Introduction
Non-small cell lung cancer (NSCLC) is the most
common type of lung cancer accounting for 80–85% of all lung cancer
cases (1). At present, traditional
cytotoxic chemotherapy has reached a therapeutic plateau with
limited survival benefits for advanced NSCLC patients and novel
combinations of available cytotoxic agents are unlikely to confer
clinically relevant survival improvement (2).
Treatment with targeted agents has improved
progression-free and overall survival in patients with a variety of
tumors, including NSCLC. Gefitinib and erlotinib, as certain
epidermal growth factor receptor tyrosine kinase inhibitors
(EGFR-TKIs) have been approved as second-line treatments for lung
cancer (3).
Marked response rates of EGFR-TKIs are associated
with activating mutations in the EGFR gene (4). Despite this patients with K-ras
mutations have been shown to confer primary resistance to gefitinib
and erlotinib therapy (5). In
addition, patients with EGFR-mutant tumors who initially respond to
treatment with EGFR-TKIs are likely to develop progressive disease
following one year of EGFR-TKI treatment, a hypothesis referred to
clinically as acquired resistance (6). Regardless of considerable efforts for
the improvement in diagnosis and treatment of lung cancer, the
majority of patients present at an advanced stage and prognosis
remains poor, with an overall 5-year survival probability of ~15%
(7,8). Therefore, there is a requirement to
identify novel treatment strategies to improve the outcomes of
patients with lung cancer.
Docetaxel is a well-established anticancer agent and
a member of the taxoid family. It is a mitotic inhibitor that
promotes the assembly of microtubules from tubulin dimers and
stabilizes microtubules by preventing depolymerization (9). In this manner, docetaxel specifically
arrests the cell cycle at the S or G2/M phase and induces apoptosis
in tumor cells (10).
At present, there is interest in assessing the
efficacy of EGFR-TKIs administered in combination with cytotoxic
chemotherapeutic agents. Gefitinib is the first EGFR tyrosine
kinase inhibitor that has been shown to be an effective monotherapy
for the treatment of chemotherapy-failed advanced non-small cell
lung cancer (11,12). Preclinical studies suggested that
the combination of gefitinib with chemotherapy was expected to
improve survival (13,14). However, despite preclinical data
suggesting additive or synergistic effects when combining EGFR-TKIs
with chemotherapy, concurrent administration of the EGFR-TKI
gefitinib with first-line chemotherapies has failed to improve
survival in unselected patients with advanced NSCLC in two large
clinical trial studies (15,16).
Two hypotheses have been proposed to explain the unexpected
negative outcomes of the EGFR-TKIs and chemotherapy combination
studies in advanced-stage NSCLC: i) Lack of patient selection with
a predictive marker; and ii) incorrect choice of chemotherapeutic
agent, dose and regimen (17,18).
Thus, one of the major challenges for an optimal use of combination
EGFR-TKIs and chemotherapy is to determine which patients are more
likely to gain a therapeutic advantage from the treatment.
In the present study, EGFR-TKI-sensitive, primary
resistant and acquired resistant human lung cancer cell lines were
used as in vitro models to define the differential effects
of gefitinib and docetaxel combination on cell proliferation,
apoptosis, cell cycle distribution and signaling pathways.
Materials and methods
Drugs
Docetaxel (Taxotere; Sanofi Aventis, Labège, France)
was purchased as a commercial product from our hospital pharmacy
and was dissolved in dimethylsulfoxide (DMSO) at 1 mM, as a stock
solution. Gefitinib (Iressa) was obtained from AstraZeneca (London,
UK) and was dissolved in DMSO to a stock concentration of 10 mM.
The drugs were stored at −20°C and diluted with culture medium
prior to use. The final concentration of DMSO in the Dulbecco’s
modified Eagle’s medium (DMEM) was maintained at <0.1%.
Cell lines
The EGFR-TKI primary resistant A549 lung cancer cell
line (mutant KRAS/wild-type EGFR) was purchased from the American
Type Culture Collection (Manassas, VA, USA). The EGFR-TKI-sensitive
PC9 (mutant EGFR/wild-type K-Ras) and the
gefitinib-acquired-resistant PC9/GR human NSCLC cell lines were
provided by Dr Xuchao Zhang, Guangdong Lung Cancer Institute
(Guangdong, China). A549, PC9 and PC9/GR cell lines were cultured
in DMEM (HyClone, Logan, UT, USA) supplemented with 10% fetal
bovine serum, penicillin (100 UI/ml) and streptomycin (100 μg/ml)
at 37°C in a humidified atmosphere with 5% CO2, and
harvested with trypsin-EDTA when the cells had reached exponential
growth.
Cell proliferation assay
The MTT assay was used to determine the antitumor
effects of each drug. In brief, cells were plated in 96-well
plates, in which the number of cells per well was 3,000 A549 cells,
3,500 PC-9 cells and 3,500 PC9/GR cells. Following overnight
culture, cells were treated with increasing doses of gefitinib or
docetaxel for 72 h. The IC50 value was the concentration
resulting in 50% cell growth inhibition by a 72 h exposure to drug
compared with the untreated control cells. Following cell exposure
to each drug for 72 h in 96-well plates, 20 μl MTT (5 mg/ml)
solution was added to each well and then the optical density (OD)
of each well was determined at 490 nm on an ELISA plate reader
(Bio-Rad Laboratories, Inc., Winooski, VT, USA) following 4 h
incubation at 37°C. The percentage of cell growth inhibition
resulting from each drug was calculated as: [(OD490control
cells−OD490treated cells)/OD490control
cells] × 100. This assay was repeated in more than three
independent experiments.
