Introduction
Dry eye is defined by the International Dry Eye
Workshop as a multifactorial disease of the tear fluid and ocular
surface that results in symptoms of discomfort, visual disturbance
and tear film instability, with potential damage to the ocular
surface. It is usually accompanied by increased osmolarity of the
tear film and inflammation of the ocular surface (1).
There is increasing evidence demonstrating that
inflammation is important in the pathogenesis of dry eye. Whatever
the initial cause of dry eye is, during the development of this
disease, a vicious cycle of inflammation may develop in the ocular
surface, which leads to ocular surface destruction. Continuous
dryness of the ocular surface may result in excessive nervous
stimulation leading to neurogenic inflammation, lymphocytic
infiltration and increased immune reactivity, and release of
inflammatory cytokines into the lacrimal glands, tear fluid and
conjunctiva. In addition, inflammatory mediators may inhibit the
neural signals to the lacrimal gland, which worsens the ocular
surface desiccation and, simultaneously, destroys the ocular
surface environment leading to tissue destruction. A previous study
has shown that a number of inflammatory cytokines, including
interleukin (IL)-6, IL-1 and tumor necrosis factor-α
(TNF-α), increase in the tear fluid of dry eye patients and
the expression of these inflammatory cytokines are also upregulated
in the corneal epithelium and conjunctiva of dry eye patients and
animals (2).
Peroxisome proliferator-activated receptor γ
(PPAR-γ) is a member of the ligand-activated nuclear receptor
superfamily and has been extensively studied in adipocytes, where
it is key in the glucose homeostasis and adipocyte differentiation
(3). In the last decade, PPAR-γ
has been found to possess anti-inflammatory activity (4). There is increasing evidence
suggesting that PPAR-γ exhibits an anti-inflammatory role by
negatively regulating the expression of pro-inflammatory cytokines
activated during the inflammatory response. PPAR-γ acts as a
heterodimer with retinoid X receptors to regulate gene expression
by recognizing and binding to PPAR response elements located in the
promoters of target genes (5).
PPAR-γ interferes with the inflammatory response at different
levels by modulating the expression of inflammatory mediators.
Following binding to ligands, PPAR-γ may inhibit the expression of
inducible nitric oxide synthase (iNOS) and MMP-9 and the production
of TNF-α, IL-6 and IL-1β (6). It
has been well documented that PPAR-γ and PPAR-γ ligands are
beneficial for airway inflammation (7) and inflammatory bowel disease in
various animal models (8). The
exact mechanisms by which PPAR-γ regulates the production of
inflammatory cytokines are complicated and not fully understood.
There is evidence showing that PPAR-γ activation may inhibit the
transcriptional activity of cytokine induced pro-inflammatory
transcription factors, including activator protein-1 and nuclear
factor (NF)-κB (9). These
transcription factors are key in the expression of inflammatory
cytokines.
PPAR-γ expression is tissue dependent. On the ocular
surface, PPAR-γ is expressed in the lacrimal gland (10) and cornea (11) at a relatively low level. The
conjunctiva contains mucosa-associated lymphoid tissue and shares a
number of common features with the mucous membrane in the airway
and digestive tract. The conjunctiva has a rich blood supply, and
may be a target of inflammation at the early stages of the immune
reaction during the development of dry eye pathology. PPAR-γ is
hypothesized to be important in the inflammatory process during the
pathogenesis of dry eye. To date, little is known concerning PPAR-γ
expression in the conjunctiva, and to the best of our knowledge no
studies have been undertaken to evaluate the change in PPAR-γ
expression on the ocular surface in dry eye. The current study
aimed to investigate the expression of PPAR-γ and inflammatory
cytokines in the conjunctiva of dry eye mice to explore the role of
PPAR-γ in the pathogenesis of dry eye.
Materials and methods
Dry eye mouse model
A total of 96 6-week-old female C57 BL/6J mice (SLAC
Laboratory Animal Center, Shanghai, China) were randomly divided
into eight groups, in which mice were sacrificed by cervical
dislocation at eight different time points, between 1 and 20 days,
following dry eye induction. A total of 12 mice per group were used
for the detection of aqueous tear production and corneal
fluorescence staining; 3 mice per group were used for the
conjunctival histopathology, detection of PPAR-γ, IL-1β and TNF-α
mRNA expression by quantitative polymerase chain reaction (qPCR),
western blot analysis and concentration of IL-1β and TNF-α in tear
fluid and the conjunctiva; and 75 mice were used for the treatment
with pioglitazone (PIO). All experiments were performed in
accordance with the Declaration of Helsinki or the NIH statement
for Use of Animals in Research. The study was approved by the
Ethics Committee of Tongji University (Shanghai, China). Animals
were cared for in accordance with Statement for the Use of Animals
in Ophthalmic and Vision Research of The Association of Research
for Vision and Ophthalmology.
Dry eye was induced as previously reported by
subcutaneous injection of 0.1 mg/0.2 ml scopolamine hydrobromide
(Sigma-Aldrich, St. Louis, MO, USA) in the hindquarters of the mice
three times daily (9, 12 am and 5 pm). Animals were exposed to air
draft for 12 h every day to maintain the ambient humidity below 40%
(12).
Detection of aqueous tear production
The aqueous tear production was measured using the
phenol-red impregnated cotton threads (Zone-Quick; Oasis, Glendora,
CA, USA). The threads were held with jeweler forceps (Katena
Products, Inc., Denville, NJ, USA) and placed in the lateral cantus
of the conjunctival fornix of the right eye for 60 sec (13). The length of moist thread was
observed under a microscope (Olympus, Tokyo, Japan), using a slide
gauge with a precision accuracy of 0.02 mm.
Corneal fluorescence staining
Corneal fluorescence staining was performed to
evaluate the corneal integrity. Briefly, 0.5 μl of 1% flourescein
(Sigma-Aldrich) was added to the inferior conjunctival sac of the
right eye with a micropipette. The cornea was examined under a slit
lamp biomicroscope (66 Vision Tech Co., Ltd., Suzhou, China) in
cobalt blue light 1 min after fluorescein addition. Corneal
fluorescein staining was classified using a grading system
developed by Park et al (13) on the basis of area of corneal
staining. No staining was graded as 0; stained area ≤1/8 of the
cornea was graded as 1; stained area ≤1/4 of the cornea was graded
as 2; stained area ≤1/2 of the cornea was graded as 3; and stained
area >1/2 of the cornea was graded as 4.
Conjunctival histopathology
Mice were sacrificed by cervical dislocation and the
eyeball containing conjunctiva was collected, fixed in 4%
paraformaldehyde and embedded in paraffin. The eyes were sectioned
in vertical plane (4-μm thick). These sections were stained using
the periodic acid-Schiff (PAS) staining system (Sigma-Aldrich)
according to the manufacturer’s instructions. The morphology of the
conjunctiva was observed and the goblet cells were counted under a
microscope (Olympus) by two investigators blinded to the study.
Three sections were collected from each sample for cell counting,
and an average was calculated.
Detection of PPAR-γ, IL-1β and TNF-α mRNA
expression by qPCR
Mice were sacrificed by cervical dislocation.
