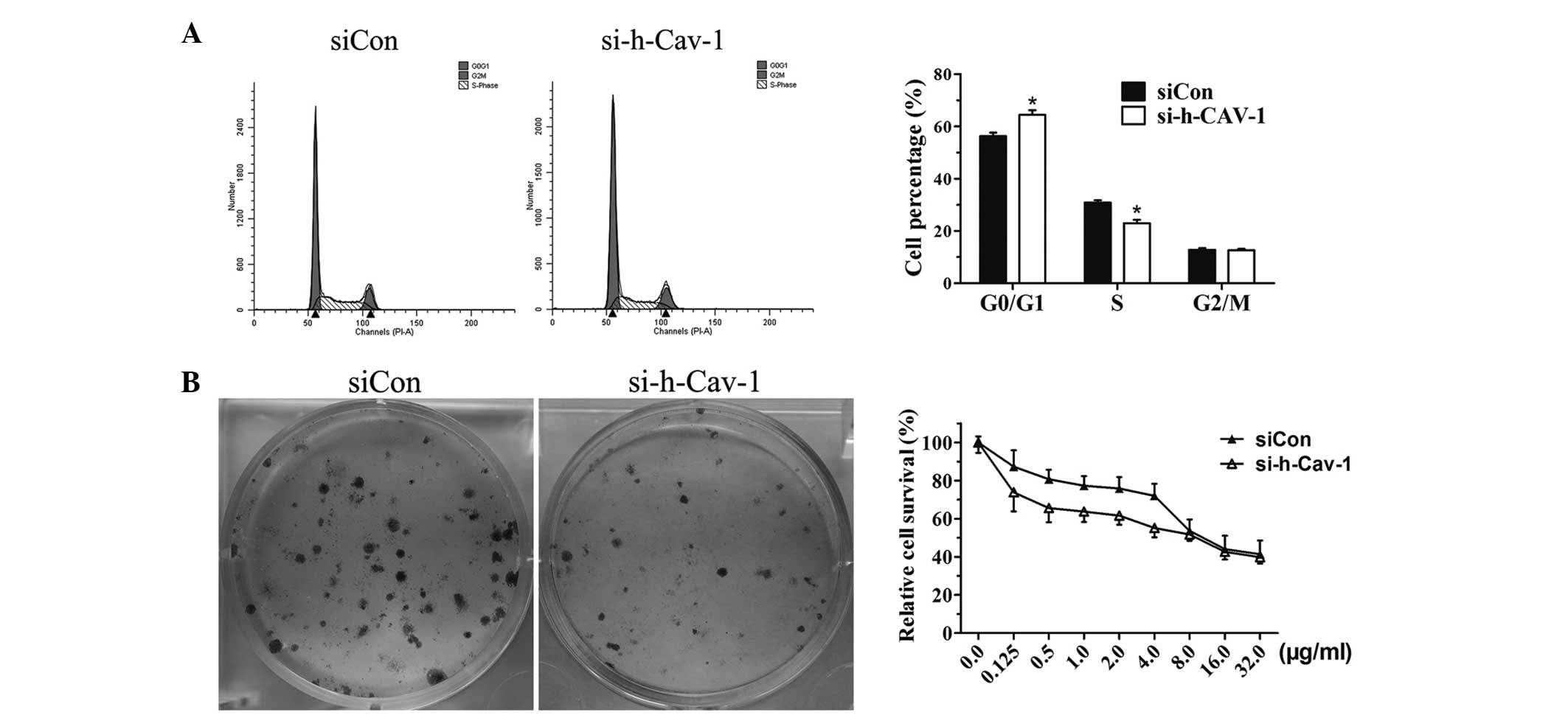
Effect of caveolin 1 knockdown in BT474
cells on colony formation, the cell cycle and Dox-induced cell
death
To further confirm the impact of caveolin 1
knockdown on cell growth, the cell cycle was analyzed using flow
cytometry. The results demonstrated that the number of BT474 cells
in G0/G1 phase increased, whilst the number of cells in the S phase
decreased following caveolin 1 knockdown (Fig. 3A). The efficiency of cell colony
formation was analyzed and it was found to decrease in caveolin 1
knockdown BT474 cells (Fig. 3B).