Analysis of interactions
To evaluate the antiproliferative effects of the
combined treatment, the A549, PC9 and PC9/GR cells were
concurrently exposed to gefitinib and docetaxel for 72 h. The two
drugs were combined in a constant ratio of doses that typically
corresponded to 0.125, 0.25, 0.5, 1, 2 and 4 times that of the
individual IC50s. Interactions between gefitinib and
docetaxel were expressed as the combination index (CI) according to
the Chou and Talaly method using CalcuSyn software (ComboSyn, Inc.,
Paramus, NJ, USA): CI>1, CI=1 and CI<1 indicate antagonistic,
additive and synergistic effects, respectively (19).
Cell cycle analysis
Cell cycle analysis was conducted using flow
cytometry. Cells (1×105/well) were seeded into six-well
plates. Following 24 h incubation, the cells were treated with
docetaxel and gefitinib as single agents or in combination at the
concentration of IC50 levels for 72 h. Next, the adhered
cells were harvested by trypsinization, washed twice with
phosphate-buffered saline (PBS) and fixed in 75% cold ethanol at
4°C overnight. DNA staining was performed using a solution with
propidium iodide (PI; 50 μg/ml), 0.1% Triton X-100 and RNase (200
μg/ml) in the dark for 30 min at room temperature. Cells were
analyzed using a FACScan cytometer (Becton Dickinson, San Jose, CA,
USA) and the percentages of cells in the G0/G1, S and G2/M phases
of the cell cycle were estimated using ModFit LT 4.0 software
(Verity Software House, Topsham, ME, USA).
Annexin V assay for assessment of
apoptosis
The effects of each individual and combination of
drugs on apoptosis were analyzed in A549, PC9 and PC9/GR cell
lines, using flow cytometry. As a standard, 2×105 cells
in the exponential growth phase were seeded in 60 mm2
dishes. After 24 h incubation, cells were treated with single or
double drugs using the IC50 concentration, as for the
growth inhibition assay. After 72 h treatment, the adherent and
floating cells were collected, washed twice with PBS, resuspended
in an Annexin V binding buffer, and incubated with 5 μl Annexin V
and 10 μl propidium iodide (40 μg/ml) at room temperature in the
dark for 15 min. Following incubation, the stained cells were
analyzed using a flow cytometer. Cells with no drug treatment were
used as a control. Data was analyzed by CellQuest software (Becton
Dickinson).
Western blot analysis
A549, PC9 and PC9/GR cells (1×106/well)
were cultured on 100 mm2 plates overnight and treated
with gefitinib and docetaxel as single agents and in combination
for 72 h, at IC50 levels. The cells were washed with
ice-cold PBS solution and scraped in lysis buffer. The lysates were
centrifuged at 13,380 × g for 30 min at 4°C and the supernatant was
collected. Equivalent cellular proteins were analyzed by sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to polyvinylidine difluoride (PVDF) membranes. The
membranes were blocked in PBS buffer containing 5% milk and 0.1%
Tween-20 for 2 h. Next, the appropriate primary antibodies against
pY1068 EGFR, EGFR, ps473AKT, AKT and β-actin purchased from Cell
Signaling Technology (Beverly, MA, USA) were used and incubated
overnight at 4°C. Visualized of proteins was performed with a
horseradish peroxidase-coupled secondary antibody from Cell
Signaling Technology at room temperature for 1 h. Specific bands
were detected using the enhanced chemiluminescence reagents
(Millipore, Billerica, MA, USA). Equal loading was assessed by
immunoblotting for β-actin, total EGFR or total AKT, as
indicated.
Statistical analysis
The results obtained from at least three independent
experiments are expressed as the mean ± standard deviation.
Student’s t-test and one-way ANOVA used to determine the
differences between control and treatment groups. P<0.05 was
considered to indicate a statistically significant result.