Conjunctiva was harvested, immediately frozen in liquid nitrogen
and stored at −80°C until RNA extraction. Total RNA was extracted
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA), and 2 μg total RNA was reverse transcribed using the ReverTra
Ace™ RT-PCR kit (Toyobo, Osaka, Japan) according to manufacturer’s
instructions. Following reverse transcription, 2 μl cDNA was used
as template in 30 μl PCR mixture and Taq platinum. Primers
were designed using DNA Star software (DNAStar, Inc., Madison, WI,
USA) according to the manufacturer’s instructions. Table I shows the primers used and the
anticipated length of products.
 | Table IPrimers used in PCR for amplification
of target genes. |
Table I
Primers used in PCR for amplification
of target genes.
| Gene | GeneBank no. | Sequence | Length (bp) |
|---|
| PPAR-γ | NM00112733 | F:
agtgccttgctgtggggatgt
R: tcagcgggaaggactttatgtatg | 180 |
| TNF-α | NM013693 | F:
tgcaccaccatcaaggactcaaat
R: ccccggccttccaaataaatacat | 289 |
| IL-1β | NM008361 | F:
actacaggctccgagatgaacaac
R: cccaaggccacaggtatttt | 144 |
| GAPDH | NM008084 | F:
accacagtccatgccatcac
R: tccaccaccctgttgctgta | 450 |
SYBR-Green qPCR was performed in a Shanghai Hongshi
Medical Technology Co, Ltd., Real-time PCR Detection system
(Hongshi, Shanghai, China). In 30 μl SYBR-Green PCR reaction
mixture, there were 2 μl cDNA, 1 μl forward primer, 1 μl reverse
primer, 15 μl 2× PCR Mastermix (QPK-201; Toyobo, Osaka, Japan) and
11 μl PCR-grade water. For the detection of mRNA expression of
PPAR-γ and TNF-α, the following conditions were used: 94°C for 3
min, 36 cycles of 94°C for 30 sec, 55°C for 30 sec and 72°C for 30
sec. For the detection of mRNA expression of IL-1β the following
conditions were used: 94°C for 3 min for 1 cycle, 40 cycles of 94°C
for 20 sec and 60°C for 40 sec. A negative control was included to
evaluate DNA contamination. Products of qPCR were analyzed using a
relative standard curve method with SLAN 5.0 software (Shanghai
Hongshi Medical Technology Co., Ltd.). The mRNA expression of
target genes were normalized to that of GAPDH.
Western blot analysis
The conjunctiva was collected and mixed in RIPA
buffer, containing 1% Nonidet P-40, 1% sodium deoxycholate, 0.1%
SDS, 50 mM sodium fluoride, 0.01 mM tetrasodium pyrophosphate, 0.2
mM orthovanadate, 2 mM EDTA, 0.15 mol/l sodium chloride and 100
units proteinase cocktail, pH 7.2. Following denaturation, proteins
were separated by 10% SDS polyacrylamide gel electrophoresis and
transferred electronically to nitrocellulose membranes (Whatman
0.45 μm; 300 mA, 90 min; Bio-Rad, Hercules, CA, USA). Nonspecific
binding was blocked with 5% non-fat milk in Tris-buffered saline
with 0.05% Tween-20 (TBST) overnight at 4°C. Next, the membranes
were incubated with mouse anti-β-actin and mouse anti-PPAR-γ
monoclonal primary antibody (1:1,000). The membranes were rinsed
three times with TBST for 10 min. Next, the membranes were
incubated with horseradish peroxidase-conjugated goat anti-mouse
secondary antibodies (1:2,000; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) for 2 h at room temperature. The immunoreactive
bands were visualized by enhanced chemiluminescence (Pierce
Biotechnology, Inc., Rockford, IL, USA). The relative densities of
target proteins were normalized against β-actin using a Gel-Pro
analyzer (Media Cybernetics, Silver Spring, MD, USA).
Concentration of IL-1β and TNF-α in tear
fluid and the conjunctiva
The concentration of IL-1β and TNF-α in the tear
fluid and the conjunctiva were determined by ELISA. Tear-washing
fluid was collected as described by Song et al (14). Mice in each group were divided into
three subgroups (four per subgroup). The tear-washing fluids of the
two eyes in the same subgroup were pooled together and stored at
−80°C until ELISA was performed. The conjunctiva was collected and
added to 200 μl phosphate-buffered saline followed by
homogenization. The homogenate was centrifuged at 35,000 × g for 20
min at 4°C, and the supernatant was collected for ELISA. The pooled
tear fluid was diluted (1:3), and the IL-1β and TNF-α concentration
was measured with a ELISA kit (R&D Systems, Minneapolis, MN,
USA) according to the manufacturer’s instructions. All samples were
assayed in duplicate and the means were calculated.
Treatment with PIO
Mice were divided into normal control, dry eye,
0.25% PIO, 0.5% PIO and 1% PIO groups. Dry eye was induced as
described in the materials and methods, and treatment was performed
12 days later. PIO powder (Wuhan Huameihua Scientific Co., Ltd,
Wuhan, China; 0.1 g) was dissolved in dimethylsulfoxide (DMSO)
under aseptic conditions and diluted with normal saline tp 1, 0.5
and 0.25%. DMSO diluted with normal saline of equal volume served
as a control. Treatment with PIO was performed three times daily on
one of the eyes for 4 weeks. In the dry eye group, mice were
treated with DMSO in normal saline. At the end of the experiment,
the concentration of IL-1β and TNF-α in the tear fluid were
measured by ELISA, and corneal fluorescein staining was performed.
Mice were sacrificed by cervical dislocation, and the conjunctiva
was collected. The mRNA and protein expression of PPAR-γ in the
conjunctiva were determined with qPCR and western blot analysis,
respectively, and PAS staining was performed to detect the goblet
cell density.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Comparisons were performed using Student’s-t test for independent
samples. Statistical analysis was performed with SPSS version 16.0.
(SPSS Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Aqueous tear production
The aqueous tear production is shown in Table II. There was a significant
decrease in aqueous tear production following the introduction of
dry eye when compared with the control group (P<0.001). The tear
production continued to decrease until 10–12 days following
introduction of dry eye. Although there was a transient increase at
15 and 20 days, the tear production remained significantly lower
compared with that of the control group.
 | Table IIAqueous tear production, corneal
fluorescein staining score and conjunctival goblet cell count of
dry eye mice and control mice. |
Table II
Aqueous tear production, corneal
fluorescein staining score and conjunctival goblet cell count of
dry eye mice and control mice.
| Variable | Aqueous tear
production, mm; n=12 | Corneal fluorescein
staining score, n=12 | Conjunctival goblet
cell count, n=3 |
|---|
| Control | 5.66±0.66 | 0.11±0.33 | 31.00±2.64 |
| Day 1 | 2.66±0.17b | 1.22±0.44b | 21.00±2.64a |
| Day 3 | 1.86±0.24b | 2.33±0.50b | 16.67±1.52b |
| Day 5 | 1.48±0.30b | 2.67±0.50b | 11.33±1.15b |
| Day 10 | 3.11±0.56b | 3.33±0.70b | 5.33±1.15b |
| Day 12 | 2.62±0.24b | 3.78±0.44b | 5.33±1.52b |
| Day 15 | 1.11±0.24b | 3.89±0.33b | 15.33±1.52a |
| Day 20 | 1.06±0.18b | 4.00±0.00b | 12.67±1.15b |
Corneal fluorescein staining
The grades of corneal fluorescein staining are shown
in Table II. At 1 min following
fluorescein treatment, no staining was observed in the corneas of
the control mice. One day following the fluorescein treatment,
scattered punctate staining was observed. Patches of punctate
staining were observed five days following fluorescein treatment
and diffuse corneal fluorescein staining was noted at 12 days
following fluorescein treatment.
