Introduction
Skin flap grafting is a form of transplantation
widely used in plastic surgery. However, ischemia/reperfusion (I/R)
injury is the main factor which reduces the survival rate of flaps
following grafting. In addition, skin necrosis may occur following
surgery (1–4). Studies (5,6) have
previously reported that hyperbaric oxygen preconditioning (HBO-PC)
may induce ischemia tolerance, which provides protection against
subsequent I/R injury. One of the first studies to examine the
effects of HBO-PC on I/R injury was conducted on the gerbil
hippocampus (7) followed by the
small intestine, testes and liver. Most recently, Wang et al
(8) designed a series of
experiments to confirm that HBO-PC reduces cerebral I/R injury by
stimulating autophagy in neurocytes. These studies suggest that
HBO-PC may potently protect against subsequent I/R injury in a
variety of tissues and organs. However, whether HBO-PC protects
grafted skin flaps against I/R injury, as well as the underlying
mechanisms, remains unknown.
High mobility group box 1 (HMGB1) and nuclear
factor-κ B (NF-κB) are two important inflammatory response
mediators involved in I/R pathogenesis. HMGB1 mediates cytokine
release and inflammation, and causes tissue damage in I/R injury by
activating innate immunity via the NF-κB-activated signal
transduction pathway (9–11). NF-κB is a transcriptional factor
that regulates the expression of pro-inflammatory cytokines,
including interleukin (IL)-1, IL-6, inducible nitric oxide synthase
(iNOS) and tumor necrosis factor (TNF). NF-κB is well known to
exhibit a critical role in cell survival (12).
In the current study, the hypothesis that
preconditioning rats with HBO-PC protects grafted skin flaps
against subsequent I/R injury was investigated. In addition, the
precise roles of two important inflammatory factors, HMGB1 and
NF-κB, in this protective process were characterized.
Materials and methods
Experimental animals
All experiments were performed in accordance with
the established ethical guidelines of the Committee for the Control
and Supervision of Experiments on Animals at Capital Medical
University (Beijing, China). Healthy adult male Sprague-Dawley rats
(250–300 g; Capital Medical University) were maintained at 25±1.0°C
in a 12/12 h light/dark cycle and allowed food and water ad
libitum.
Experimental groups
A total of 40 rats were randomly assigned to the
following five groups: Sham surgery [SH; 21% O2 at 1.0
atmosphere absolute (ATA)] (n=8); ischemia followed by reperfusion
3 days following surgery (I/R3d; 21% O2 at 1.0 ATA;
n=8); ischemia followed by reperfusion 5 days following surgery
(I/R5d; 21% O2 at 1.0 ATA; n=8); hyperbaric oxygen
preconditioning and ischemia followed by reperfusion 3 days
following surgery (HBO-PC+3d; 100% O2 at 2.0 ATA; n=8);
and hyperbaric oxygen preconditioning and ischemia followed by
reperfusion 5 days following surgery (HBO-PC+5d; 100% O2
at 2.0 ATA; n=8).
Epigastric pedicle skin flap model
All procedures were performed aseptically under
anesthesia using intraperitoneal injections of 10% chloral hydrate
(350 mg/kg). Following shaving and washing of the abdomen, the rats
were fixed on wooden shelves. Single inferior epigastric vessel
pedicled skin flaps were designed and elevated (9×6 cm). The right
inferior epigastric artery and vein pedicle were skeletonized,
while the contralateral inferior epigastric vessel was ligated by
suturing, and the feeding vessels of the skin flaps were clamped
using a microvascular clamp to achieve ischemia. For reperfusion,
the microvascular clamp was removed 3 h later, restoring blood
flow. The flaps were repositioned above a silicone sheet of the
same dimensions as the flap with continuous 5–0 monofilament nylon
sutures to block vascular supply other than that from the pedicle.
The SH group underwent the same surgery but was not subjected to
ischemia. All rats received a single intramuscular injection of
penicillin sodium (0.8 mg/g) postoperatively.
Hyperbaric oxygen preconditioning
The rats in the HBO-PC+3d and HBO-PC+5d groups were
placed into a custom-made transparent acrylic plastic pressure
chamber (701 Space Research Institute, Beijing, China) immediately
following surgery and received 1 h HBO therapy at 2.0 ATA with 100%
O2 twice per day (at 8 h intervals) for 3 consecutive
days. Compressed air was supplied at 1 kg/cm2/min to 2.0
ATA/100% oxygen and maintained for 1 h. The chamber was flushed
with 100% oxygen at 5 l/min to avoid carbon dioxide accumulation.
Decompression was performed at 0.2 kg/cm2/min. During
HBO exposure, the oxygen and carbon dioxide contents were monitored
continuously and maintained at ≥98 and ≤0.03%, respectively. The
chamber temperature was maintained between 22 and 25°C. To minimize
the effects of diurnal variation, all HBO exposures began at ~8:00
AM and 4:00 PM. The rats in the SH, I/R3d and I/R5d groups were
treated postoperatively with normobaric air at 1.0 ATA in 21%
oxygen at an ambient temperature of 22–25°C.
Laser Doppler perfusion imaging
(LDPI)
The blood flow in the pedicled skin flaps on the
epigastric zone was mapped with a high-resolution PIM-2 LDPI
(Perimed Inc., North Royalton, OH, USA) with a scanning area of
70×80 mm, a high resolution, and a distance of 42.3 cm between the
scanner head and the wound. The images and perfusion values were
analyzed using the LDISOFT software package, version 1.5 (Perimed
AB, Stockholm, Sweden).
Histological analysis
Each flap was evaluated 3 and 5 days
postoperatively. For each flap, 3–4 μm tissue blocks sectioned from
the viable region were fixed in a standard manner in 10% formalin
and embedded in paraffin for hematoxylin-eosin staining. Images
were subsequently captured using an Olympus BX51 microscope
(Olympus Optical Co., Tokyo, Japan) with a ×40 objective. According
to the Zdichavsky et al (13) score for skin injury and Rongione
et al (14) histological
score for acute pancreatitis, the degree of microscopic injury was
scored on the basis of the following histological changes:
Congestion, epidermal edema and leukocyte infiltration. The
severity of injury was graded for each variable as follows: No
injury, 0; 1, injury ≤25% of the field; 2, injury >25% to ≤55%
of the field; 3, injury >55% to ≤75% of the field; 4, diffuse
injury. All evaluations were performed in a double-blinded
manner.