Results
Different antiproliferative effects of
gefitinib and docetaxel in EGFR-TKI-sensitive and
EGFR-TKI-resistant NSCLC cell lines
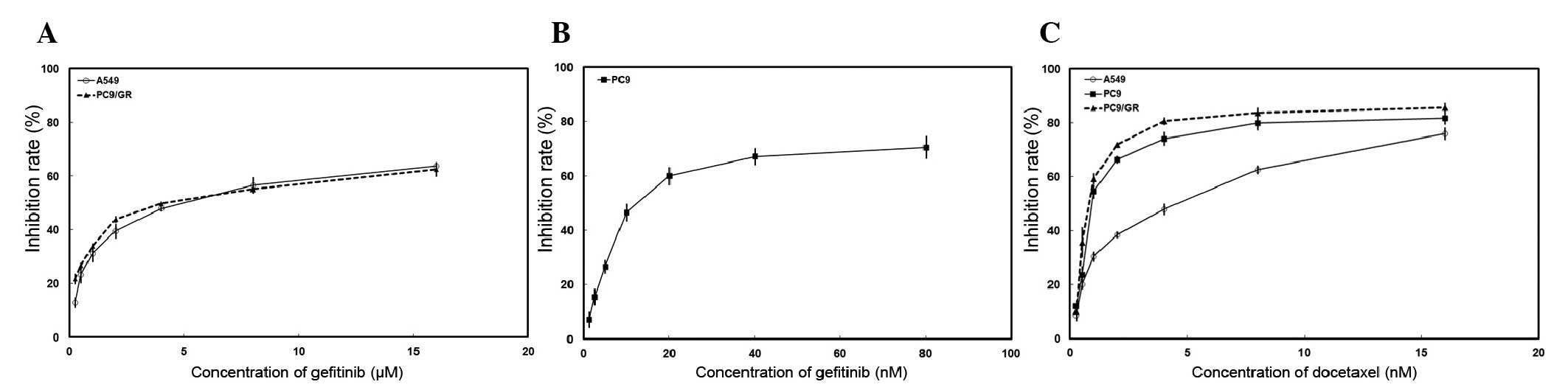
The effects of gefitinib and docetaxel on the
proliferation of the three NSCLC cells were determined using an MTT
assay. Dose-dependent growth inhibitory effects of gefitinib or
docetaxel were observed in NSCLC cell lines (Fig. 1). Table I summarizes the IC50 of
these two drugs. To investigate the effects of combined treatment,
PC9, A549 and PC9/GR cells were exposed to various concentrations
of gefitinib and docetaxel concomitantly for 72 h. In
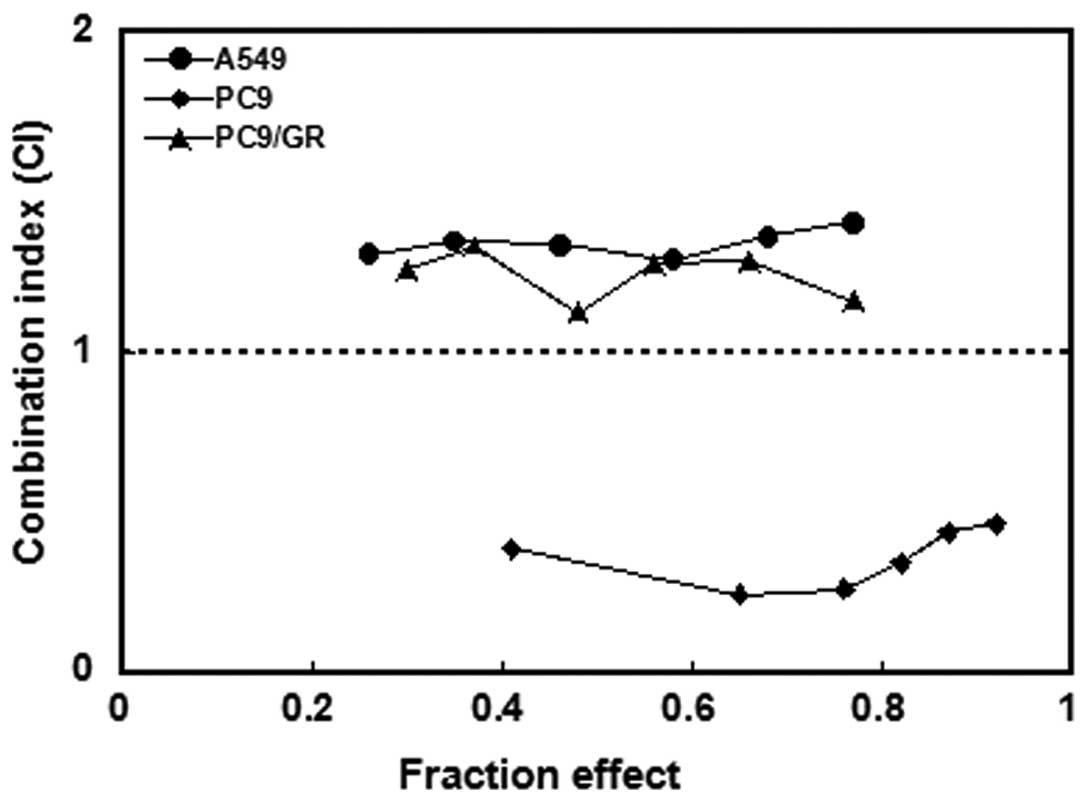
EGFR-TKI-sensitive PC9 cells concurrent administration of the drugs
resulted in synergistic effects (CI<1; Fig. 2). By contrast, antagonistic
activity was observed in EGFR-TKI primary resistant A549 cells and
acquired resistant PC9/GR cell lines (CI>1).
 | Table IIC50 values of gefitinib
and docetaxel were determined by MTT. |
Table I
IC50 values of gefitinib
and docetaxel were determined by MTT.