Conjunctival goblet cell count
The number of goblet cells in the conjunctiva of dry
eye mice reduced gradually 1 day following introduction of dry eye.
A significant difference was observed between dry eye mice and
control mice at days 1–20 (P<0.01; Table II).
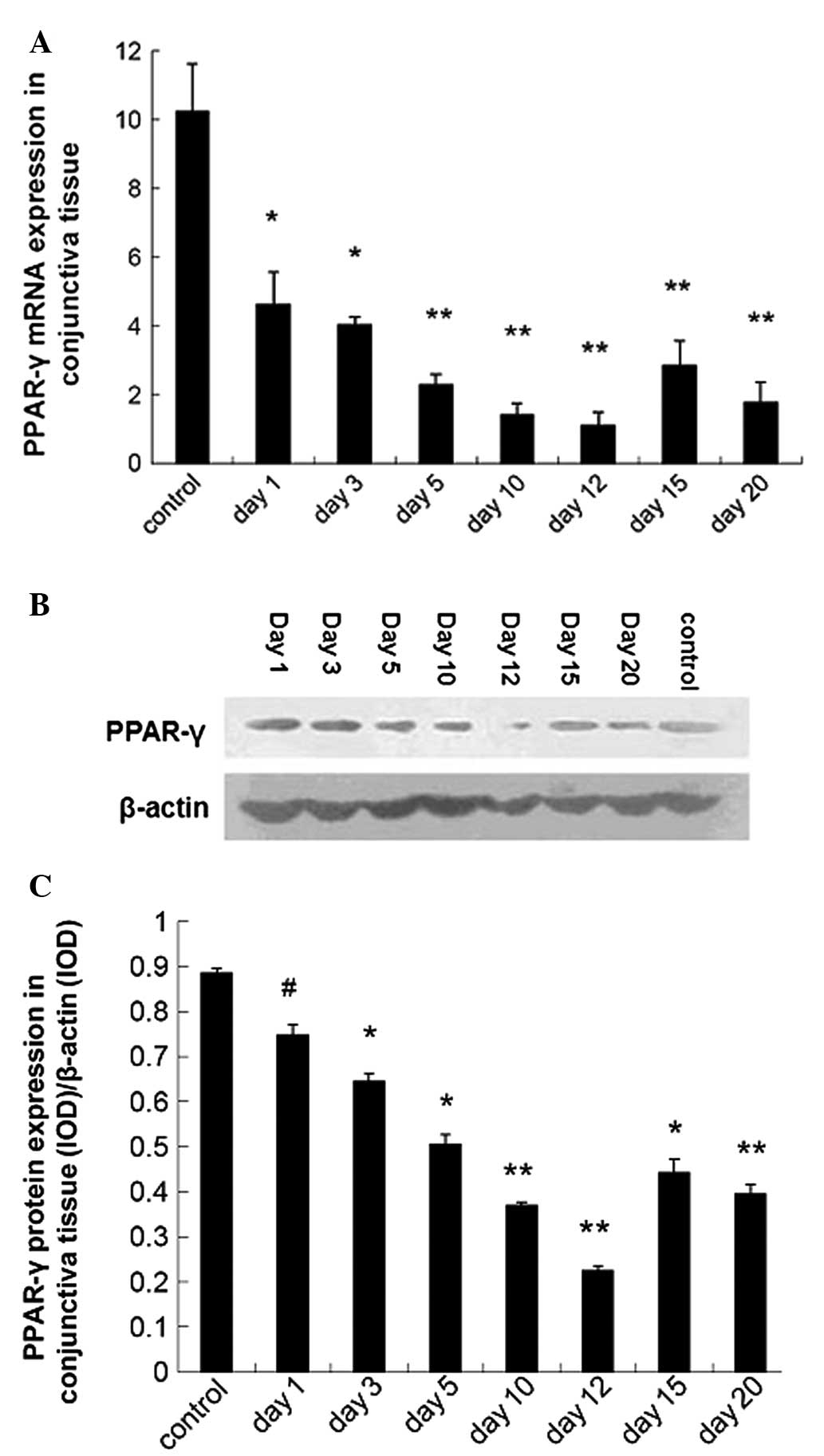
Downregulated PPAR-γ expression in the
conjunctiva of dry eye mice
The PPAR-γ mRNA and protein expression in the
conjunctiva were detected using qPCR and western blot analysis,
respectively. Results showed the PPAR-γ mRNA expression was
downregulated in dry eye mice as compared with control mice
(P<0.01; Fig. 1A). Western blot
analysis revealed a similar trend in the PPAR-γ mRNA expression
(Fig. 1B and C).
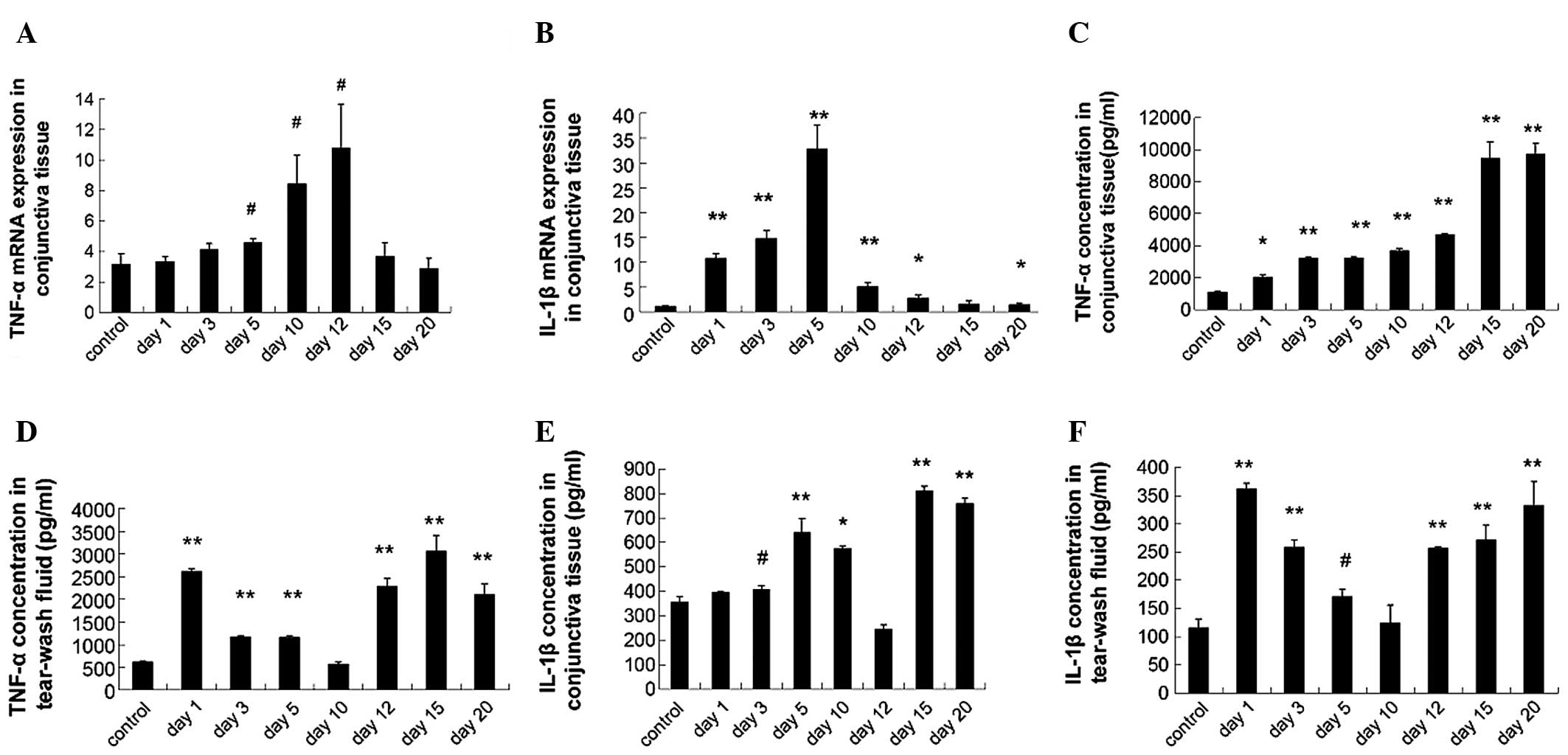
Expression of TNF-α and IL-1β
The mRNA expression of TNF-α and IL-1β detected by
qPCR is shown in Fig. 2A and B,
respectively. The IL-1β mRNA expression increased markedly in the
conjunctiva of dry eye mice as compared with control mice
(P<0.01) except on day 15. The TNF-α mRNA expression increased
between days 5 and 12 (P<0.05). ELISA showed that the TNF-α
concentration of the conjunctiva (days 1–20) was: 2,006.33±165.79,