Immunohistochemical staining
Histological sections of tissue (3–4 μm thick) were
obtained, fixed in 10% formalin, and embedded in paraffin. The
sections were deparaffinized in xylene and rehydrated in ethanol,
and endogenous peroxidase was blocked by immersion in methanol
containing 0.3% hydrogen peroxidase for 20 min. Prior to
incubation, the sections were permeabilized and blocked with normal
goat serum. The sections were incubated overnight at 4°C with
primary rat monoclonal antibodies (Histostain-Plus kit; Sunbio,
Beijing, China). On the following day, the sections were incubated
with secondary antibodies and horseradish peroxidase enzyme markers
for 10–15 min followed by staining with diaminobenzidine. The
slides were examined using a Nikon i50 microscope. The proportion
of positively stained cells was calculated as the number of
positive cells divided by the total number of cells.
Protein preparation
The flap tissues were frozen in liquid nitrogen and
stored at −80°C until use. The tissues were homogenized in ice-cold
isolation solution containing 250 mmol/l sucrose, 10 mmol/l
triethanolamine, 1 μg/ml leupeptin and 0.1 mg/ml
phenylmethylsulfonyl fluoride. The homogenates were centrifuged at
15,000 × g for 10 min at 4°C to separate the incompletely
homogenized tissue. The supernatants were obtained and the protein
concentrations were measured using a protein assay kit (Sunbio,
Beijing, China). An N-glycosidase F Deglycosylation kit
(Roche Diagnostics GmbH, Mannheim, Germany) was used to
deglycosylate the proteins.
Western blotting
Total proteins (50 μg/sample) were diluted in 5X
loading buffer [0.25 mol/l Tris HCl (pH 6.8), 10% sodium dodecyl
sulfate (SDS), 0.5% bromophenol blue, 50% glycerol and 0.5 mol/l
dithiothreitol] and boiled for 5 min. SDS-polyacrylamide gel
electrophoresis (PAGE) was performed on 12% gradient gels. The
proteins were transferred electrophoretically to polyvinylidene
difluoride membranes (Millipore, Billerica, MA, USA) pre-treated
with methanol and blocked for 1 h at room temperature in
Tris-buffered saline with 0.1% Tween-20 containing 5% non-fat dry
milk (TBS-T). They were subsequently incubated overnight at 4°C
with anti-HMGB1 antibody (1:100) and anti-NF-κB antibody (1:500;
both from Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TBS-T
containing 5% non-fat dry milk. Following washing in TBS-T, the
membranes were incubated with horseradish peroxidase-labeled
anti-rabbit antibody (1:3,000; Santa Cruz Biotechnology) for 2–3 h
at room temperature. The blots were developed with enhanced
chemiluminescence agents (ECL Plus; Sunbio) prior to exposure to
X-rays. To confirm equivalent loading of the samples, the same
membranes were incubated with anti-β-actin antibody (1:300; Santa
