Introduction
Ischemia reperfusion injury (IRI) is a common
complication observed in patients who undergo liver resection or
transplantation, or suffer from toxic liver injury and
veno-occlusive diseases (1,2). It
is the major cause of the increasing mortality reported after liver
injury, as it is linked with kidney, lung and other organs
(3). Previous studies proposed
that the excessive inflammatory response potentiated by the immune
system is responsible for IRI (4,5).
Free radicals, interleukins and other inflammatory factors are
released and cause damage to the local tissue. Therefore,
inhibition of the inflammatory response may reduce IRI damage
(6,7). Numerous protective strategies against
IRI have been adopted, including therapeutic hypothermia and
ischemic preconditioning (8).
However, few of them focus on the role of regulatory T cells
(Tregs) during the process of liver IRI.
Tregs are a subpopulation of T cells that plays a
critical role in the modulation of the immune system and of
self-tolerance (9). Specifically,
Tregs suppress immune responses mediated by other cell types, and
in order to increase the immune response, they are downregulated
during infection. It was shown that Tregs ameliorate kidney injury
caused by IRI (10), an effect
that may result from their immunosuppressive ability. Heat shock
proteins (HSPs) are involved in numerous physiological processes,
particularly stress. HSP27, a member of the HSP family, is able to
mediate cytoskeletal stability and prevent cell apoptosis (11). Although in a recent study, the use
of HSP27 proved to be helpful for tumor detection and diagnosis
(12), our understanding of the
roles of human HSP27 on reperfusion injury, particularly with
regards to cell and tissue protection, is limited. A few studies on
animal models showed that HSP27 confers protection from reperfusion
injury (13,14). Chen et al (15) reported that mice overexpressing
human HSP27 are protected from IRI. However, how the immune system
affects IRI remains unclear. This study aimed to explore the
interaction of HSP27 with Tregs and its effect on IRI.
Materials and methods
Animal procedures
Male Wistar rats (body weight, 200–230 g) were
purchased from the Shanghai Animal Center (Chinese Academy of
Science, Shanghai, China). The rats were kept in standard animal
care conditions and bred with rat chow and water. These procedures
were approved by the Animal Care Committee of the Zhejiang
University, in accordance with the Principles of Laboratory Animal
Care (NIH publication 85-23, revised 1985).
To clone the human HSP27 full length gene
(NCBI Reference Sequence: NM_001540.3; open reading frame),
HSP27 cDNA were amplified from a HCC cDNA library using the
following primers: Forward, ACTGCTCGAGGCCACCATGACCGAGCGCCGCG and
reverse, TACTGAATTCTTACTTGGCGGCAGTCTCAT). The PCR product was
inserted into the XhoI/EcoRI site of the pIRES2-EGFP
vector (Catalog #6029-1; Clontech Laboratories, Inc., Mountain
View, CA, USA) and sequenced. Subsequently, HSP27 as well as
the vector were packed with adenovirus vector-pAD/CMV/V5-DEST
(Shanghai R&S Biotechnology Co., Ltd., Shanghai, China). The
human HSP27 gene was synthesized and inserted into the
genome of the inactivated adenovirus. The adenovirus was amplified
within human embryonic kidney 293 (HEK 293) cells and dissolved in
phosphate-buffered saline. Rats received anesthetic (4% chloral
hydrate, 0.7 ml/100 g) intraperitoneally, and a midline laparotomy
was performed. The ileocecal vein (a branch of the portal vein) was
exposed, and 0.5 ml of liquid containing 2.5×1012 viral
particles (VP) were injected into the rats via the ileocecal vein.
The abdominal cavity was closed with 3-0 silk sutures. Five days
later, the abdominal cavity was opened again and the median, left
portal vein, hepatic artery and bile ducts were clamped in order to
induce partial hepatic ischemia. The right vessels and bile ducts
were not impeded, and intestinal congestion was thus avoided. A
sixty-minute blockage was considered safe for the animals, since it
did not cause severe liver failure or death. One hour later, the
clip was removed to initiate hepatic reperfusion. The abdominal
cavity was then closed with 3-0 silk sutures.
Animals were placed into 4 groups, all of which
shared in common the induction of partial hepatic ischemia/ischemia
reperfusion (IR) during the second surgery 5 days later: i) HSP27
group, where adenovirus bearing the HSP27 gene was injected;
ii) HSP27+gadolinium trichloride (GdCl3) group, where
adenovirus bearing the HSP27 gene was injected along with
GdCl3 (Sigma-Aldrich, St. Louis, MO, USA) through the
ileocecal vein; iii) vector group, where adenovirus without the
HSP27 gene (empty vector) was injected; and iv)
vector+GdCl3 group, where GdCl3 was injected
along with the empty vector.