| IC50 | A549 | PC9 | PC9/GR |
|---|
| Docetaxel | 3.76±0.32 nM | 1.41±0.18 nM | 1.05±0.14 nM |
| Gefitinib | 4.92±0.79 μM | 17.16±2.62 nM | 4.55±0.54 μM |
Cell cycle effects of gefitinib and
docetaxel
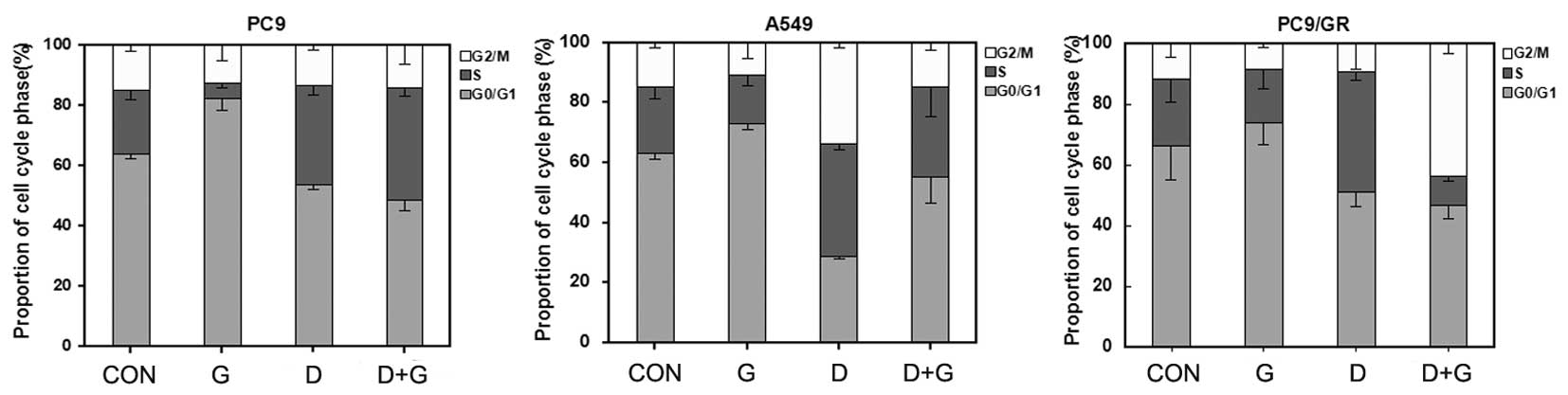
Flow cytometry was used to evaluate the cell cycle
phase distribution in the NSCLC cells following single-drug and
concurrent administration of gefitinib and docetaxel for 72 h. Cell
cycle analysis in the PC9 cell line demonstrated that treatment
with gefitinib alone increased the population of cells in the G0/G1
phase with a concomitant decrease in S phase (P<0.05; Fig. 3). However, in A549 and PC9/GR cell
lines, when treated with gefitinib alone marked additional G0/G1
phase arrest was observed. Following administration of docetaxel
alone, the S or G2 phase fraction significantly increased in PC9,
A549 and PC9/GR cell lines (P<0.05). When EGFR-TKI-sensitive PC9
cells were exposed to docetaxel combined with gefitinib, a similar
cell cycle arrest pattern (mainly S phase arrest) as observed with
docetaxel administered alone and a corresponding reduction of
arrest at the G0/G1 phase (P<0.05) was observed. However, in
EGFR-TKI-resistant A549 and PC9/GR cells the concurrent
administration of docetaxel and gefitinib resulted in alterations
in the cell cycle phase distributions and overlapping effects from
the two agents.
Effects of docetaxel or gefitinib alone,
or in combination on cell apoptosis
To further evaluate whether observed growth
inhibition is due to enhanced apoptosis, cell apoptosis analyses
were performed using an Annexin V/PI assay. In EGFR-sensitive PC9
cell lines, combined treatment with gefitinib and docetaxel
resulted in a significant increase in the apoptotic population
compared with cells treated with each single agent (Fig. 4). By contrast, when
EGFR-TKI-resistant A549 and PC9/GR cells were exposed to
cotreatment with docetaxel and gefitinib, a decrease in apoptosis
was observed compared with cells treated with docetaxel alone.
Collectively, these results indicate that gefitinib antagonizes
docetaxel-induced apoptosis in NSCLC cell lines with primary and
acquired resistance to EGFR-TKI, and has the opposite effect on the
EGFR-TKI-sensitive cell line.