Cruz Biotechnology) and visualized by enhanced chemiluminescence as
described above. For quantification, films of the western blot
analysis were scanned using a Minolta scanner (Konica Minolta,
Inc., Tokyo, Japan) and Adobe Photoshop software. The labeling
density was quantitated using Lab Works software (UVP, Upland, CA,
USA). The values of the relative densities of HMGB1 and NF-κB bands
were normalized to the density of actin to represent the quantity
of HMGB1 and NF-κB protein, respectively.
Statistical analysis
Statistical analysis was performed using SPSS
version 15.0 (SPSS Inc., Chicago, IL, USA). All quantitative data
are expressed as the mean ± standard deviation. A one-way analysis
of variance was used to test the significance of differences in
HMGB1 and NF-κB western blot analysis, and survival area. The
associations between skin injury scores, and the expression of
HMGB1 and NF-κB were analyzed by calculating Pearson product-moment
correlation coefficients. P<0.05 was considered to indicate a
statistically significant difference.
Results
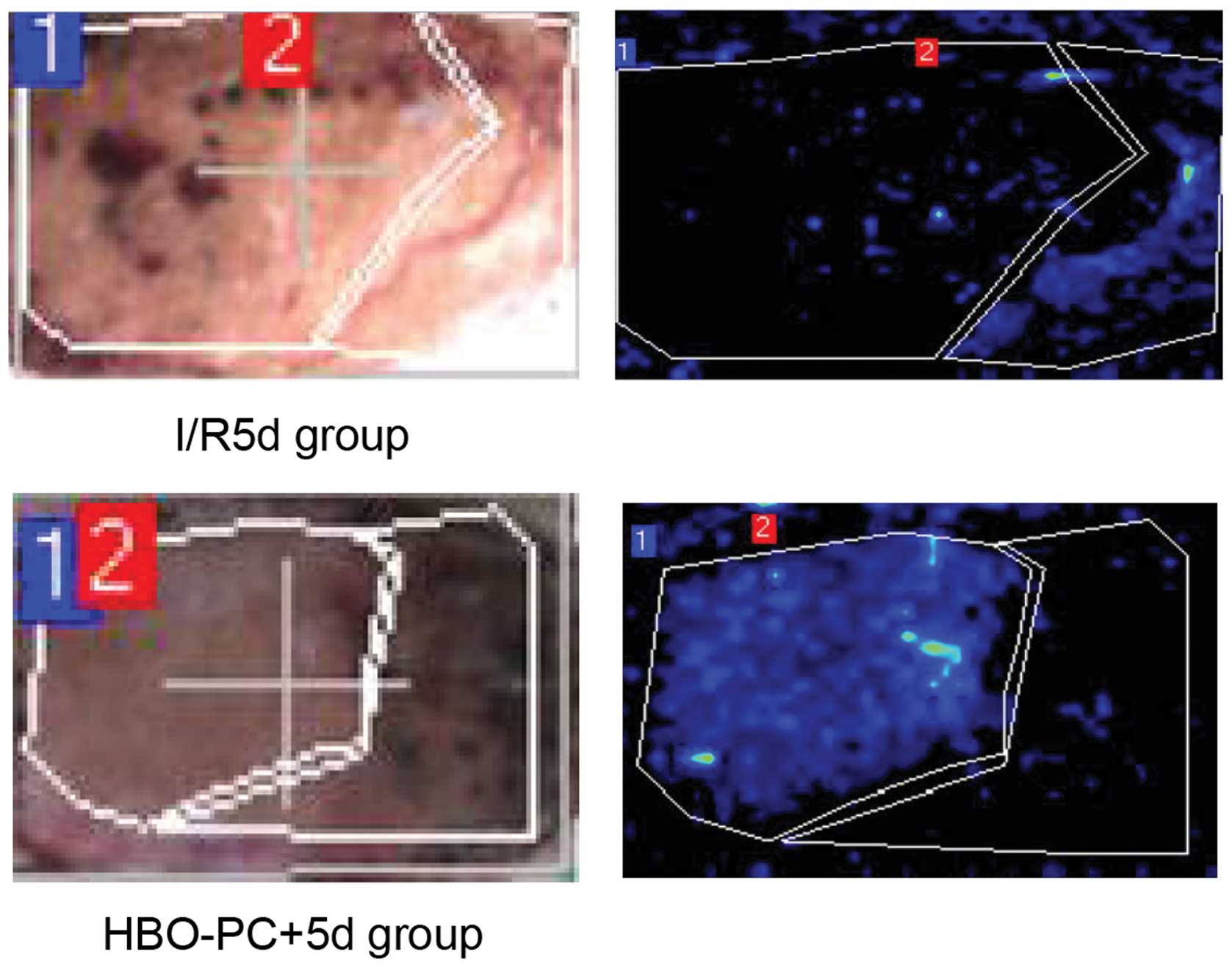
HBO-PC increases blood perfusion in
epigastric skin flaps
LDPI is a noninvasive and relatively new technique
for measuring skin blood perfusion (15). LPDI was initially performed 5 days
following skin grafting to detect blood perfusion. As shown in
Fig. 1, HBO-PC increased blood
perfusion (dark blue) compared with the groups with I/R alone
(P<0.05). Notably, the LDPI data were consistent with the
histological data, which indicated that HBO-PC protects against I/R
injury during epigastric skin flap grafting.
HBO-PC attenuates I/R injury following
skin flap grafting
Following grafting, the skin flaps from the I/R
groups exhibited typical edema, effusion and necrosis, and were
dark purple in color. However, the skin flaps from the HBO-PC
groups exhibited a low level of effusion demonstrated by pink skin
flap coloration. Histological examination of the skin flap sections
from the I/R groups showed significant edema and congestion
(Fig. 2). The injury scores, which
were calculated from congestion, epidermis edema and neutrophil
infiltration, were significantly higher in the I/R groups compared
with the SH group. However, the injury scores of the HBO-PC groups
were significantly lower compared with the I/R groups. These data
indicate that HBO-PC may attenuate I/R injury during skin flap
grafting.
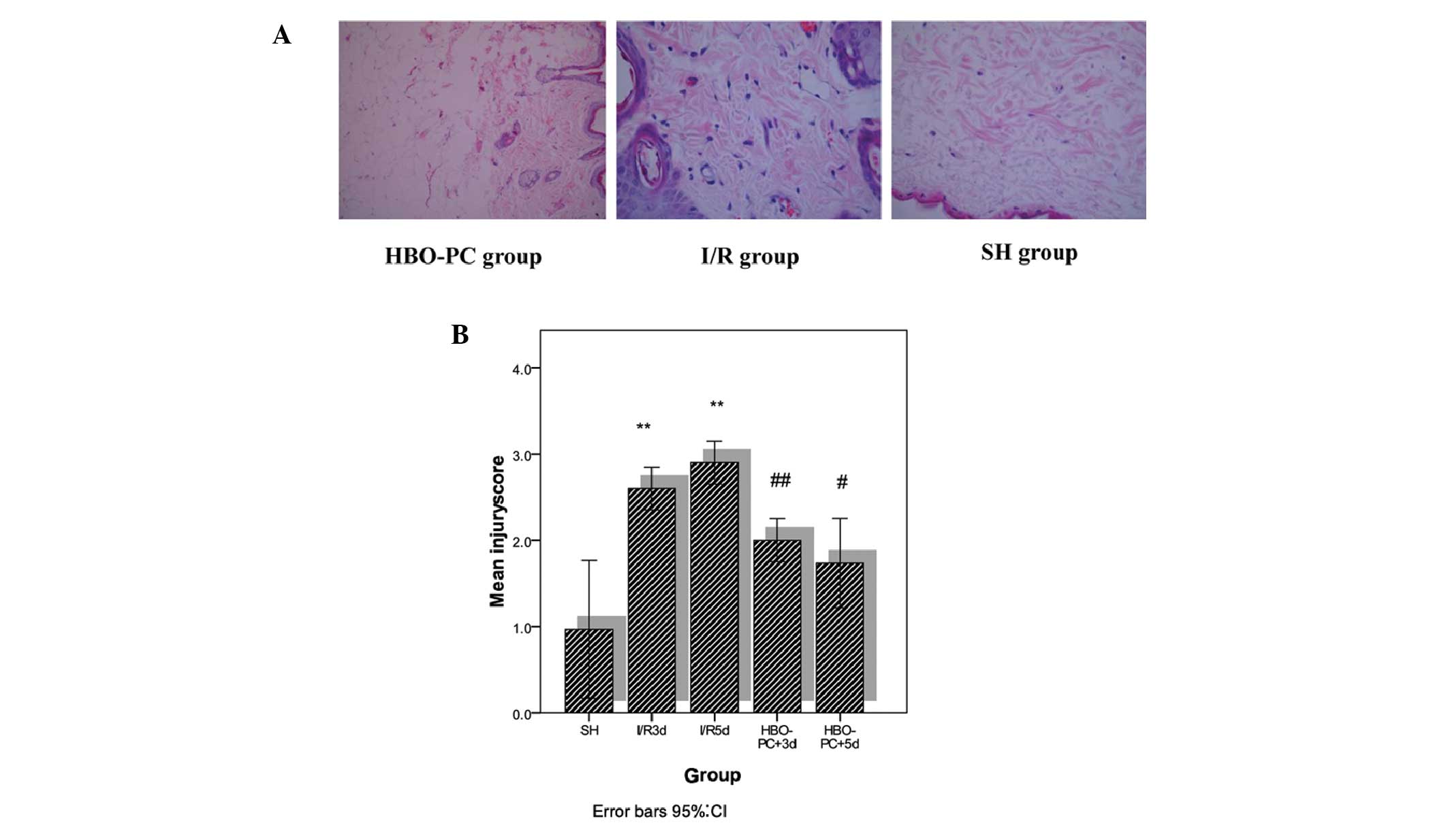 | Figure 2(A) Representative skin flap
microscopic images: HBO-PC group rats (left), I/R group rats
(middle) and SH group rats (right). (B) Injury scores of the SH
group (n=8), I/R3d group (n=8), I/R5d group (n=8), HBO-PC+3d group
(n=8), and HBO-PC+5d group (n=8). Data are presented as the mean ±
standard deviation **P<0.01 for SH versus I/R groups.