All animals were sacrificed at different times after
reperfusion (0.5, 2, 4, 12 and 24 h; n=6 in each group per time
point).Blood samples were collected from the inferior vena cava of
the rats for biochemical examination and flow cytometry. The liver
of each rat was harvested, and one part was immediately frozen in
liquid nitrogen and stored at −80°C to be further analyzed by
quantitative polymerase chain reaction (qPCR), and another part was
fixed in formalin for hematoxylin-eosin staining.
Alanine transaminase (ALT), aspartate
aminotransferase (AST), glutathione (GSH) and superoxide dismutase
(SOD) assays
Blood samples were immediately centrifuged, and
serum was collected and stored at −80°C for the ALT and AST assays.
A total of 100 μl of each sample was diluted in 500 μl
double-distilled water. These dilutions were used to assess ALT and
AST using an automated clinical analyzer (7600; Hitachi, Tokyo,
Japan). Liver damage was evaluated by measuring the GSH and SOD
levels in the liver tissue homogenate using the corresponding
commercial kits (Nanjing Jiancheng Bioengineering Institute,
Nanjing, China) according to the manufacturer’s instructions.
Hematoxylin-eosin staining
Explanted rat livers were fixed in 10% formalin for
5 days. After automated dehydration, the samples were embedded in
paraffin, sectioned (4 μm) and stained with hematoxylin-eosin.
Sections were examined under a light microscope (BX41; Olympus
Optical Co., GmbH, Hamburg, Germany).
Flow cytometry
Rat blood was harvested at various time points
following reperfusion. Peripheral blood mononuclear cells were
isolated using rat lymphocyte separation medium (Borunlaite Science
and Technology Co., Ltd., Beijing, China). Following cell surface
staining with fluorescein isothiocyanate (FITC)-conjugated anti-rat
anti-CD4 and allophycocyanin (APC)-conjugated anti-rat anti-CD25
primary antibodies (eBioscience, San Diego, CA, USA), cells were
fixed and permeabilized using Fix & Perm reagents (Caltag
Medsystems, Buckingham, UK) according to the manufacturer’s
instructions. Cells were then incubated with phycoerythrin
(PE)-conjugated anti-mouse/rat anti-forkhead box protein P3 (Foxp3)
as the secondary antibody (eBioscience). Stained cells were
analyzed using a flow cytometer (FC500; Beckman Coulter, Inc.,
Brea, CA, USA) and the CellQuest software (BD Biosciences, Franklin
Lakes, NJ, USA).
qPCR
We extracted total RNA from each frozen sample using
the TRIzol® reagent (Invitrogen Life Technologies,
Carlsbad, CA, USA) according to a standard protocol. Complementary
DNA (cDNA) was synthesized from the extracted RNAs using Moloney
murine leukemia virus reverse transcriptase (Promega Corp.,
Madison, WI, USA). The following qPCR primers were designed based
on the reported DNA sequences: GAPDH (Gene ID: 24383) forward,
5′-GGGCTCTCTGCTCCTCCCTGTTCT and reverse, 5′-GCCGCCTGCTTCACCACCTTC;
macrophage inflammatory protein-2 (MIP-2) (Gene ID: 114105)
forward, 5′-CCTCCAGCAAGCTCCCTCCTGT and reverse,
5′-GTGGGGTCCTGGAGGGGTCAC; monocyte chemotactic protein-1 (MCP-1)
(Gene ID: 24770) forward, 5′-ACAGAGGCCAGCCCAGAAACCA and reverse,
5′-ACAGGCCCAGAAGCGTGACAGA. The PCR reaction was performed in a
final volume of 10 μl, containing SYBR-Green PCR Master mix
(containing SYBR® Green I dye, AmpliTaq Gold®
DNA Polymerase, dNTPs with dUTP, Passive Reference 1 and optimized
buffer; Applied Biosystems). The amplifications were conducted in
an ABI 7500 Real-Time PCR system (Applied Biosystems, Foster City,
CA, USA) with the following thermal cycling conditions: 95°C for 10
min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
GADPH was used as an internal control. Samples were assayed in
triplicate. Quantification of the mRNAs from the amplified products
was performed using the comparative threshold cycle method as
described in the manufacturer’s manual.