The cells were treated with Dox for 12 h, prior to being
transfected with si-h-Cav-1 or siCon for 36 h. BT474 cells in the
si-h-Cav-1 group demonstrated higher sensitivity to the Dox
treatment compared with cells in the siCon group (Fig. 3C).
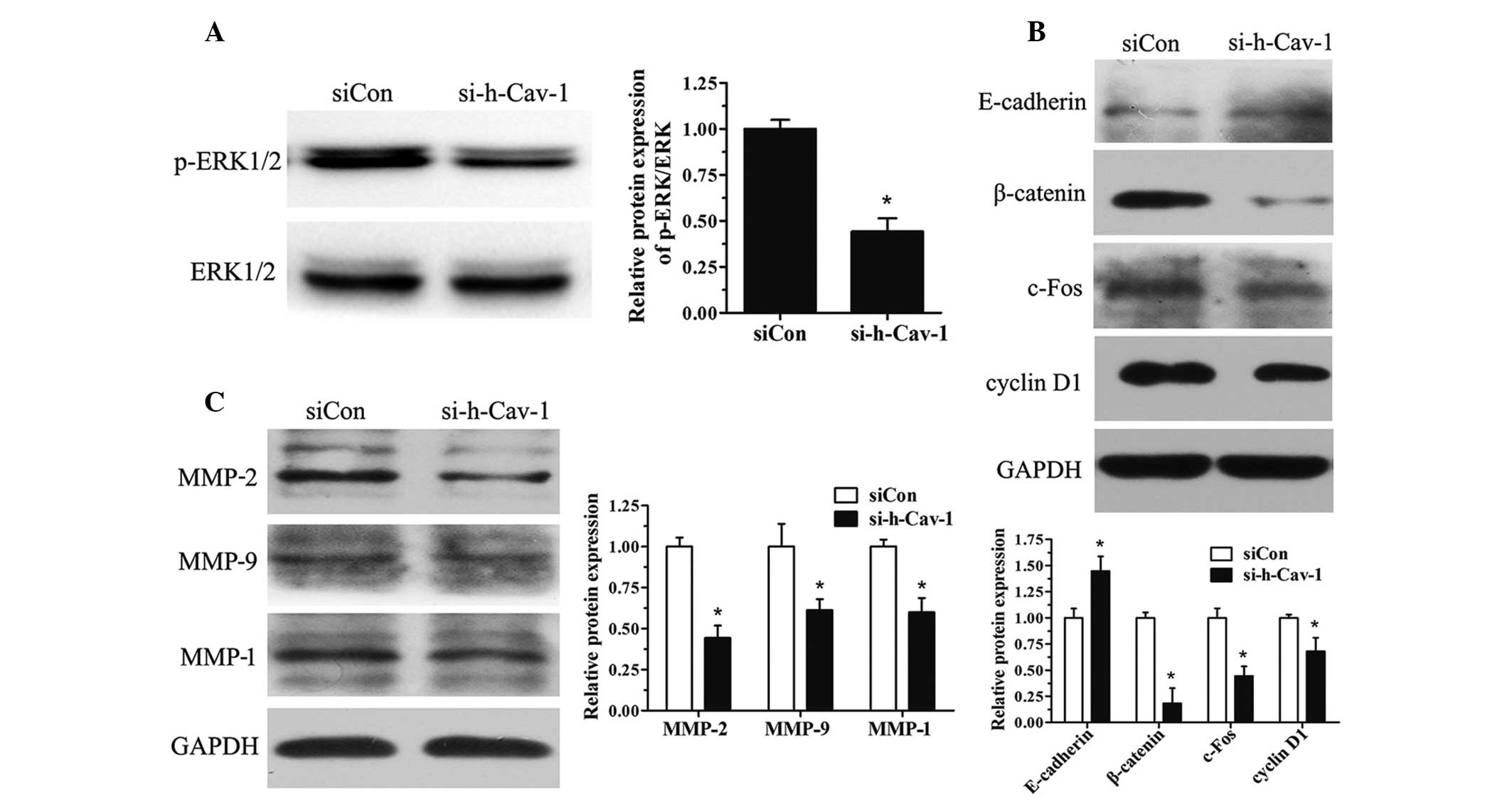
Effect of caveolin 1 knockdown in BT474
cells on the expression of proteins involved in the cell cycle,
migration and invasion
BT474 cells were further investigated from a
mechanistic perspective. Caveolin 1 knockdown reduced the
activation of the ERK1/2 pathway (Fig.