#P<0.01 for HBO-PC+5d group versus I/R5d group,
##P<0.05 for HBO-PC+3d group versus I/R3d group. SH,
sham surgery; I/R3d, ischemia followed by reperfusion 3 days
following surgery; I/R5d, ischemia followed by reperfusion 5 days
following surgery; HBO-PC+3d, hyperbaric oxygen preconditioning and
ischemia followed by reperfusion 3 days following surgery;
HBO-PC+5d, hyperbaric oxygen preconditioning and ischemia followed
by reperfusion 5 days following surgery. |
HBO-PC suppresses HMGB1 and NF-κB
expression in skin flaps
The potential mechanisms underlying the protective
effect of HBO-PC were investigated. The expression of HMGB1 and
NF-κB were examined by western blotting (Fig. 3) and immunohistochemical staining
(Figs. 4 and 5).
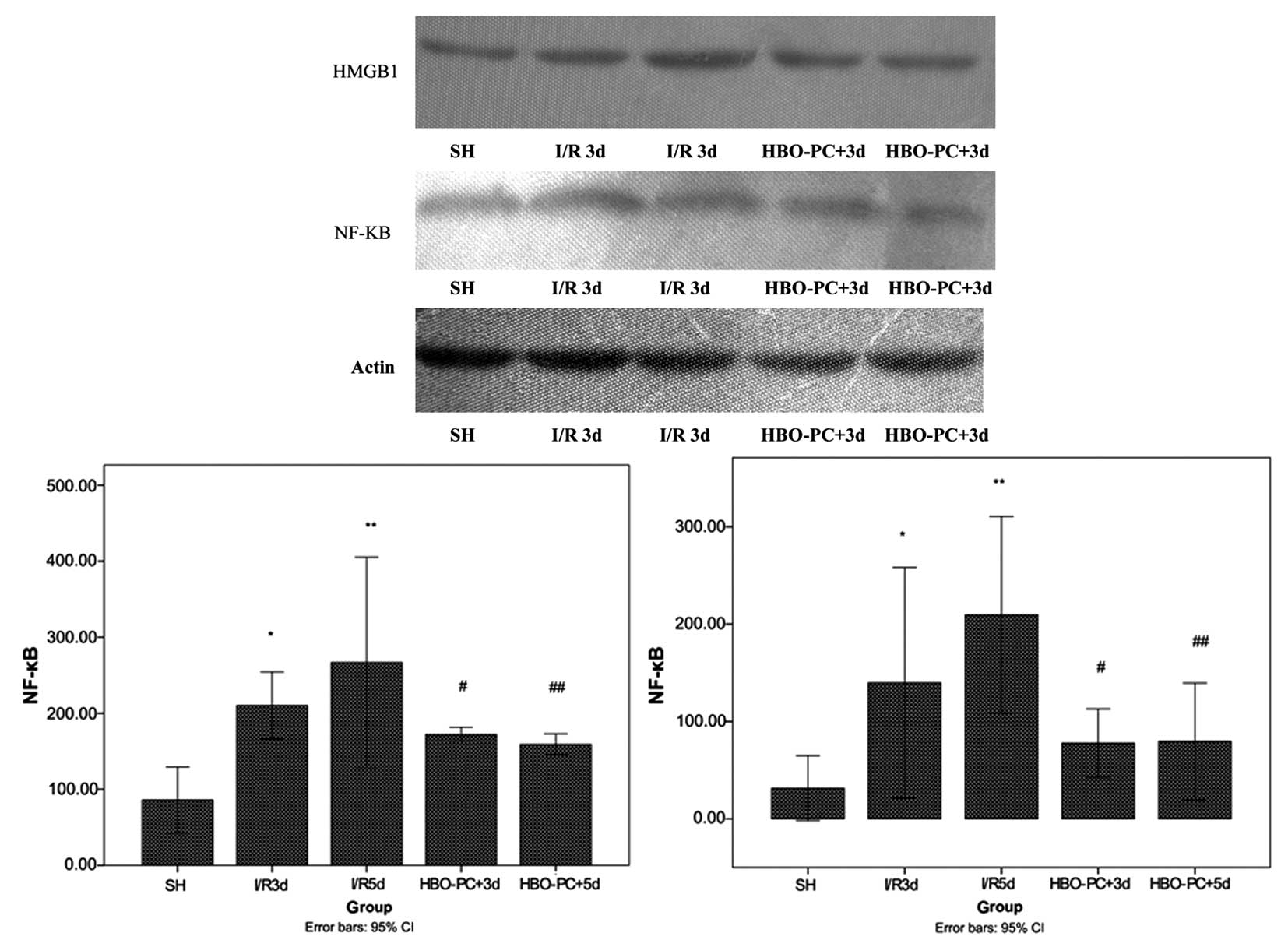 | Figure 3Expression of HMGB1 and NF-κB protein
in the SH group (n=7), I/R groups [by I/R3d and I/R5d groups, n=7],
and HBO-PC groups (HBO-PC+3d and HBO-PC+5d groups, n=7). Data are
presented as the mean ± standard deviation. **P<0.01
for the SH group vs. the I/R5d group, *P<0.05 for the
SH group vs. the I/R3d group, #P<0.05 for the I/R3d
group vs. the HBO-PC+3d group, ##P<0.01 for the I/R5d