Results
Inflammatory infiltration
All rats in this study survived ischemia and
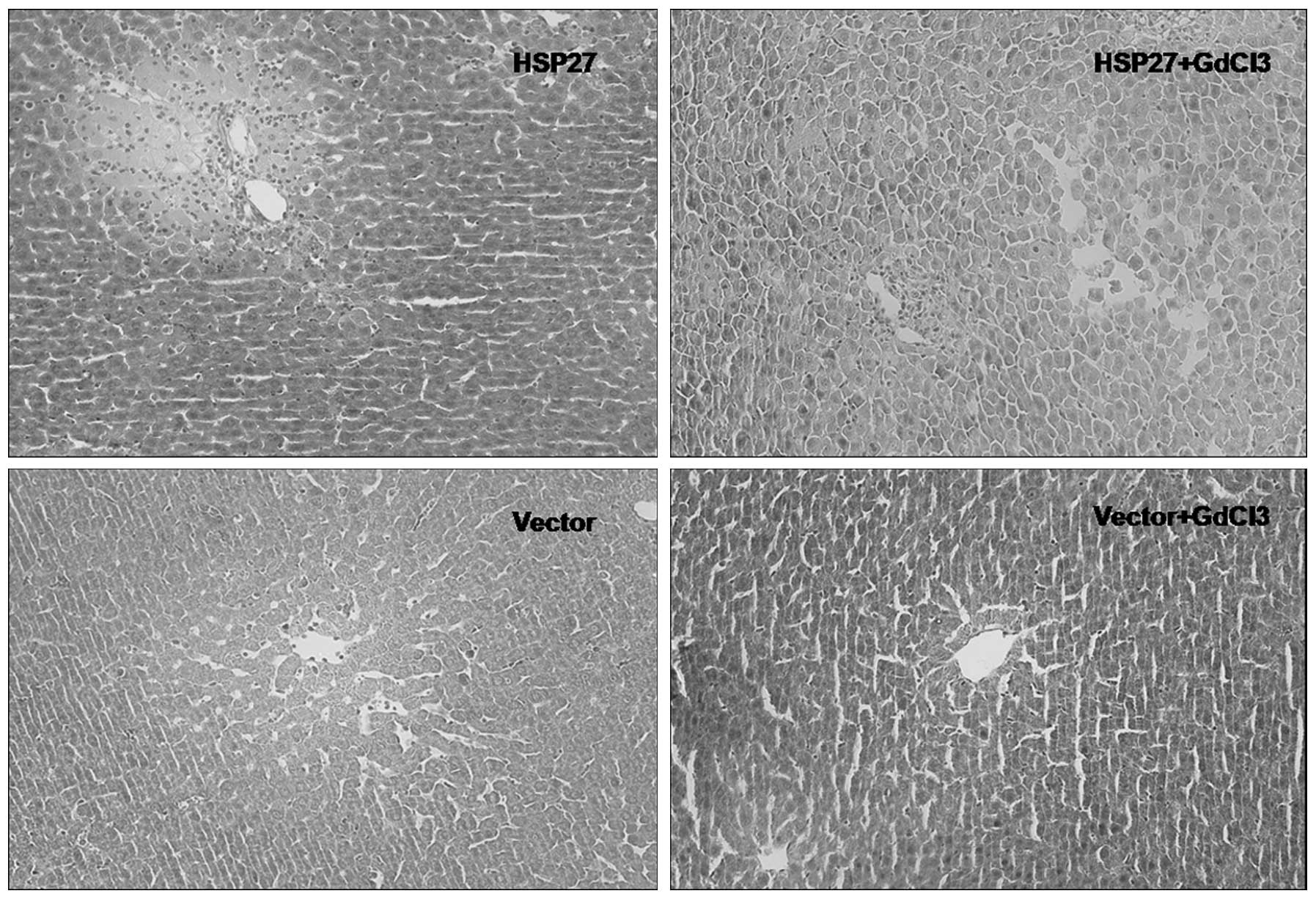
reperfusion. Fig. 1 shows
hematoxylin-eosin stained liver sections following liver ischemia
and reperfusion of the liver for 2 h. Inflammatory cell
infiltrations were more severe in the HSP27 group. In the control
group, where rats were injected with an empty vector, reduced
leukocyte infiltration was observed around the blood vessels. In
the vector treated group, there were also fewer inflammatory cells
compared to the HSP27 group. The vector+GdCl3 group was
the least infiltrated with inflammatory cells.
Liver injury following IR
We found that the AST level was consistent with the
inflammation levels in the different groups. The highest level was
observed in the HSP27 group (data not shown). The combination of
HSP27 and GdCl3 appeared to protect from IRI compared to
the HSP27 group. The liver injury was more severe in the
HSP27-injected groups (HSP27 and HSP27+GdCl3) compared
to groups injected with the empty vector (vector and
vector+GdCl3). HSP27 appeared thus to exacerbate IRI at
2 h following reperfusion. The liver function was best in the
vectors+GdCl3 treated group. Twenty four hours after ischemia, AST
was still detected at a relatively high level in the vector group,
while its level returned to normal in the other three groups. These
results indicate that HSP27 might have a certain protective effect
at later stages of ischemia.