4A) and decreased the expression of proteins involved in the
cell cycle, including cyclin D1, c-Fos and β-catenin (Fig. 4B). Caveolin 1 knockdown in BT474
cells also led to the upregulation of E-cadherin (Fig. 4B). Furthermore, the protein
expression of the MMP family (MMP-2, MMP-9 and MMP-1) was also
investigated and it was found that MMP expression decreased with
caveolin 1 knockdown (Fig.
4C).
Discussion
Caveolin 1 has been demonstrated to have a
suppressing and promoting role in pancreatic cancer, lung cancer,
esophageal squamous cell carcinoma, renal cell carcinoma, prostate
cancer and melanoma (6–8,12–14).
Previous studies have found that patients with a high caveolin 1
expression have more progressive diseases, and caveolin 1 has been
demonstrated to have tumor promoting and pro-survival functions in
more advanced disease stages (15,16).
By contrast, several studies have also revealed that caveolin 1
functions as a tumor suppressor in breast cancer, which has been
confirmed in breast cancer MCF7 cells and several animal models
(17,18). However, there is also evidence that
caveolin 1 serves as a tumor promoter in breast cancer (19). In the present study, it was
demonstrated that caveolin 1 had a tumor promoting role in BT474
cells. Knockdown of caveolin 1 resulted in the suppression of cell
proliferation, migration and invasion of BT474 cells.
It has been demonstrated that caveolin 1 is able to
negatively regulate cell proliferation. Knockdown of caveolin 1
resulted in a decrease in the number of cells in the G0/G1 phase
population and an increase in the number of cells in the S phase
population, through driving the expression of cyclin D1, an
essential factor in the G1/S transition and in tumor formation
(20–22). However, caveolin 1 knockdown had a
different effect on cell growth and the cell cycle in BT474 cells,
resulting in a significant reduction in cell growth (Fig. 2A) associated with decreased cyclin
D1 expression, and increased G0/G1 phase population and reduced S
phase population. Furthermore, c-Fos, as well as β-catenin, has
previously been demonstrated to function as a nuclear transcription
factor (23,24). In addition, in the present study,
their expression was found to be decreased by caveolin 1 knockdown
in BT474 cells (Fig. 4B).
Not only has caveolin 1 been identified as a
tumorigenic activity-associated gene, but it has also been
suggested that it is involved in multiple-drug resistance to
chemotherapy in numerous types of carcinoma (25,26).
In the present study, it was demonstrated that caveolin 1 knockdown
increased Dox-induced cell death in BT474 cells and therefore
sensitized those cells to Dox treatment (Fig. 3C).
E-cadherin has previously been demonstrated to be
important in tumor metastasis through modulating the process of
cell-cell adhesion, establishment of cell polarity and cytoskeletal
rearrangement (27).
Downregulation of caveolin 1 has been demonstrated to be associated
with a reduction in E-cadherin expression and, therefore, cell
motility, as well as enhancing the metastatic ability of tumor
cells (28). However, in contrast
to previous studies, the inhibition of caveolin 1 in the present
study resulted in an induction in the protein level of E-cadherin
in BT474 cells (Fig. 4B).
In addition to E-cadherin, MMPs, a family of
zinc-containing proteolytic enzymes, are important in tumor cell
invasion through the degradation of proteins in the extracellular
matrix and the basement membrane, for example, collagen and
fibronectin. MMP-2 and MMP-9, as well as MMP-1, have been found to
be important in cancer progression and metastasis (29). In the present study, it was
revealed that MMP expression was downregulated by caveolin 1
knockdown in BT474 cells (Fig.
4C). In combination, these results suggest that the inhibition
of migration and invasion of BT474 cells associated with caveolin 1
deprivation may be attributed to the upregulation of E-cadherin and
downregulation of MMPs (MMP-2, -9, -1).