group vs. the HBO-PC+5d group. HMGB1, high mobility group protein
1; NF-κB, nuclear factor-κ B; .SH, sham surgery; I/R3d, ischemia
followed by reperfusion 3 days following surgery; I/R5d, ischemia
followed by reperfusion 5 days following surgery; HBO-PC+3d,
hyperbaric oxygen preconditioning and ischemia followed by
reperfusion 3 days following surgery; HBO-PC+5d, hyperbaric oxygen
preconditioning and ischemia followed by reperfusion 5 days
following surgery. |
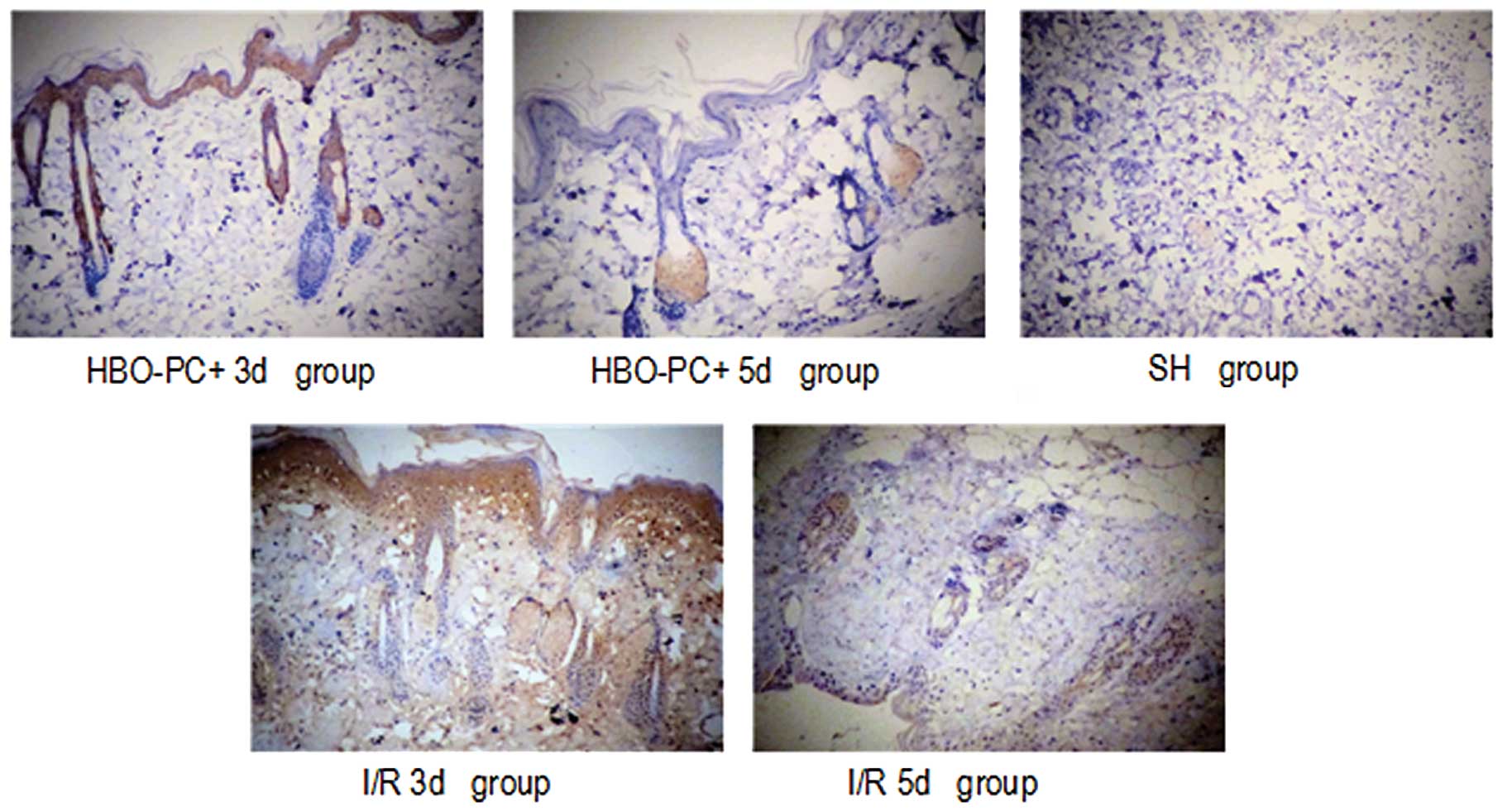
HMGB1-positive cells were rarely observed in the
skin flap tissue in the SH group. By contrast, there were
significantly more HMGB1-positive cells in the ischemic skin flaps
in the I/R groups. The percentages of positive staining in the SH
group, and the I/R3d and I/R5d were 7.8, 34.5 and 42.3%,
respectively. However, HBO-PC significantly attenuated the I/R
injury-elicited increase in HMGB1 expression (P<0.01). The
percentages of positive staining in the HBO-PC+3d and HBO-PC+5d
groups were 31.6 and 28.2%, respectively. There was no significant
difference in the numbers of HMGB1-positive cells between the
HBO-PC+3d and HBO-PC+5d groups (P>0.05).