Assessment of oxidative stress
The antioxidant enzyme SOD, as well as GSH, play an
important role in protecting cells from oxidative stress and
attenuating the effects of injury. They are crucial for maintaining
the balance between reactive oxygen species and antioxidants, which
also help prevent damages caused by oxidative stress (16). The depletion of SOD and GSH occurs
at the initial phase of liver necrosis (17). The levels of the stress-related
markers SOD and GSH were significantly decreased in the HPS27 group
compared to the vector+GdCl3 group, indicating that
HSP27 may exacerbate reperfusion injury in rats. Overall, the SOD
and GSH levels were consistent with the AST level, i.e. the more
damaged the liver was, the lower were the SOD and GSH levels.
Amplification of inflammatory factor
genes
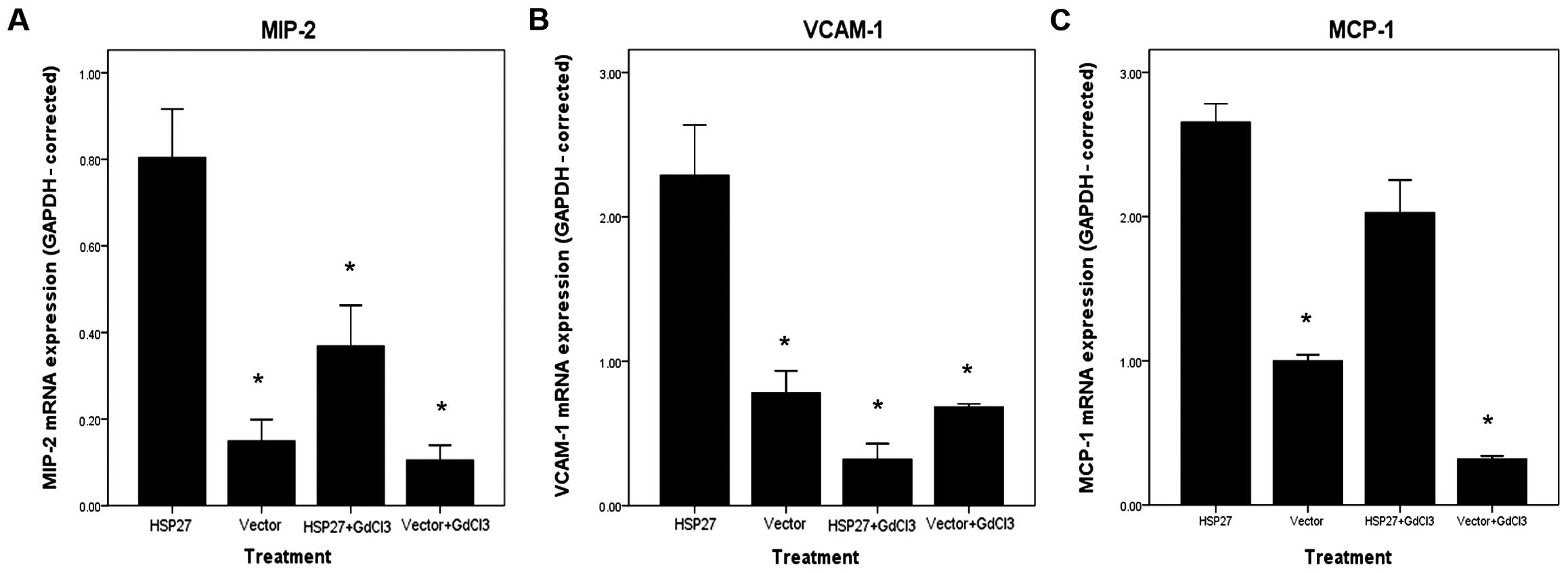
Fig. 2 shows the
changes in expression of pro-inflammatory factors in the four
groups of rats following reperfusion. The mRNA levels of genes
encoding the vascular cell adhesion protein-1 (VCAM-1), MCP-1 and
MIP-2 were increased in rats of the HSP27 group compared with the
other groups. These results suggested that HSP27 enhances the
inflammatory response in the rat liver following IR.