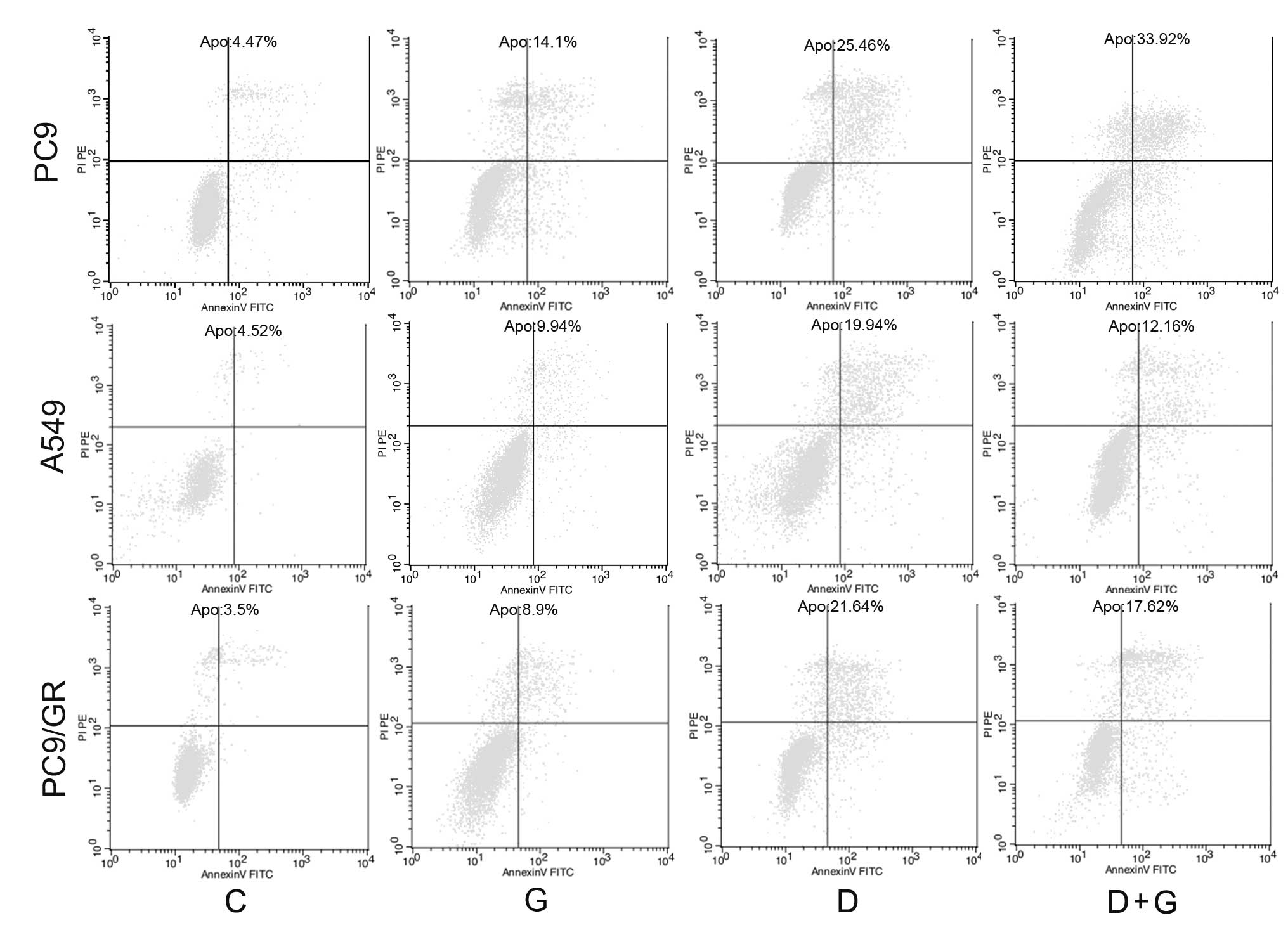 | Figure 4Effect of gefitinib and docetaxel on
cell apoptosis. The effects of each single drug and the combination
on apoptosis were analyzed in PC9, A549 and PC9/GR cell lines using
flow cytometry. Following 72 h treatment, adherent and floating
cells were collected and incubated with Annexin V and PI (x-axis,
Annexin V; y-axis, PI). The apoptotic rate was determined using the
CellQuest software. PI, propidium iodide. CON, G, D and D+G refer
to control, gefitinib, docetaxel and concurrent administration,
respectively. |
Effect of gefitinib or docetaxel, or
their combination on EGFR-mediated signaling pathways
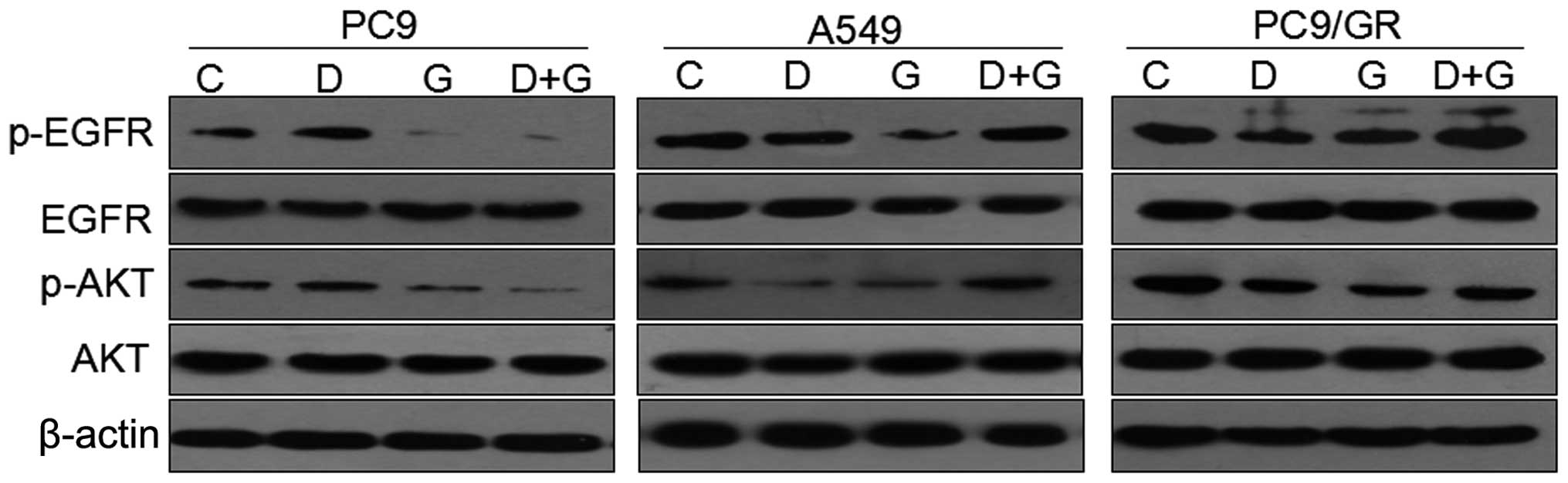
To determine the effects of single or combined drugs
on the EGFR signaling pathway, the levels of phosphorylated EGFR
and phosphorylated AKT in PC9, A549 and PC9/GR cell lines were
analyzed by immunoblotting. Cells were exposed to the
IC50 concentration of each drug for 72 h. Gefitinib
inhibited the activation of EGFR and its downstream signaling
mediator, AKT, effectively in gefitinib-sensitive PC9 cells,
whereas the inhibitory effects on these signaling pathways in
EGFR-TKI-resistant A549 and PC9/GR cells were significantly less
than in PC9 cells (Fig. 5). In
addition, when the three NSCLC cell lines were exposed to docetaxel
at its IC50-value dose for 72 h, the levels of p-EGFR
and p-AKT were increased in PC9 cells, while in A549 and PC9/GR
cells, docetaxel decreased the levels of p-EGFR and p-AKT compared
with those of unexposed cells.
Additionally, when the PC9 cells were exposed to a
combination of docetaxel and gefitinib for 72 h, the levels of
p-EGFR and p-AKT were significantly decreased compared with their
levels in the control, whereas, concurrent administration of the
two drugs increased the levels of p-AKT and p-EGFR in the A549 and
PC9/GR cells. However, compared with the control, there was no
significant alteration in the total EGFR and AKT expression
(Fig. 5).
Discussion
EGFR-targeted anticancer agents, including gefitinib
and erlotinib, have improved the survival rates in patients whose
tumors harbor activating mutations within the EGFR gene (3,4).
However, the presence of primary or acquired resistance to