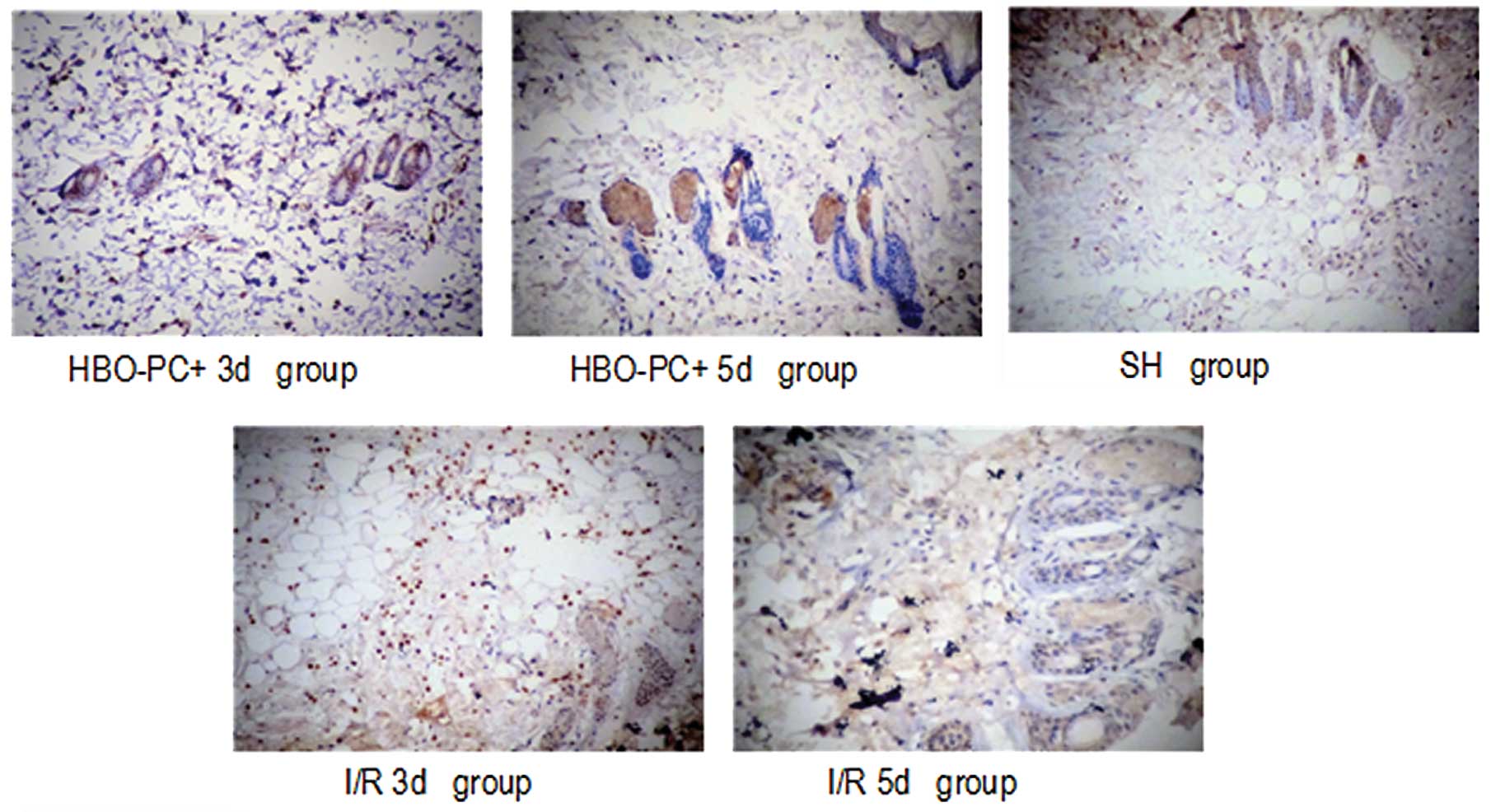
The percentages of positive staining for NF-κB in
the I/R3d, I/R5d, HBO-PC+3d, and HBO-PC+5d groups were 45.2, 57.9,
37.6 and 31.2%, respectively. Thus, HBO-PC significantly attenuated
NF-κB expression (P<0.01).
As shown in Figs. 4
and 5, the expression of HMGB1 and
NF-κB were distributed in the cytoplasm and nucleus in the I/R
groups and primarily in the cytoplasm following HBO-PC
treatment.
Western blotting was performed to further verify the
expression of these two proteins. Concordant with the results of
immunohistochemical staining, HBO-PC significantly decreased the
expression of HMGB1 and NF-κB proteins compared with their
expression in I/R injury following skin flap grafting (P<0.01;
Fig. 3).
Discussion
I/R injury is a severe injury that occurs when
circulation is re-established following ischemia in skin flap graft
surgery. It induces a cascade of pathophysiological changes,
including neutrophil influx, interstitial edema and increased
permeability, resulting in skin flap necrosis or graft loss
(16,17). In the current study, to induce I/R
injury, inferior epigastric vessel pedicled skin flaps were
designed in which the feeding vessels of the skin flaps were
clamped and removed 3 h later. I/R-induced reactive oxygen species
(ROS) initiate subsequent injury (18,19).
The formation of ROS contributes to the activation of adhesion
molecules, which leads to leukocyte infiltration (20) and causes a series of changes that
impair microcirculation (21,22).
Accumulating evidence has demonstrated that HBO-PC
is a promising strategy for protecting cells against I/R injury
(23–25). HBO-PC increases the levels of
antioxidants, including GSH and superoxide dismutase (SOD) to
prevent ROS-induced lipid peroxidation in membranes (26). Li et al (27) further confirmed that HBO-PC
improves ROS scavenging. It was hypothesized that an endogenous
antioxidative protective pathway is initiated through HBO-PC when
patients are exposed to ROS during cardiopulmonary bypass surgery.
However, the molecular mechanism of HBO-PC remains unknown. ROS are
an initiating factor, while the ROS scavenging ability of HBO-PC
may inhibit the interactions between specific I/R-associated
mediators and their corresponding receptors. Thus, in the current
study, it was hypothesized that HBO-PC attenuates I/R injury
following skin flap grafting by mediating two important
inflammatory mediators, HMGB1 and NF-κB, which inhibits the
subsequent inflammatory reaction.
HMGB1 is a highly conserved non-histone DNA-binding
protein that stabilizes DNA and is crucial in the inflammatory
response during I/R injury (28).
HMGB1 may be passively released from necrotic and damaged cells or
actively secreted from macrophages and monocytes (29–32).