Treg levels in the serum
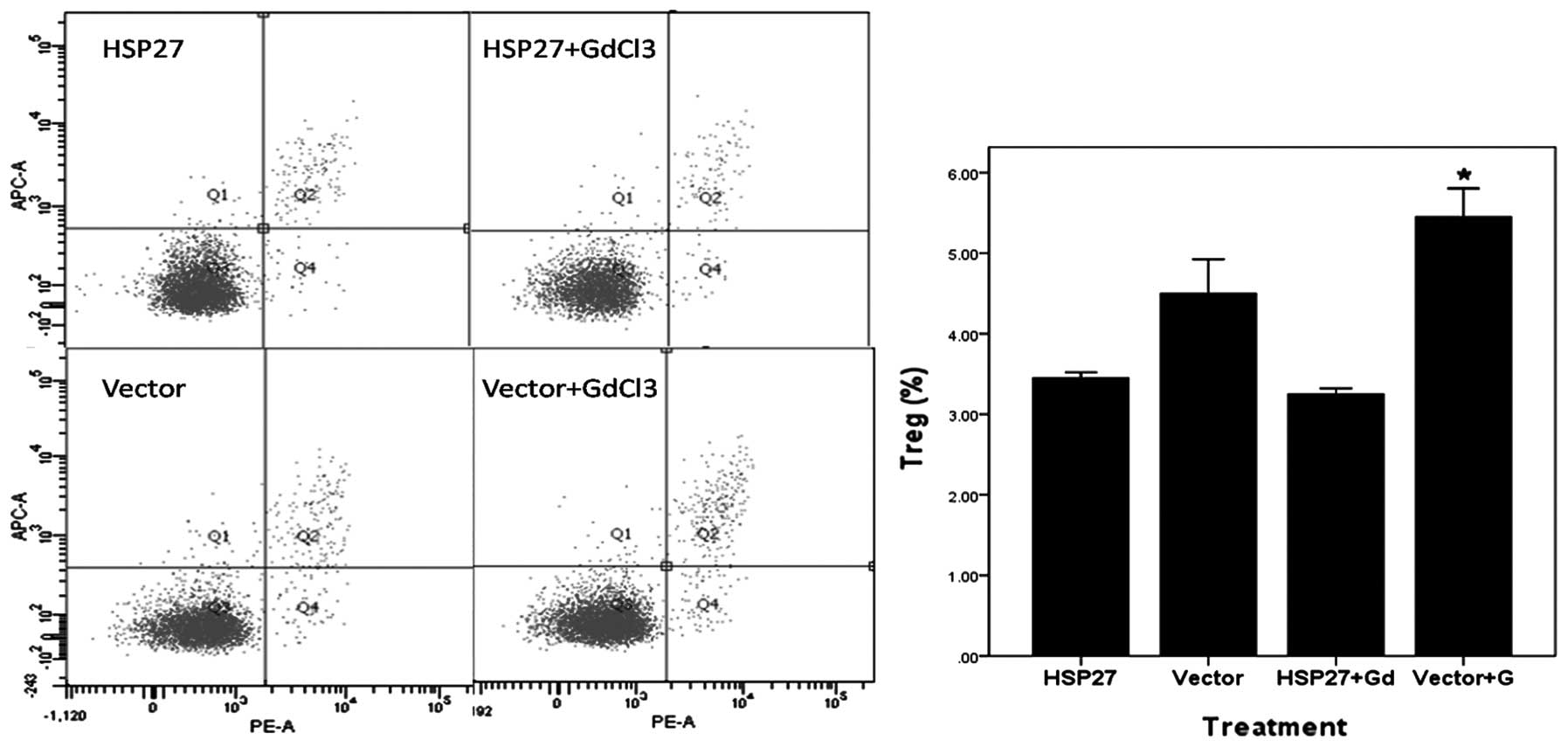
Fig. 3 shows the
levels of Tregs in the different groups after 2 h of IR. Tregs were
fewer in number in the HSP27 groups, which might be the cause of
the more severe inflammation in these groups. The percentage of
Tregs in the serum of rats from the HSP27 group was much lower
compared to that observed in the vector and vector+GdCl3
groups. Therefore, HSP27 may suppress Tregs. By contrast, in the
groups that were not injected with HSP27, the percentage of
Tregs was higher compared to the groups injected with
HSP27.