EGFR-TKIs has limited the effects of these targeted drugs. Thus,
the development of novel treatment strategies for patients with
NSCLC is an urgent clinical objective.
Previous in vitro and in vivo studies
suggest that EGFR-TKIs enhance the anticancer effects of specific
conventional cytotoxic drugs, which may lead to less toxic and more
effective cancer treatment options (20–22).
However, the concomitant use of EGRF-TKIs with
cytotoxic chemotherapy in four clinical randomized phase III trials
of INTACT-1, INTACT-2, TALENT and TRIBUTE showed no survival
improvement over chemotherapy alone in patients with advanced NSCLC
(15,16,23,24).
The unexpected negative results may have been explained by either
the lack of patient selection or antagonism between EGFR-TKIs and
chemotherapeutic agents (17,18).
The present study was performed in
EGFR-TKI-sensitive PC9 (EGFR mutant/wild-type K-Ras),
EGFR-TKI-primary resistant A549 (wild-type EGFR/mutant K-Ras) and
EGFR-TKI acquired-resistant PC9/GR human lung cancer cell lines to
investigate the antiproliferative effects of gefitinib and
docetaxel as single agents or in combined treatment.
Docetaxel and gefitinib exhibited dose-dependent
antiproliferative effects when used as single agents to treat A549,
PC9 and PC9/GR lung cancer cells. However, the IC50
values of gefitinib in A549 and PC9/GR cell lines were higher
compared with gefitinib-sensitive PC9 cells. Notably, synergism was
only observed in the PC9 cells when gefitinib was combined with
docetaxel. By contrast, in the EGFR-TKI-resistant A549 and PC9/GR
cells, antagonistic interactions were observed upon concomitant
administration. The antiproliferative effects in
EGFR-TKIs-resistant cell lines were consistent with those observed
in a previous study (25).
A similar antitumor effect of combination therapy on
cell apoptosis was also observed in the three NSCLC cell lines.
Gefitinib induced minimal apoptosis in A549 and PC9/GR cells,
whereas PC9 cells were more sensitive. Docetaxel alone induced
marked apoptosis in all three cells examined. Notably, the
combination of gefitinib and docetaxel exhibited superior rates of
apoptosis only in the PC9 cell line with an EGFR mutation.