Previous reports demonstrated that HBO treatment is appropriate for
decreasing HMGB1 levels in patients with cerebral injury (33). In the present study, the elevated
expression of HMGB1 protein in the skin flap tissue of the I/R
groups was attenuated by HBO-PC. The majority of samples from the
I/R groups showed marked HMGB1 cytoplasmic staining. Thus, it was
hypothesized that HBO-PC protects membrane proteins from lipid
peroxidation through ROS scavenging. The I/R-elicited ROS may
promote HMGB1 translocation from the nucleus to the cytoplasm as
well as its release into the extracellular space. However, this
pathway is blocked by HBO-PC through the removal of ROS, which
terminated I/R-induced cell necrosis and macrophage activation.
As a transcription factor, NF-κB controls the
expression of pro-inflammatory proteins. Under normal conditions,
NF-κB is in an inactive state and mainly located in the cytoplasm.
Activated NF-κB translocates from the cytoplasm to the nucleus
through nuclear pores. NF-κB subsequently combines with the K
structure area of target genes, initiating transcription of the
corresponding genes encoding inflammatory mediators, including
tumor necrosis factor and interleukin-6 (34–36).
Concordant with the results reagrding HMGB1, the expression of
NF-κB in the I/R group was higher compared with that in the SH
group. These changes in NF-κB expression paralleled those of HMGB1
expression, suggesting a possible association between HMGB1 and
NF-κB activation in the I/R process following skin flap grafting.
Wang et al (37) reported
that tanshinone IIA effectively decreases tissue injury under
cerebral ischemic conditions through the downregulation of the
NF-κB activation pathway. The current data suggest that HBO-PC
inhibits the release of HMGB1 into the extracellular space, and is
likely to prevent HMGB1 from binding to cell-surface receptors,
including toll-like receptor 4 (TLR4) and blocking MyD88-dependent
transduction pathways. The inactivation of the TLR4 pathway
inhibits the translocation and DNA binding activity of NF-κB,
consequently decreasing pro-inflammatory cytokine production.
Immunochemical analysis revealed that NF-κB expression in the
HBO-PC group was significantly reduced in the cytoplasm and
nucleus. In agreement with this hypothesis, a previous study
demonstrated that the expression of TLR4 and NF-κB protein is
increased by I/R injury and downregulated by ischemic
preconditioning through the mediation of the TLR4/NF-κB pathway
(38).
In the present study, LDPI was used to monitor skin
blood perfusion in different experimental groups. Skin blood flow
to surface tissue was significantly higher in the HBO-PC groups
compared with the I/R groups. Therefore, this technique provides
invaluable evidence demonstrating that HBO-PC can improve
microcirculation of skin flap grafts.
On the basis of the results of the current study, it
was concluded that HBO-PC effectively promotes the survival of skin
flap grafts by eliciting an endogenous protective mechanism. HBO-PC
reduces HMGB1 and NF-κB expression in skin flaps, suggesting that
the inhibition of HMGB1 and NF-κB expression is associated with
blocking the TLR4/NF-κB pathway during I/R injury. In addition,
HBO-PC inhibits the inflammatory response at the early stage of I/R
injury, providing an attractive therapeutic avenue for skin graft
surgery.
References
|
1
|
Kerrigan CL and Stotland MA: Ischemia
reperfusion injury: a review. Microsurgery. 14:165–175. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siemionow M and Arslan E:
Ischemia/reperfusion injury: a review in relation to free tissue
transfers. Microsurgery. 24:468–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yoshida WB and Campos EB: Ischemia and
reperfusion in skin flaps: effects of mannitol and vitamin C in
reducing necrosis area in a rat experimental model. Acta Cir Bras.
20:358–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo YR, Wang FS, Jeng SF, Huang HC, Wei FC
and Yang KD: Nitrosoglutathione modulation of platelet activation
and nitric oxide synthase expression in promotion of flap survival
after ischemia/reperfusion injury. J Surg Res. 119:92–99. 2004.
View Article : Google Scholar
|
|
5
|
Xiong LZ, Zhu ZH, Dong HL, Hu W, Hou L and
Chen S: Hyperbaric oxygen preconditioning induces neuroprotection
against transient not permanent middle cerebral artery occlusion
rat model. Chin Med J (Engl). 113:836–839. 2000.
|
|
6
|
Dong H, Xiong L, Zhu Z, Chen S, Hou L and
Sakabe T: Preconditioning with hyperbaric oxygen and hyperoxia
induces tolerance against spinal cord ischemia in rabbits.
Anesthesiology. 96:907–912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wada K, Miyazawa T, Nomura N, Tsuzuki N,
Nawashiro H and Shima K: Preferential conditions for and possible
mechanisms of induction of ischemic tolerance by repeated
hyperbaric oxygenation in gerbil hippocampus. Neurosurgery.
49:160–167. 2001.PubMed/NCBI
|
|
8
|
Wang YC, Zhang S, Du TY, Wang B and Sun
XQ: Hyperbaric oxygen preconditioning reduces ischemia-reperfusion
injury by stimulating autophagy in neurocyte. Brain Res.
1323:149–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang H, Hreggvidsdottir HS, Palmblad K,
Wang H, Ochani M, Li J, et al: A critical cysteine is required for
HMGB1 binding to Toll-like receptor 4 and activation of macrophage
cytokine release. Proc Natl Acad Sci USA. 107:11942–11947. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pavare J, Grope I, Kalnins I and Gardovska
D: High-mobility group box-1 protein, lipopolysaccharide-binding
protein, interleukin-6 and C-reactive protein in children with
community acquired infections and bacteraemia: a prospective study.
BMC Infect Dis. 10:282010. View Article : Google Scholar
|
|
11
|
Lotze MT, Zeh HJ, Rubartelli A, Sparvero
LJ, Amoscato AA, Washburn NR, et al: The grateful dead:
damage-associated molecular pattern molecules and
reduction/oxidation regulate immunity. Immunol Rev. 220:60–81.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Q and Verma IM: NF-kappaB regulation in
the immune system. Nat Rev Immunol. 2:725–734. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zdichavsky M, Jones JW, Ustuner ET, Ren X,
Edelstein J, Maldonado C, et al: Scoring of skin rejection in a
swine composite tissue allograft model. J Surg Res. 85:1–8. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rongione AJ, Kusske AM, Kwan K, Ashley SW,
Reber HA and McFadden DW: Interleukin 10 reduces the severity of
acute pancreatitis in rats. Gastroenterology. 112:960–967. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosato E, Borghese F, Pisarri S and
Salsano F: Laser Doppler perfusion imaging is useful in the study
of Raynaud’s phenomenon and improves the capillaroscopic diagnosis.