3,205.3±100.07, 3,225±102.34, 3,676.67±158.82, 4,675±76.02,
9,442.60±576.39 and 9,717±704.32 which was significantly higher
compared with the control group (1,066.27±106.59; P<0.001;
Fig. 2C). The TNF-α concentration
in the tear fluid samples (days 1–20) was 2,609±68.57,
1,164.67±34.27, 1,158±36.37, 564.4±44.09, 2,276.33±183.02,
3,052.33±354.88 and 2,099.33±233.02 in dry eye groups, and 613±19.0
in control group (Fig. 2D). The
IL-1β concentration of the conjunctiva (days 1–20) was 393.63±5.47,
407.47±16.4, 639.33±59.14, 573.03±14.18, 243±19.58, 810.2±19.75 and
757.73±23.74 in dry groups and 353.6±26.36 in the control group
(Fig. 2E). The TNF-α concentration
in the tear fluid (days 1–20) was 362.37±9.26, 258.30±12.32,
171.10±13.08, 124.17±31.93, 256.33±2.73, 270.67±26.53 and
332.17±42.88 in dry eye groups and 115.17±16.56 in the control
group (Fig. 2F).
Influence of PIO treatment on dry
eye
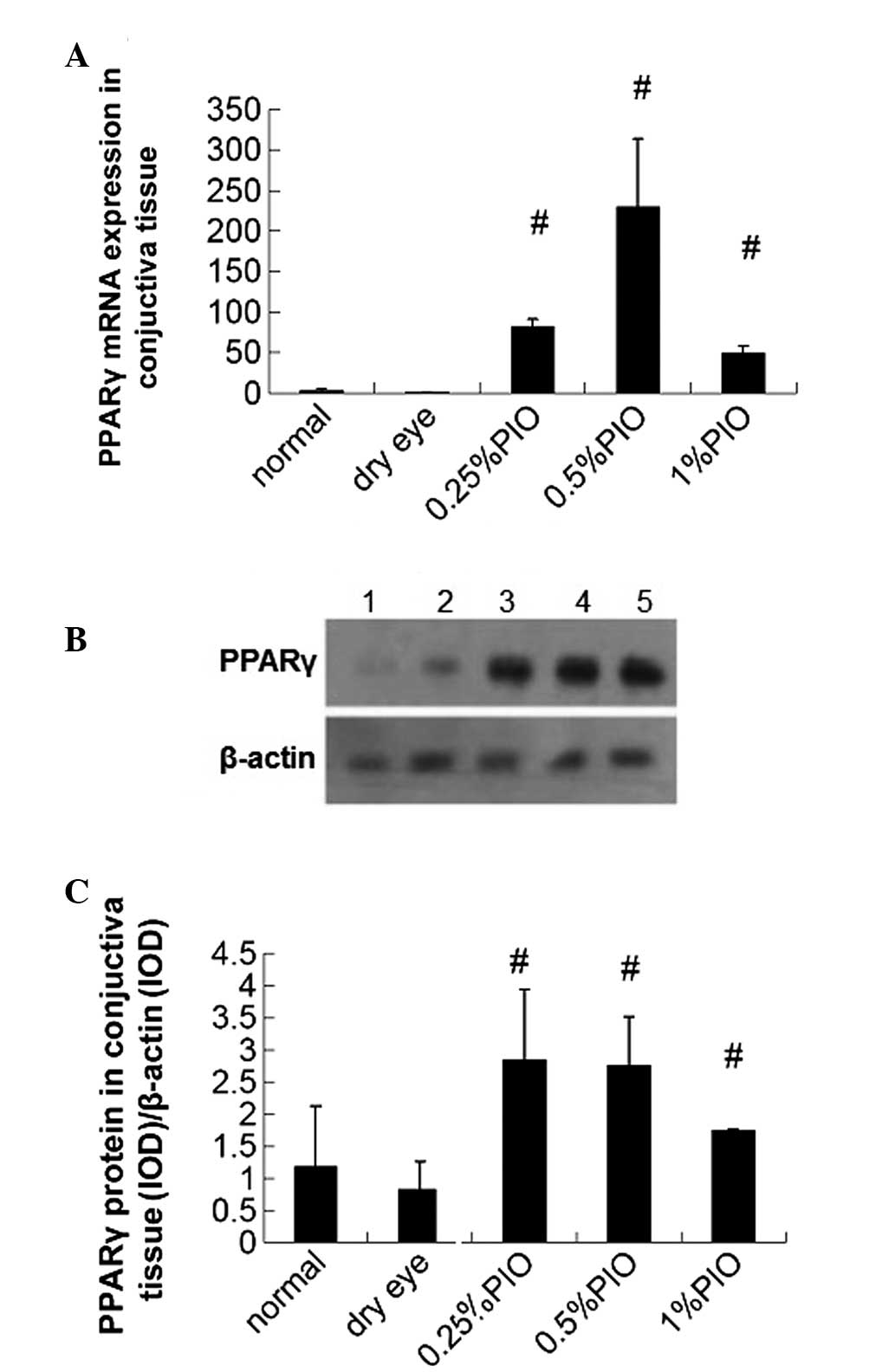
The PPAR-γ mRNA expression in dry eye groups was
significantly lower compared with the control group; however, PIO
treatment markedly increased the PPAR-γ mRNA expression when
compared with the control and dry eye groups (P<0.05; Fig. 3A).