Discussion
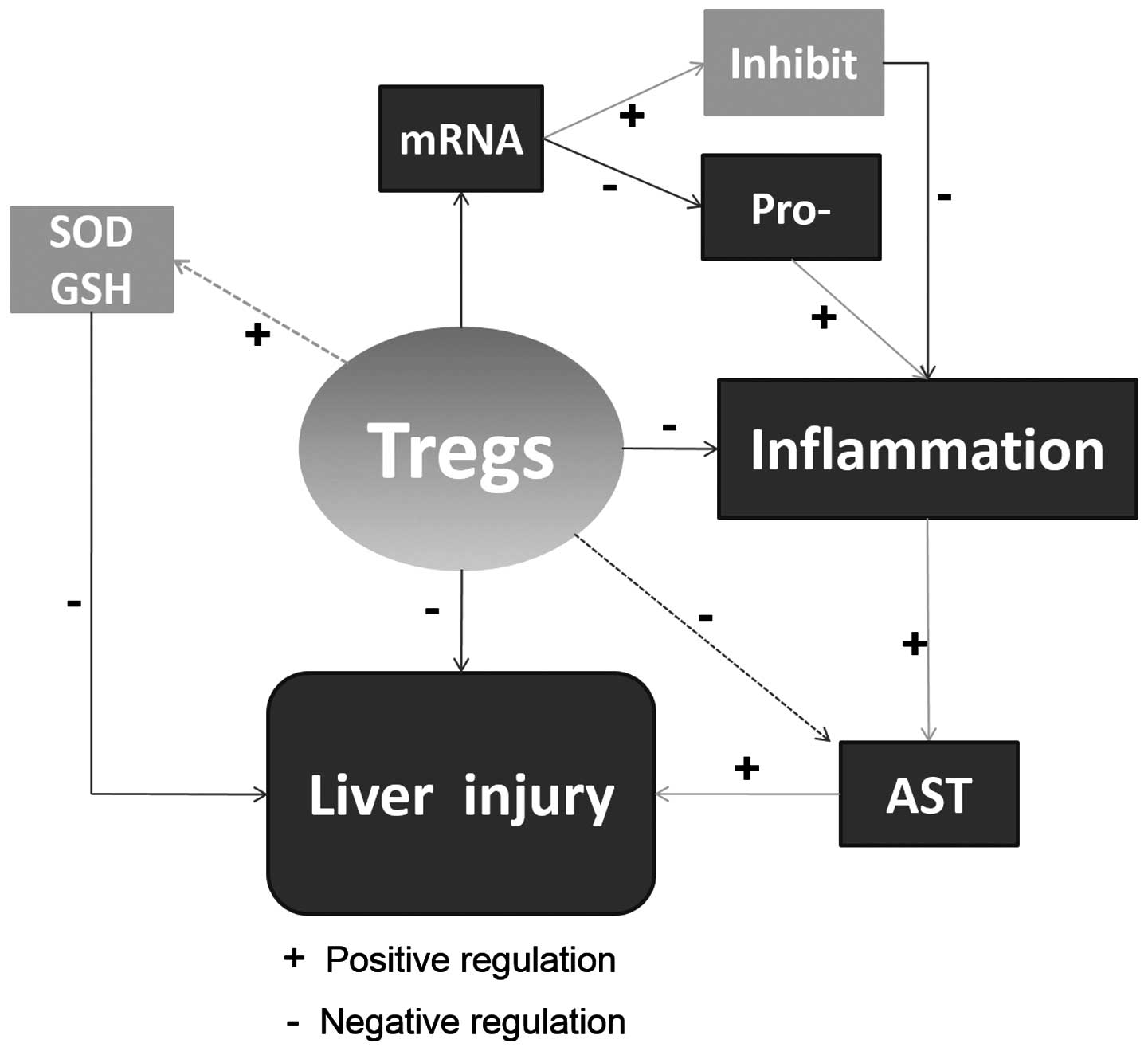
The major finding of our study was that rats
overexpressing the HSP27 gene can exacerbate liver injury
following IR. This might be partly due to the downregulation of
Tregs (Fig. 4). In addition to the
regular function of HSP in folding and unfolding proteins,
induction of its expression in response to stress has been
suggested to be protective for the cells (18,19).
A proteomic analysis showed that the blockage of chaperones such as
HSP may contribute to increased rates of apoptosis and necrosis
(20). Hence, we investigated the
roles of HSP in response to hepatocyte injury.
In this study, we transfected rat hepatocytes with
an adenovirus bearing the human HSP27 gene, so that its
expression would increase when IRI occurs. We chose to use the
human instead of the rat HSP27 gene for two reasons: first,
Chen et al (15) showed
that overexpressed HSP27 in mice can protect liver from IRI,
and we thus aimed to investigate whether the human gene may have a
certain protective function in rats as well. Second, in case this
proved to be true, we may assume that the human HSP27 protein has a
universal protective function in liver IRI, which is very promising
for clinical patients who undergo liver surgery.