The ERK1/2 pathway is required for tumor survival
(30), invasion and metastasis
(31). The inhibition of cell
motility by caveolin 1 knockdown in BT474 cells may therefore be
attributed to the suppression of the ERK1/2 pathway (Fig. 4A).
In conclusion, the results from the present study
indicate the potential capacity of caveolin 1 as a tumor promoter
in BT474 cells. The role of caveolin 1 in cancer progression has
been demonstrated to be controversial and complex. The present
study provides novel insights into the function of caveolin 1 in
breast cancer. It was demonstrated that caveolin 1 has a tumor
promoting role in BT474 cells and the results suggest that caveolin
1 may be used as a metastatic marker in carcinomas. However, this
requires further investigation before it may be used as a practical
diagnostic and prognostic marker.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81001171) and the Key
Technologies R&D Program of Hubei Province (grant no.
2007AA302B07).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Shaul PW and Anderson RG: Role of
plasmalemmal caveolae in signal transduction. Am J Physiol.
275:L843–L851. 1998.PubMed/NCBI
|
|
3
|
Williams TM and Lisanti MP: Caveolin-1 in
oncogenic transformation, cancer, and metastasis. Am J Physiol Cell
Physiol. 288:C494–C506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cokakli M, Erdal E, Nart D, et al:
Differential expression of Caveolin-1 in hepatocellular carcinoma:
correlation with differentiation state, motility and invasion. BMC
Cancer. 9:652009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cerezo A, Guadamillas MC, Goetz JG, et al:
The absence of caveolin-1 increases proliferation and
anchorage-independent growth by a Rac-dependent, Erk-independent
mechanism. Mol Cell Biol. 29:5046–5059. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang G, Truong LD, Wheeler TM and Thompson
TC: Caveolin-1 expression in clinically confined human prostate
cancer: a novel prognostic marker. Cancer Res. 59:5719–5723.
1999.PubMed/NCBI
|
|
7
|
Joo HJ, Oh DK, Kim YS, Lee KB and Kim SJ:
Increased expression of caveolin-1 and microvessel density
correlates with metastasis and poor prognosis in clear cell renal
cell carcinoma. BJU Int. 93:291–296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato K, Hida Y, Miyamoto M, et al:
Overexpression of caveolin-1 in esophageal squamous cell carcinoma
correlates with lymph node metastasis and pathologic stage. Cancer.
94:929–933. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu P, Wang X, Li F, Qi B, Zhu H, et al:
Growth suppression of MCF-7 cancer cell-derived xenografts in nude
mice by caveolin-1. Biochem Biophys Res Commun. 376:215–220. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urra H, Torres VA, Ortiz RJ, et al:
Caveolin-1-enhanced motility and focal adhesion turnover require
tyrosine-14 but not accumulation to the rear in metastatic cancer
cells. PLoS One. 7:e330852012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elsheikh SE, Green AR, Rakha EA, et al:
Caveolin 1 and Caveolin 2 are associated with breast cancer
basal-like and triple-negative immunophenotype. Br J Cancer.
99:327–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han F, Gu D, Chen Q and Zhu H: Caveolin-1
acts as a tumor suppressor by down-regulating epidermal growth
factor receptor-mitogen-activated protein kinase signaling pathway
in pancreatic carcinoma cell lines. Pancreas. 38:766–774. 2009.
View Article : Google Scholar
|
|
13
|
Sunaga N, Miyajima K, Suzuki M, et al:
Different roles for caveolin-1 in the development of non-small cell
lung cancer versus small cell lung cancer. Cancer Res.
64:4277–4285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Felicetti F, Parolini I, Bottero L, et al:
Caveolin-1 tumor-promoting role in human melanoma. Int J Cancer.