Western blot analysis revealed a weak band in the
control and dry eye groups, suggesting a low PPAR-γ protein
expression; however, the PPAR-γ protein expression increased
markedly following PIO treatment. The PPAR-γ protein expression was
normalized to β-actin protein expression, and results showed the
changes in the PPAR-γ protein expression were similar to those in
PPAR-γ mRNA expression. In the dry eye groups, the PPAR-γ protein
expression was significantly lower compared with the control group,
however, the PIO treatment markedly increased the PPAR-γ protein
expression as compared with the control group and dry eye groups
(P<0.05; Fig. 3B and C).
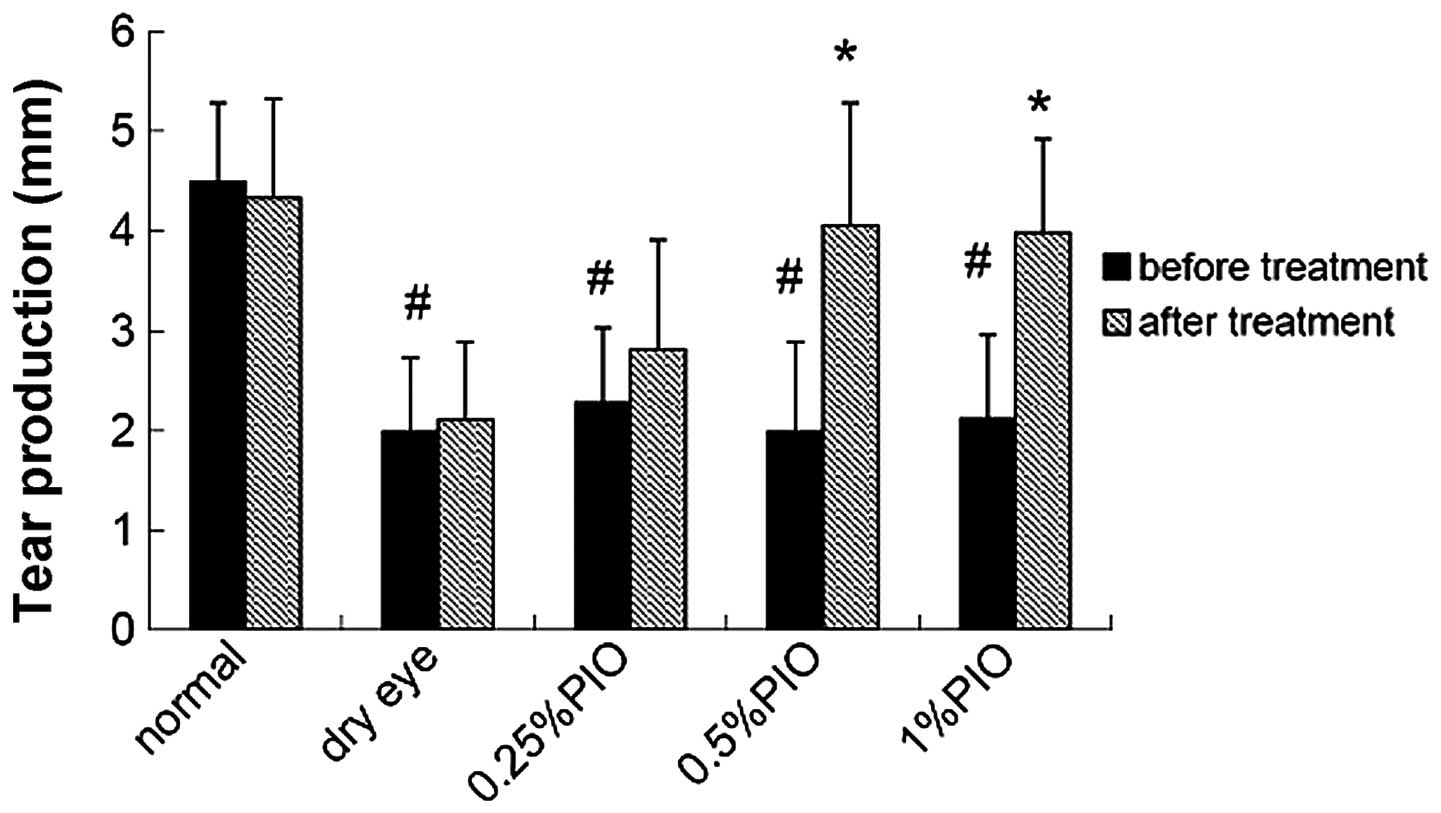
Four weeks following PIO treatment, the tear
production increased when compared with that of the dry eye group,
and a significant difference was observed between the 0.5 and 1%
PIO groups (P<0.05; Fig.
4).
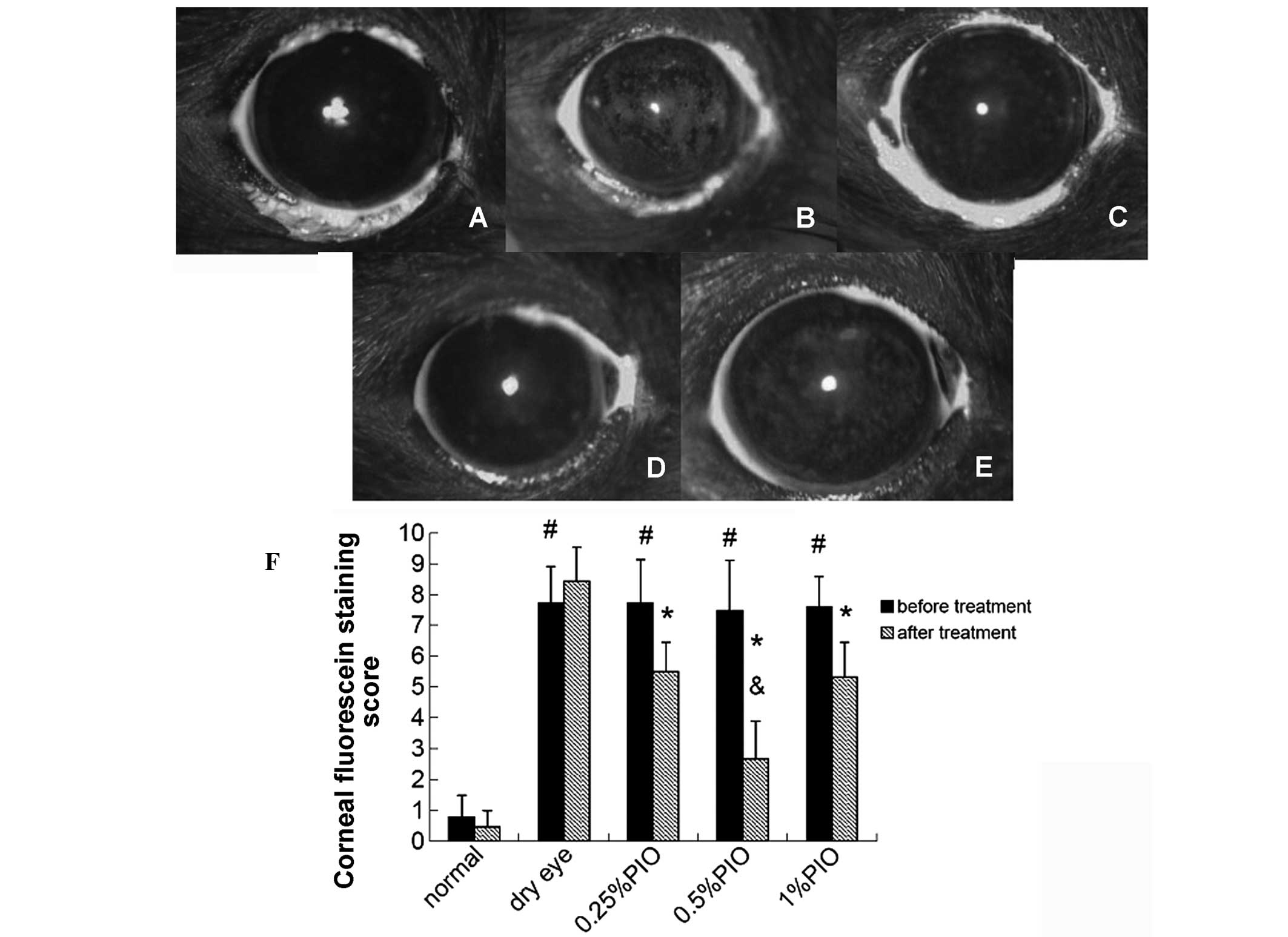
Four weeks following the PIO treatment, the scores
of corneal fluorescein staining were markedly lower compared with
the dry eye groups (P<0.05), and significant differences were
observed among the 0.5, 0.25 and 1% PIO groups (P<0.05; Fig. 5).