The mechanisms of the synergistic and antagonistic
effects in different cell lines may be attributed to cell cycle
distributions. In the current study, gefitinib arrested cells at
the G0/G1 phase and docetaxel caused mainly S or G2/M phase
accumulation in three cell lines. In the PC9 cells, when exposed to
a combination of docetaxel and gefitinib, a cell cycle arrest
pattern was observed that was similar to that resulting from
treatment with docetaxel administered alone. However, in the A549
and PC9/GR cells, the concurrent administration of docetaxel and
gefitinib resulted in alterations in the cell cycle phase
distribution and overlapping effects from the two agents. Other
studies also suggested that concurrent administration of EGFR-TKIs
and chemotherapy resulted in an antagonistic interaction resulting
from mutual cell cycle interference (26,27).
The differences in the antiproliferative effects of
gefitinib combined with docetaxel may also result from their
effects on growth signaling pathways. AKT is an important
downstream target of the EGFR pathway and is known to inhibit
apoptosis in several ways (28).
The results from the current study showed that in the PC9 cells,
which are highly sensitive to gefitinib, there was a marked
inhibition of p-AKT and p-EGFR following treatment with gefitinib.
By contrast, in the EGFR wild-type A549 and
gefitinib-acquired-resistant PC9/GR cell lines, there was no
significant inhibition of p-EGFR and p-AKT following treatment with
gefitinib. In the PC9 EGFR mutant cells, an increase in p-EGFR and
p-AKT was observed following docetaxel treatment. By contrast, in
the EGFR-TKI-resistant A549 and PC9/GR cells, docetaxel exposure
did not result in increased EGFR or AKT phosphorylation. The
results were inconsistent with those of previous studies, which
reported that docetaxel increased the levels of phosphorylated AKT
in EGFR-TKI-resistant cell lines (29). The conflicting results may be in
part due to the different heritage characteristics of the cell
lines and a lower concentration of docetaxel.
When gefitinib was combined with docetaxel, a
significant decrease in p-AKT and p-EGFR levels was observed in the
PC9 cells, as compared with the control. However, in the A549
EGFR-TKI-primary resistant cells and PC9/GR EGFR-TKI-acquired
resistant cells, the levels of p-EGFR and p-AKT increased when
gefitinib and docetaxel were applied together. These observations
of p-EGFR and p-AKT in NSCLC cells indicate that the interactions
between gefitinib and docetaxel may be associated with the effect
of docetaxel on EGFR phosphorylation and AKT phosphorylation.
Similar to the current results, previous studies reported that EGFR
phosphorylation levels following chemotherapy determine the
response to combined gefitinib/chemotherapy treatment in NSCLC
cells (30,31).
In conclusion, the present study demonstrated that
docetaxel in combination with gefitinib, resulted in differences in
inhibition of tumor cell proliferation and rates of apoptosis in
three NSCLC cell lines in vitro. The observation of
synergistic and antagonistic effects between gefitinib and
docetaxel in the three NSCLC cell lines may indicate that
coadministration of gefitinib and docetaxel may be beneficial to
NSCLC patients with EGFR mutant tumors. However, to be able to
generalize, confirmation of this observation in a larger number of
NSCLC cell lines is necessary and further studies are required to
explore in vivo concurrent administration of gefitinib plus
docetaxel in patients with EGFR-TKI-resistant NSCLC.
Acknowledgements
This study was supported by a grant from the Anhui
Provincial Natural Science Research Program of Higher Education
Institutions Foundation of China (grant no. KJ2012A157) and
supported by the Central Laboratory of the Third Affiliated
Hospital of Anhui Medical University. The authors would like to
thank Dr Xuchao Zhang for providing the cell lines.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Schiller JH, Harrington D, Belani CP, et
al: Comparison of four chemotherapy regimens for advanced
non-small-cell lung cancer. N Engl J Med. 346:92–98. 2002.
View Article : Google Scholar
|
|
3
|
Lynch TJ, Bell DW, Sordella R, et al:
Activating mutations in the epidermal growth factor receptor
underlying responsiveness of non-small-cell lung cancer to
gefitinib. N Engl J Med. 350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Paez JG, Jänne PA, Lee JC, et al: EGFR
mutations in lung cancer: correlation with clinical response to
gefitinib therapy. Science. 304:1497–1500. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pao W, Wang TY, Riely GJ, et al: KRAS
mutations and primary resistance of lung adenocarcinomas to
gefitinib or erlotinib. PLoS Med. 2:e172005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jackman D, Pao W, Riely GJ, et al:
Clinical definition of acquired resistance to epidermal growth
factor receptor tyrosine kinase inhibitors in non-small-cell lung
cancer. J Clin Oncol. 28:357–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
8
|
Gloeckler Ries LA, Reichman ME, Lewis DR,
Hankey BF and Edwards BK: Cancer survival and incidence from the
Surveillance, Epidemiology, and End Results (SEER) program.
Oncologist. 8:541–552. 2003.
|
|
9
|
Schiff PB, Fant J and Horwitz SB:
Promotion of microtubule assembly in vitro by taxol. Nature.
277:665–667. 1979. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gligorov J and Lotz JP: Preclinical
pharmacology of the taxanes: implications of the differences.