J Rheumatol. 36:2257–2263. 2009.PubMed/NCBI
|
|
16
|
Wong HP, Zamboni WA and Stephenson LL:
Effect of hyperbaric oxygen on skeletal muscle necrosis following
primary and secondary ischemia in a rat model. Surg Forum.
47:705–707. 1996.
|
|
17
|
Elktzschig HK and Collard CD: Vascular
ischemia and reperfusion injury. Br Med Bull. 70:71–86. 2004.
View Article : Google Scholar
|
|
18
|
Aydogan H, Gurlek A, Parlakpinar H, Askar
I, Bay-Karabulut A, Aydogan N, et al: Beneficial effects of caffeic
acid phenethyl ester (CAPE) on the ischaemia-reperfusion injury in
rat skin flaps. J Plast Reconstr Aesthet Surg. 60:563–568. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng GM, Yang WG, Huan-Tang CS, Chu YM,
Tsai LM, Chang TM, et al: Periodic alterations of jejunal mucosa
morphology following free microvascular transfer for
pharyngoesophageal reconstruction. J Plast Reconstr Aesthet Surg.
59:1312–1317. 2006. View Article : Google Scholar
|
|
20
|
Cetin C, Köse AA, Aral E, Colak O, Erçel
C, Karabağli Y, et al: Protective effect of fucoidin (a neutrophil
rolling inhibitor) on ischemia reperfusion injury: experimental
study in rat epigastric island flaps. Ann Plast Surg. 47:540–546.
2001. View Article : Google Scholar
|
|
21
|
Tomur A, Etlik O and Gundogan NU:
Hyperbaric oxygenation and antioxidant vitamin combination reduces
ischemia-reperfusion injury in a rat epigastric island skin-flap
model. J Basic Clin Physiol Pharmacol. 16:275–285. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reichenberger MA, Heimer S, Schaefer A,
Lass U, Gebhard MM, Germann G, et al: Adipose derived stem cells
protect skin flaps against ischemia-reperfusion injury. Stem Cell
Rev. 8:854–862. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim CH, Choi H, Chun YS, Kim GT, Park JW
and Kim MS: Hyperbaric oxygenation pretreatment induces catalase
and reduces infarct size in ischemic rat myocardium. Pflugers Arch.
442:519–525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Prass K, Wiegand F, Schumann P, Ahrens M,
Kapinya K, Harms C, et al: Hyperbaric oxygenation induced tolerance
against focal cerebral ischemia in mice is strain dependent. Brain
Res. 871:146–150. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamada T, Taguchi T, Hirata Y, Suita S and
Yagi H: The protective effect of hyperbaric oxygenation on the
small intestine in ischemia-reperfusion injury. J Pediatr Surg.
30:786–790. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu SY, Chiu JH, Yang SD, Yu HY, Hsieh CC,
Chen PJ, et al: Preconditioned hyperbaric oxygenation protects the
liver against ischemia-reperfusion injury in rats. J Surg Res.
128:28–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Dong H, Chen M, Liu J, Yang L, Chen
S, et al: Preconditioning with repeated hyperbaric oxygen induces
myocardial and cerebral protection in patients undergoing coronary
artery bypass graft surgery: a prospective, randomized, controlled
clinical trial. J Cardiothorac Vasc Anesth. 25:908–916. 2011.
View Article : Google Scholar
|
|
28
|
Andrassy M, Volz HC, Igwe JC, Funke B,
Eichberger SN, Kaya Z, et al: High-mobility group box-1 in
ischemia-reperfusion injury of the heart. Circulation.
117:3216–3226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tsung A, Sahai R, Tanaka H, Nakao A, Fink
MP, Lotze MT, et al: The nuclear factor HMGB1 mediates hepatic
injury after murine liver ischemia-reperfusion. J Exp Med.
201:1135–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Scaffidi P, Misteli T and Bianchi ME:
Release of chromatin protein HMGB1 by necrotic cells triggers
inflammation. Nature. 418:191–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, et al: HMG-1 as a late mediator of endotoxin
lethality in mice. Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andersson U, Wang H, Palmblad K, Aveberger
AC, Bloom O, Erlandsson-Harris H, et al: High mobility group 1
protein (HMG-1) stimulates proinflammatory cytokine synthesis in
human monocytes. J Exp Med. 192:565–570. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duan XF, Huang YM, Zhou YQ, Xu JJ and
Zhang Q: Study of hyperbaric oxygenation on serum resistin and
HMGB1 in patients with severe craniocerebral injury. Chin J
Clinicians. 5:3189–3192. 2011.
|
|
34
|
Chen YQ, Liu DH, Yang GT, et al:
Expression of nuclear factor kappa B in rats after acute global
cerebral ischemia-reperfusion and its significance. J Clin Res.
121:772–774. 2004.
|
|
35
|
Qin C, Xiao YB, Zhong QJ, Chen L and Wang
XF: Anti-inflammatory effect of erythropoietin pretreatment on
cardiomyocytes with hypoxia/reoxygenation injury and the possible
mechanism. Chin J Traumatol. 11:352–358. 2008.PubMed/NCBI
|
|
36
|
Ghosh S and Baltimore D: Activation in
vitro of NF-kappa B by phosphorylation of its inhibitor I kappa B.
Nature. 344:678–682. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Zhang X, Liu L, Cui L, Yang R, Li
M, et al: Tanshinone II A down-regulates HMGB1, RAGE, TLR4,
NF-kappaB expression, ameliorates BBB permeability and endothelial
cell function, and protects rat brains against focal ischemia.
Brain Res. 1321:143–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang J, Li Y and Hu C: Ischemic
preconditioning protects against myocardial ischemia-reperfusion
injury through inhibiting toll-like receptor 4/NF-κB signaling
pathway in rats. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 36:972–978.
2011.PubMed/NCBI
|