Although the detailed mechanism leading to IRI has
not been fully explored yet, it is widely accepted that several
factors contribute to IRI, including free radicals, cytokines and
numerous pro-inflammatory factors (21). From this perspective, liver injury
following ischemia is initiated by inflammatory infiltration and
correlates with the severity of inflammation, and the degree of
liver function can be determined by the inflammatory response
following reperfusion (4). In the
present study, the increase of inflammatory infiltration was
corroborated by hematoxylin-eosin staining. We found that
inflammation was more severe in the HSP27 group, followed by that
observed in the HSP27+GdCl3, vector and
vector+GdCl3 groups.
We further investigated liver function following IRI
in the different groups. A lower AST level was observed in both
groups treated with the empty vector. As inflammatory cells were
recruited, the increase in necrosis, apoptosis and other processes
of cell death resulted in severe damage of liver cells. Therefore,
we conclude that the human HSP27 protein induces inflammation in
the rat liver at the early stage of IRI. Nevertheless, the
observation that AST remained at a relatively high level in the
empty vector group at later stages of IRI, while it was decreased
in the other three groups after 24 h, is surprising. This might be
due to the fact that the human HSP27 protein contributes to tissue
repair at later stages of IRI. HSP27 apart from its role in
attacking inflammatory cells at the early stage, might contribute
in maintaining the liver structure at later stages; inflammation
represents indeed a defense mechanism, protecting the cells from
further damage.
Oxygen, free radicals, cytokines and other
pro-inflammatory factors are involved in the processes of liver
ischemia and reperfusion (22). In
the present study, SOD and GSH levels in rat liver tissues of the
HSP27 group were lower compared to the vector+GdCl3
group, indicating that HSP27 has an unfavorable antioxidant
activity.
Treatment with HPS27 has been associated with
decreased expression of transforming growth factor-β (TGF-β). The
cytokine TGF-β is important for the assembly of liver cells and
promotes growth of hepatocytes (23,24).
In our study, overexpression of the human HSP27 gene in rat
liver was associated with a decrease in the level of the
TGF-β mRNA, suggesting that HSP27 has a negative role in
liver protection at the early stage of IRI. The increased mRNA
expression of pro-inflammatory factors such as VCAM-1, MCP-1 and
MIP-2 further revealed that reperfusion injury is associated with
the upregulation of signaling pathways involved in inflammatory
responses. It was previously demonstrated that T cells are key
mediators of inflammatory responses in the liver following IR
(25), and that depletion of T
cells confers protection from IRI (26). Compared to other subgroups of T
cells, Tregs can directly suppress the activation of
monocytes/macrophages, and can also produce inhibitory cytokines
(27). The deprivation or
downregulation of Tregs may lead to inflammatory response and cause
liver damage. In our study, we demonstrated that Tregs are reduced
in the HSP27 group, which led to reduced protection of the liver
from IRI.
In conclusion, numerous factors are related to liver
IRI. For example, hepatic stellate cells may also play a crucial
role in the progression of IRI (28). The human HSP27 gene does not
protect rat liver from IRI at the early stages, however, it might
exert protective effects at a later stage.
Acknowledgements
This study was supported by grants from the Zhejiang
Provincial Natural Science Foundation for Young Distinguished
Scholars (no. R2110125), the National Natural Science Foundation of
China (no. 81272281) and the National High Technology Research and
Development Program 863 of China (no. 2012AA021002). The funding
institutes had no role in the study design, collection, analysis
and interpretation of data, preparation or submission of the
manuscript.
References
|
1
|
Teoh NC and Farrell GC: Hepatic ischemia
reperfusion injury: pathogenic mechanisms and basis for
hepatoprotection. J Gastroenterol Hepatol. 18:891–902. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fondevila C, Busuttil RW and
Kupiec-Weglinski JW: Hepatic ischemia/reperfusion injury - a fresh
look. Exp Mol Pathol. 74:86–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wobbes T, Bemelmans BL, Kuypers JH,
Beerthuizen GI and Theeuwes AG: Risk of postoperative septic
complications after abdominal surgical treatment in relation to
perioperative blood transfusion. Surg Gynecol Obstet. 171:59–62.
1990.
|
|
4
|
Daemen MA, de Vries B and Buurman WA:
Apoptosis and inflammation in renal reperfusion injury.
Transplantation. 73:1693–1700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Serracino-Inglott F, Habib NA and Mathie
RT: Hepatic ischemia-reperfusion injury. Am J Surg. 181:160–166.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pundik S, Xu K and Sundararajan S:
Reperfusion brain injury: focus on cellular bioenergetics.