Four weeks following the PIO treatment, the number
of goblet cells in the control and PIO groups was significantly
higher compared with the dry eye group, and a significant
difference was also noted between the PIO and dry eye groups
(P<0.05; Table III).
 | Table IIIConjunctival goblet cell count of
mice in different groups. |
Table III
Conjunctival goblet cell count of
mice in different groups.
| Group | Goblet cell count,
n=5 |
|---|
| Control | 51.00±7.68 |
| Dry eye | 22.20±5.45 |
| 0.25% PIO | 43.80±5.36a |
| 0.5% PIO | 48.20±5.40a |
| 1% PIO | 48.00±8.89a |
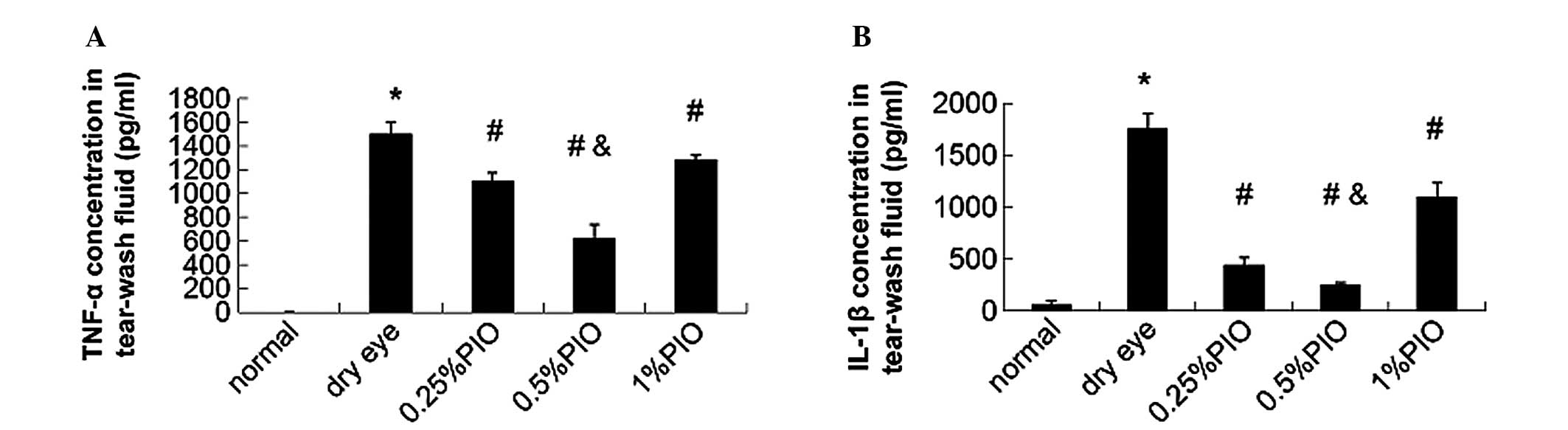
In addition, the IL-1β and TNF-α concentrations of
tear fluid in PIO groups were significantly lower compared with
those in the dry eye groups (P<0.05; Fig. 6).
Discussion
In the present study, dry eye was induced by
systemic administration of an anticholinergic agent, scopolamine,
and exposure to a low humidity environment (12). Scopolamine is a competitive
inhibitor of acetylcholine for muscarinic cholinergic receptors.
Scopolamine-induced dry eye, which was demonstrated by a
significant reduction in tear production and conjunctiva goblet
cell count, and an increase in cornea fluorescein positive areas.
These changes on the ocular surface are similar to those of human
dry eye.
Dry eye is a complicated condition and its
pathogenesis remains to be clearly elucidated. However, there is
increasing evidence that dry eye is associated with ocular surface
inflammation and relevant inflammatory cytokines. The initiation of
the inflammatory response is associated with changes in local blood
flow and accumulation of various inflammatory cells. Thus, the
conjunctiva is hypothesized to be a crucial target at the early
stages of inflammation, due to its rich blood supply. Notably, a
large number of patients with dry eye present with lesions in the
conjunctiva earlier than those in the cornea. The changes in the
composition and hyperosmolarity of tear fluid and the dysfunction
of the tear gland may promote inflammation of the ocular surface.
Pflugfelder et al (15)
reported that the mRNA expression of IL-1β, IL-6, IL-8, TNF-α and
transforming growth factor-β1 increased significantly in the
conjunctival epithelium of patients with Sjogren’s syndrome when
compared with healthy subjects. In non-Sjogren’s dry eye patients,
Yoon et al (16) also found
similar findings. The present results showed that mice with dry eye
presented with upregulated expression of pro-inflammatory cytokines
in the conjunctiva, which was similar to the findings in the study
of Luo et al (17) on
scopolamine-treated mice. However, the current results showed that
the expression of inflammatory cytokines increased following
induction of dry eye. This may be partly due to the desiccated
environment. The TNF-α mRNA expression increased in the conjunctiva
on days 5, 10 and 12, and IL-1β mRNA expression was upregulated at
all time points, with the exception of day 15. The concentration of
inflammatory cytokines in the conjunctiva and tear fluid of dry eye
mice was markedly higher compared with those in the control group.
Notably however, the concentrations of TNF-α and IL-1β in the
conjunctiva were significantly higher compared with those in the
tear fluid. In the conjunctiva, inflammatory mediators are
primarily released by local immune cells. The inflammatory
cytokines in the tear fluid are mainly secreted by the lacrimal
gland. This suggests that decreased lacrimal gland secretion is
partly attributed to the development of dry eyes. However, in the
conjunctiva, the response to decreased tear production and the
stress of desiccation may initiate and trigger the inflammatory
process.