Oncologist. 9(Suppl 2): 3–8. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukuoka M, Yano S, Giaccone G, et al:
Multi-institutional randomized phase II trial of gefitinib for
previously treated patients with advanced non-small-cell lung
cancer (The IDEAL 1 Trial) [corrected]. J Clin Oncol. 21:2237–2246.
2003.
|
|
12
|
Kris MG, Natale RB, Herbst RS, et al:
Efficacy of gefitinib, an inhibitor of the epidermal growth factor
receptor tyrosine kinase, in symptomatic patients with non-small
cell lung cancer: a randomized trial. JAMA. 290:2149–2158. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ciardiello F, Caputo R, Bianco R, et al:
Antitumor effect and potentiation of cytotoxic drugs activity in
human cancer cells by ZD-1839 (Iressa), an epidermal growth factor
receptor-selective tyrosine kinase inhibitor. Clin Cancer Res.
6:2053–2063. 2000.
|
|
14
|
Sirotnak FM, Zakowski MF, Miller VA, Scher
HI and Kris MG: Efficacy of cytotoxic agents against human tumor
xeno-grafts is markedly enhanced by coadministration of ZD1839
(Iressa), an inhibitor of EGFR tyrosine kinase. Clin Cancer Res.
6:4885–4892. 2000.PubMed/NCBI
|
|
15
|
Herbst RS, Giaccone G, Schiller JH, et al:
Gefitinib in combination with paclitaxel and carboplatin in
advanced non-small-cell lung cancer: a phase III trial - INTACT 2.
J Clin Oncol. 22:785–794. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giaccone G, Herbst RS, Manegold C, et al:
Gefitinib in combination with gemcitabine and cisplatin in advanced
non-small-cell lung cancer: a phase III trial - INTACT 1. J Clin
Oncol. 22:777–784. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gandara D, Narayan S, Lara PN Jr, et al:
Integration of novel therapeutics into combined modality therapy of
locally advanced non-small cell lung cancer. Clin Cancer Res.
11:5057s–5062s. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandara DR and Gumerlock PH: Epidermal
growth factor receptor tyrosine kinase inhibitors plus
chemotherapy: case closed or is the jury still out? J Clin Oncol.
23:5856–5858. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Magné N, Fischel JL, Dubreuil A, et al:
Sequence-dependent effects of ZD1839 (‘Iressa’) in combination with
cytotoxic treatment in human head and neck cancer. Br J Cancer.
86:819–827. 2002.
|
|
21
|
Xu JM, Azzariti A, Colucci G and Paradiso
A: The effect of gefitinib (Iressa, ZD1839) in combination with
oxaliplatin is schedule-dependent in colon cancer cell lines.
Cancer Chemother Pharmacol. 52:442–448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins B, Kolinsky K, Smith M, et al:
Antitumor activity of erlotinib (OSI-774, Tarceva) alone or in
combination in human non-small cell lung cancer tumor xenograft
models. Anticancer Drugs. 15:503–512. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herbst RS, Prager D, Hermann R, et al:
TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774)
combined with carboplatin and paclitaxel chemotherapy in advanced
non-small-cell lung cancer. J Clin Oncol. 23:5892–5929. 2005.
View Article : Google Scholar
|
|
24
|
Gatzemeier U, Pluzanska A, Szczesna A, et
al: Phase III study of erlotinib in combination with cisplatin and
gemcitabine in advanced non-small-cell lung cancer: the Tarceva
Lung Cancer Investigation Trial. J Clin Oncol. 25:1545–1552. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng H, An SJ, Zhang XC, et al: In vitro
sequence-dependent synergism between paclitaxel and gefitinib in
human lung cancer cell lines. Cancer Chemother Pharmacol.
67:637–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davies AM, Ho C, Lara PN Jr, Mack P,
Gumerlock PH and Gandara DR: Pharmacodynamic separation of
epidermal growth factor receptor tyrosine kinase inhibitors and
chemotherapy in non-small-cell lung cancer. Clin Lung Cancer.
7:385–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li T, Ling YH, Goldman ID and Perez-Soler
R: Schedule-dependent cytotoxic synergism of pemetrexed and
erlotinib in human non-small cell lung cancer cells. Clin Cancer
Res. 13:3413–3422. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cross TG, Scheel-Toellner D, Henriquez NV,
Deacon E, Salmon M and Lord JM: Serine/threonine protein kinases
and apoptosis. Exp Cell Res. 256:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan F, Tian J, Zhang X, et al: Synergistic
interaction between sunitinib and docetaxel is sequence dependent
in human non-small lung cancer with EGFR TKIs-resistant mutation. J
Cancer Res Clin Oncol. 137:1397–1408. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Schaeybroeck S, Karaiskou-McCaul A,
Kelly D, et al: Epidermal growth factor receptor activity
determines response of colorectal cancer cells to gefitinib alone
and in combination with chemotherapy. Clin Cancer Res.
11:7480–7489. 2005.
|
|
31
|
Van Schaeybroeck S, Kyula J, Kelly DM, et
al: Chemotherapy-induced epidermal growth factor receptor
activation determines response to combined gefitinib/chemotherapy
treatment in non-small cell lung cancer cells. Mol Cancer Ther.
5:1154–1165. 2006.
|