Neurology. 79:S44–S51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song X, Zhang N, Xu H, Cao L and Zhang H:
Combined preconditioning and postconditioning provides synergistic
protection against liver ischemic reperfusion injury. Int J Biol
Sci. 8:707–718. 2012. View Article : Google Scholar
|
|
8
|
Gurusamy KS, Gonzalez HD and Davidson BR:
Current protective strategies in liver surgery. World J
Gastroenterol. 16:6098–6103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hori S, Nomura T and Sakaguchi S: Control
of regulatory T cell development by the transcription factor Foxp3.
Science. 299:1057–1061. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lai LW, Yong KC and Lien YH: Pharmacologic
recruitment of regulatory T cells as a therapy for ischemic acute
kidney injury. Kidney Int. 81:983–992. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rane MJ, Pan Y, Singh S, et al: Heat shock
protein 27 controls apoptosis by regulating Akt activation. J Biol
Chem. 278:27828–27835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nagata Y, Kudo M, Nagai T, et al: Heat
shock protein 27 expression is inversely correlated with atrophic
gastritis and intraepithelial neoplasia. Dig Dis Sci. 58:381–388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park SW, Chen SW, Kim M, D’Agati VD and
Lee HT: Human heat shock protein 27-overexpressing mice are
protected against acute kidney injury after hepatic ischemia and
reperfusion. Am J Physiol Renal Physiol. 297:F885–F894. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim M, Park SW, Kim M, Chen SW, Gerthoffer
WT, D’Agati VD and Lee HT: Selective renal overexpression of human
heat shock protein 27 reduces renal ischemia-reperfusion injury in
mice. Am J Physiol Renal Physiol. 299:F347–F358. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SW, Park SW, Kim M, Brown KM, D’Agati
VD and Lee HT: Human heat shock protein 27 overexpressing mice are
protected against hepatic ischemia and reperfusion injury.
Transplantation. 87:1478–1487. 2009. View Article : Google Scholar
|
|
16
|
Taniguchi M, Takeuchi T, Nakatsuka R,
Watanabe T and Sato K: Molecular process in acute liver injury and
regeneration induced by carbon tetrachloride. Life Sci.
75:1539–1549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams AT and Burk RF: Carbon
tetrachloride hepatotoxicity: an example of free radical-mediated
injury. Semin Liver Dis. 10:279–284. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharma A, Upadhyay AK and Bhat MK:
Inhibition of Hsp27 and Hsp40 potentiates 5-fluorouracil and
carboplatin mediated cell killing in hepatoma cells. Cancer Biol
Ther. 8:2106–2113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
O’Neill S, Ross JA, Wigmore SJ and
Harrison EM: The role of heat shock protein 90 in modulating
ischemia-reperfusion injury in the kidney. Expert Opin Investig
Drugs. 21:1535–1548. 2012.PubMed/NCBI
|
|
20
|
Tiriveedhi V, Conzen KD, Liaw-Conlin J, et
al: The role of molecular chaperonins in warm ischemia and
reperfusion injury in the steatotic liver: a proteomic study. BMC
Biochem. 13:172012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Montalvo-Jave EE, Escalante-Tattersfield
T, Ortega-Salgado JA, Pina E and Geller DA: Factors in the
pathophysiology of the liver ischemia-reperfusion injury. J Surg
Res. 147:153–159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weerachayaphorn J, Chuncharunee A,
Jariyawat S, et al: Protection of centrilobular necrosis by
Curcuma comosa Roxb. in carbon tetrachloride-induced mice
liver injury. J Ethnopharmacol. 129:254–260. 2010.PubMed/NCBI
|
|
23
|
Breitkopf K, Godoy P, Ciuclan L, Singer MV
and Dooley S: TGF-beta/Smad signaling in the injured liver. Z
Gastroenterol. 44:57–66. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michalopoulos GK: Liver regeneration. J
Cell Physiol. 213:286–300. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zwacka RM, Zhang Y, Halldorson J,
Schlossberg H, Dudus L and Engelhardt JF: CD4(+) T-lymphocytes
mediate ischemia/reperfusion-induced inflammatory responses in
mouse liver. J Clin Invest. 100:279–289. 1997.
|
|
26
|
Yokota N, Daniels F, Crosson J and Rabb H:
Protective effect of T cell depletion in murine renal
ischemia-reperfusion injury. Transplantation. 74:759–763. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taams LS, van Amelsfort JM, Tiemessen MM,
et al: Modulation of monocyte/macrophage function by human
CD4+CD25+ regulatory T cells. Hum Immunol. 66:222–230. 2005.
|
|
28
|
Jameel NM, Thirunavukkarasu C, Murase N,
et al: Constitutive release of powerful antioxidant-scavenging
activity by hepatic stellate cells: protection of hepatocytes from
ischemia/reperfusion injury. Liver Transpl. 16:1400–1409. 2010.
View Article : Google Scholar
|