There is an increasing body of evidence indicating
that PPAR-γ and its activators are important in the regulation of
inflammatory processes (18). A
previous study showed that PPAR-γ is involved in the control of
inflammation, particularly in the modulation of the production of a
number of inflammatory mediators, including TNF-α and IL-1β
(19). This is partly associated
with the inhibited activation of NF-κB, which is an important
transcription factor in the inflammatory response. PPAR-γ
interferes with inflammatory pathways, including the NF-κB pathway
by interacting with p50 and p65 in vitro (20). In the present study, the results
showed that the mRNA expression of PPAR-γ in the conjunctiva of dry
eye mice was downregulated significantly one day following dry eye
induction. Although the PPAR-γ expression reduced, its expression
was significantly different to that in the control group. The
PPAR-γ protein expression in the conjunctiva showed a similar trend
to the PPAR-γ mRNA expression. This suggests that decreased tear
production and the desiccated environment may inhibit PPAR-γ
activity. Notably, these results were closely associated with the
features of dry eye mice in terms of aqueous tear production,
corneal fluorescein staining scale and goblet cell count of the
conjunctiva. By contrast, the expression of TNF-α and IL-1β
increased to different extents. It is worth noting that, although
the mRNA expression of TNF-α was not fully consistent with the
changes in PPAR-γ expression, the TNF-α protein expression in the
conjunctiva was consistent with the trend of the changes in PPAR-γ
expression. In the tear fluid, the TNF-α content of dry eye mice at
different time points was higher compared with that in the control
group. This suggests that, in the development of dry eyes, the
conjunctival inflammation and the changes on the ocular surface
were associated with the changes in PPAR-γ expression. It has been
suggested by Gao et al (21) that TNF-α may inhibit the PPAR-γ
activity at two different levels. The mRNA expression of PPAR-γ may
be downregulated by TNF-α, which was confirmed by Ruan et al
(22). By contrast, it has been
suggested that extracellular signal-regulated kinase and c-JUN NH2
terminal kinase in the TNF-α signaling pathway may inhibit the
transcriptional activity of PPAR-γ through phosphorylating serine
residues of PPAR-γ and may suppress ligand-dependent PPAR-γ
activity without decreasing PPAR-γ expression (23). Thus, PPAR-γ may negatively modulate
the production of inflammatory cytokines, including TNF-α, and by
contrast, these components may interfere with the PPAR-γ activity.
However, the exact mechanism requires further investigation.
PPAR-γ has two types of ligands, namely endogenous
ligands and chemically synthesized ligands. The endogenous ligands
are mainly composed of polyunsaturated fatty acids and fatty acid
derivatives, and the chemically synthesized ligands, including
diketones thiazolidines (rosiglitazone, pioglitazone) and tyrosine
derivatives (GW7845). Natural agonists of PPAR-γ usually have poor
specificity and chemically synthesized agonists present with
relatively high specificity. Glitazones are a group of derives with
a thiazolidinedione structure. Studies have shown that glitazones
may selectively activate PPAR-γ to increase the peripheral
sensitivity to insulin and reduce the blood glucose level in
patients with type 2 diabetes. In previous years, increasing
numbers of studies have been conducted to investigate the roles of
PPAR-γ in the regulation of inflammation, immunity and cell
proliferation, and PIO has been used in the treatment of
inflammatory diseases and tumors. There is evidence showing that
PIO may inhibit the secretion of inflammatory cytokines, including
TNF-α and IL-6, by macrophages to improve the symptoms of asthma
patients. In addition, PIO has been obseved to inhibit the release
of MMP-9 (a pro-inflammatory cytokine) lipid peroxidation and
accumulation of polymorphonuclear leukocytes to improve
ischemia/reperfusion-induced lung injury. PIO can also inhibit
vascular endothelial adhesion molecule expression and interfere
with the inflammatory response to alleviate ulcerative colitis; PIO
may inhibit the production of IL-1β, TNF-α, COX-2 and iNOS in the
ulcerative mucosa and blood and increase heat shock protein 70
production to promote gastric ulcer healing (24–26).
In previous years, PIO has been used in the
treatment of corneal neovascularization and diabetic retinopathy
(10,27,28).
Traditionally, PIO is used by intravitreal injection or
subconjunctival injection. However, these injections are invasive
and not suitable for the treatment of dry eye. Thus, PIO drops are
more suitable in the treatment of eye diseases. PIO has a small
molecular weight, is fat-soluble (insoluble in water but soluble in
organic solvents, including ethanol and acetonitrile) and has a low
toxicity. In addition, the pH value may be adjusted to be neutral.
Thus, it is proposed that PIO has good corneal permeability. In
this study, results showed that following treatment with 0.25, 0.5
or 1% of PIO for 4 weeks, the PPAR-γ expression increased markedly
when compared with healthy mice and dry eye mice, and the PPAR-γ
mRNA and protein expression showed a similar tendency. This
suggests that PIO drops may be absorbed by the ocular surface and
may activate PPAR-γ expression on the ocular surface. Detection of
PPAR-γ expression under different conditions may be used to
evaluate the inflammation in dry eye conditions, which may be
beneficial for the investigation of the role of PPAR-γ in
inflammatory regulation and elucidation of the clinical
significance of PIO.
Following treatment with PIO drops, the PPAR-γ
expression in the conjunctiva increased, accompanied by an increase
in tear production, improvement of corneal fluorescein staining
scores, elevation of goblet cell count, reduction in TNF-α and
IL-1β concentration in the tear fluid and improvement of
conjunctival pathology when compared with dry eye mice without
treatment. This demonstrates that upregulated PPAR-γ expression may
reduce the expression of inflammatory cytokines to inhibit the
inflammatory process, increase the tear production, improve the
tear film stability and attenuate the damage to the ocular surface,
which then exerts therapeutic effects on dry eyes.
In the present study, PIO was used at three
concentrations, showing differences in the therapeutic efficacy.
Although the symptoms of dry eye and the damage to the ocular
surface were improved to different extents, the improvement of
corneal fluorescein staining scores was the most evident in the
0.5% PIO group (P<0.05, vs. 0.25% PIO group and 1% PIO group).
In addition, TNF-α and IL-1β expression was the lowest in the 0.5%
PIO group followed by the 0.25% PIO group and highest in the 1% PIO
group. The PPAR-γ mRNA expression was highest in the 0.5% PIO group
and lowest in the 1% PIO group. The PPAR-γ protein expression was
comparable between 0.5% PIO and 0.25% PIO groups, however, the
PPAR-γ protein expression in the former two groups was
significantly higher compared with the 1% PIO group. Results showed
the therapeutic efficacy and stability of 0.25% PIO and 1% PIO are
inferior to those of 0.5% PIO. Thus, 0.5% is hypothesized as an
ideal concentration of PIO for the treatment of dry eye. However,
few studies have been conducted to investigate the therapeutic
efficacy of PIO drops. Thus, the concentration, safety and side
effects of PIO drops require further elucidation.
In conclusion, the results showed the ocular
manifestations of mice with dry eye. The increased expression of
inflammatory cytokines, including TNF-α and IL-1β in the
conjunctiva and tear fluid may be associated with the
downregulation of PPAR-γ expression on the ocular surface. However,
the exact mechanism underlying the changes in PPAR-γ expression
during the development of dry eye is poorly understood. We
hypothesize that there is an interaction between PPAR-γ and
inflammatory mediators.