125:1514–1522. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Savage K, Lambros MB, Robertson D, et al:
Caveolin 1 is overexpressed and amplified in a subset of basal-like
and metaplastic breast carcinomas: a morphologic, ultrastructural,
immunohistochemical, and in situ hybridization analysis. Clin
Cancer Res. 13:90–101. 2007. View Article : Google Scholar
|
|
16
|
Van den Eynden GG, Van Laere SJ, Van der
Auwera I, et al: Overexpression of caveolin-1 and -2 in cell lines
and in human samples of inflammatory breast cancer. Breast Cancer
Res Treat. 95:219–228. 2006.PubMed/NCBI
|
|
17
|
Fiucci G, Ravid D, Reich R and Liscovitch
M: Caveolin-1 inhibits anchorage-independent growth, anoikis and
invasiveness in MCF-7 human breast cancer cells. Oncogene.
21:2365–2375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Williams TM, Medina F, Badano I, et al:
Caveolin-1 gene disruption promotes mammary tumorigenesis and
dramatically enhances lung metastasis in vivo. Role of Cav-1 in
cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion.
J Biol Chem. 279:51630–51646. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salatino M, Beguelin W, Peters MG, et al:
Progestin-induced caveolin-1 expression mediates breast cancer cell
proliferation. Oncogene. 25:7723–7739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Williams TM, Lee H, Cheung MW, et al:
Combined loss of INK4a and caveolin-1 synergistically enhances cell
proliferation and oncogene-induced tumorigenesis: role of
INK4a/CAV-1 in mammary epithelial cell hyperplasia. J Biol Chem.
279:24745–24756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Williams TM, Sotgia F, Lee H, et al:
Stromal and epithelial caveolin-1 both confer a protective effect
against mammary hyperplasia and tumorigenesis: Caveolin-1
antagonizes cyclin D1 function in mammary epithelial cells. Am J
Pathol. 169:1784–1801. 2006. View Article : Google Scholar
|
|
22
|
Boström P, Söderström M, Palokangas T, et
al: Analysis of cyclins A, B1, D1 and E in breast cancer in
relation to tumour grade and other prognostic factors. BMC Res
Notes. 2:1402009.PubMed/NCBI
|
|
23
|
Arteaga CL and Holt JT: Tissue-targeted
antisense c-fos retroviral vector inhibits established breast
cancer xenografts in nude mice. Cancer Res. 56:1098–1103.
1996.PubMed/NCBI
|
|
24
|
Wend P, Runke S, Wend K, et al:
WNT10B/β-catenin signalling induces HMGA2 and proliferation in
metastatic triple-negative breast cancer. EMBO Mol Med. 5:264–279.
2013.
|
|
25
|
Selleri S, Arnaboldi F, Palazzo M, Hussein
U, Balsari A and Rumio C: Caveolin-1 is expressed on multipotent
cells of hair follicles and might be involved in their resistance
to chemotherapy. Br J Dermatol. 153:506–513. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho CC, Kuo SH, Huang PH, Huang HY, Yang CH
and Yang PC: Caveolin-1 expression is significantly associated with
drug resistance and poor prognosis in advanced non-small cell lung
cancer patients treated with gemcitabine-based chemotherapy. Lung
Cancer. 59:105–110. 2008. View Article : Google Scholar
|
|
27
|
Tiwari N, Gheldof A, Tatari M and
Christofori G: EMT as the ultimate survival mechanism of cancer
cells. Semin Cancer Biol. 22:194–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu Z, Ghosh S, Wang Z and Hunter T:
Downregulation of caveolin-1 function by EGF leads to the loss of
E-cadherin, increased transcriptional activity of beta-catenin, and
enhanced tumor cell invasion. Cancer Cell. 4:499–515. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu D, Guo H, Li Y, Xu X, Yang K and Bai
Y: Association between polymorphisms in the promoter regions of
matrix metalloproteinases (MMPs) and risk of cancer metastasis: a
meta-analysis. PLoS One. 7:e312512012. View Article : Google Scholar
|
|
30
|
Balmanno K and Cook SJ: Tumour cell
survival signalling by the ERK1/2 pathway. Cell Death Differ.
16:368–377. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prasad CP, Chaurasiya SK, Axelsson L and
Andersson T: WNT-5A triggers Cdc42 activation leading to an ERK1/2
dependent decrease in MMP9 activity and invasive migration of
breast cancer cells. Mol Oncol. 7:870–883. 2013. View Article : Google Scholar : PubMed/NCBI
|