Acknowledgements
This study was supported by grants from the Shanghai
Health Hospital Development Center Foundation Project (grant no.
SHDC12007104); the Natural Science Foundation of Shanghai (grant
no. 11ZR1427900); the Youth Research Project of Shanghai Municipal
Health Bureau (grant no. 2010Y164); the Young Talent Training Plan
of Tongji University (grant no. 2010KJ018); and the Young Talent
Training Plan of Shanghai Tenth People’s Hospital (grant no.
11RQ108).
References
|
1
|
No authors listed. The definition and
classification of dry eye disease: report of the Definition and
Classification Subcommittee of the International Dry Eye WorkShop
(2007). Ocul Surf. 5:75–92. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Massingale ML, Li X, Vallabhajosyula M,
Chen D, Wei Y and Asbell PA: Analysis of inflammatory cytokines in
the tears of dry eye patients. Cornea. 28:1023–1027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Berger JP, Akiyama TE and Meinke PT:
PPARs: therapeutic targets for metabolic disease. Trends Pharmacol
Sci. 26:244–251. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang C, Ting AT and Seed B: PPAR-gamma
agonists inhibit production of monocyte inflammatory cytokines.
Nature. 391:82–86. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tugwood JD, Issemann I, Anderson RG,
Bundell KR, McPheat WL and Green S: The mouse peroxisome
proliferator activated receptor recognizes a response element in
the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J.
11:433–439. 1992.PubMed/NCBI
|
|
6
|
Chinetti G, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors and inflammation: from
basic science to clinical applications. Int J Obes Relat Metab
Disord. 27(Suppl 3): S41–S45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Standiford TJ, Keshamouni VG and Reddy RC:
Peroxisome proliferator-activated receptor-{gamma} as a regulator
of lung inflammation and repair. Proc Am Thorac Soc. 2:226–231.
2005.
|
|
8
|
Ramakers JD, Verstege MI, Thuijls G, Te
Velde AA, Mensink RP and Plat J: The PPARgamma agonist
rosiglitazone impairs colonic inflammation in mice with
experimental colitis. J Clin Immunol. 27:275–283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ricote M, Li AC, Willson TM, Kelly CJ and
Glass CK: The peroxisome proliferator-activated receptor-gamma is a
negative regulator of macrophage activation. Nature. 391:79–82.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beauregard C and Brandt PC: Peroxisome
proliferator-activated receptor agonists inhibit
interleukin-1beta-mediated nitric oxide production in cultured
lacrimal gland acinar cells. J Ocul Pharmacol Ther. 19:579–587.
2003. View Article : Google Scholar
|
|
11
|
Sarayba MA, Li L, Tungsiripat T, et al:
Inhibition of corneal neovascularization by a peroxisome
proliferator-activated receptor-gamma ligand. Exp Eye Res.
80:435–442. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dursun D, Wang M, Monroy D, et al: A mouse
model of keratoconjunctivitis sicca. Invest Ophthalmol Vis Sci.
43:632–638. 2002.PubMed/NCBI
|
|
13
|
Park CY, Zhuang W, Lekhanont K, et al:
Lacrimal gland inflammatory cytokine gene expression in the
botulinum toxin B-induced murine dry eye model. Mol Vis.
13:2222–2232. 2007.PubMed/NCBI
|
|
14
|
Song XJ, Li DQ, Farley W, et al:
Neurturin-deficient mice develop dry eye and keratoconjunctivitis
sicca. Invest Ophthalmol Vis Sci. 44:4223–4229. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pflugfelder SC, Jones D, Ji Z, Afonso A
and Monroy D: Altered cytokine balance in the tear fluid and
conjunctiva of patients with Sjögren’s syndrome
keratoconjunctivitis sicca. Curr Eye Res. 19:201–211.
1999.PubMed/NCBI
|
|
16
|
Yoon KC, Jeong IY, Park YG and Yang SY:
Interleukin-6 and tumor necrosis factor-alpha levels in tears of
patients with dry eye syndrome. Cornea. 26:431–437. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo L, Li DQ, Doshi A, Farley W, Corrales
RM and Pflugfelder SC: Experimental dry eye stimulates production
of inflammatory cytokines and MMP-9 and activates MAPK signaling
pathways on the ocular surface. Invest Ophthalmol Vis Sci.
45:4293–4301. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fahmi H, Di Battista JA, Pelletier JP,
Mineau F, Ranger P and Martel-Pelletier J: Peroxisome proliferator
- activated receptor gamma activators inhibit
interleukin-1beta-induced nitric oxide and matrix metalloproteinase
13 production in human chondrocytes. Arthritis Rheum. 44:595–607.
2001. View Article : Google Scholar
|
|
19
|
Li M, Pascual G and Glass CK: Peroxisome
proliferator-activated receptor gamma-dependent repression of the
inducible nitric oxide synthase gene. Mol Cell Biol. 20:4699–4707.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chung SW, Kang BY, Kim SH, et al: Oxidized
low density lipoprotein inhibits interleukin-12 production in
lipopolysaccharide-activated mouse macrophages via direct
interactions between peroxisome proliferator-activated
receptor-gamma and nuclear factor-kappa B. J Biol Chem.
275:32681–32687. 2000. View Article : Google Scholar
|
|
21
|
Gao Z, He Q, Peng B, Chiao PJ and Ye J:
Regulation of nuclear translocation of HDAC3 by IkappaBalpha is
required for tumor necrosis factor inhibition of peroxisome
proliferator-activated receptor gamma function. J Biol Chem.
281:4540–4547. 2006. View Article : Google Scholar
|
|
22
|
Ruan H, Hacohen N, Golub TR, Van Parijs L
and Lodish HF: Tumor necrosis factor-alpha suppresses
adipocyte-specific genes and activates expression of preadipocyte
genes in 3T3-L1 adipocytes: nuclear factor-kappaB activation by
TNF-alpha is obligatory. Diabetes. 51:1319–1336. 2002. View Article : Google Scholar
|
|
23
|
Camp HS, Tafuri SR and Leff T: c-Jun
N-terminal kinase phosphorylates peroxisome proliferator-activated
receptor-gamma1 and negatively regulates its transcriptional
activity. Endocrinology. 140:392–397. 1999.
|
|
24
|
Delerive P, Fruchart JC and Staels B:
Peroxisome proliferator-activated receptors in inflammation
control. J Endocrinol. 169:453–459. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moraes LA, Piqueras L and Bishop-Bailey D:
Peroxisome proliferator-activated receptors and inflammation.
Pharmacol Ther. 110:371–385. 2006. View Article : Google Scholar
|
|
26
|
Houseknecht KL, Cole BM and Steele PJ:
Peroxisome proliferator-activated receptor gamma (PPARgamma) and
its ligands: a review. Domest Anim Endocrinol. 22:1–23. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xin X, Yang S, Kowalski J and Gerritsen
ME: Peroxisome proliferator-activated receptor gamma ligands are
potent inhibitors of angiogenesis in vitro and in vivo. J Biol
Chem. 274:9116–9121. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Murata T, Hata Y, Ishibashi T, et al:
Response of experimental retinal neovascularization to
thiazolidinediones. Arch Ophthalmol. 119:709–717. 2001. View Article : Google Scholar : PubMed/NCBI
|