Introduction
Inflammation is a key mediator of renal
ischemia-reperfusion (IR) injury (1). Following ischemic injury, vascular
endothelial cells and/or tubular epithelial cells are
morphologically or functionally changed. The endothelial and
tubular cells of injured kidneys generate inflammatory mediators,
including cytokines and chemokines, which contribute to the
recruitment of inflammatory cells into the kidneys (2). These inflammatory responses to
ischemic injury have an important role not only in renal damage,
but also in the reparative process.
A factor affecting the inflammatory process is the
hormonal environment, particularly the sex hormones. Sex hormones
have been reported to have an important role in IR-induced
inflammatory processes in the kidneys (3). Meta-analysis data have shown that
males exhibit rapid progression of non-diabetic kidney diseases,
including membranous nephropathy, immunoglobulin (Ig) A nephropathy
and autosomal dominant polycystic kidney disease (4). Previous studies have demonstrated
that testosterone has an important role in increasing the
susceptibility to ischemic renal injury compared with those of the
depletion of estrogen or the absence of male sex hormones, which
induced a reduction in the levels of post-ischemic oxidative stress
in the kidneys (5,6). By contrast, other experimental
results suggest that estrogen has a protective effect in ischemic
renal injury via suppression of endothelin-1 production and
activation of the phopsphatidylinositol-3 kinase/protein kinase B
signaling pathway (7,8). Therefore, these data present
conflicting results in regard to the role that sex hormones have in
the pathophysiology of acute kidney injury (AKI).
Based on the aforementioned information, the present
study was designed to investigate how depletion and/or repletion of
sex hormones affect the renal IR-induced inflammatory process in
male and female mice.
Materials and methods
Animal experiment
Male and female C57BL/6 mice (four weeks old;
weight, 14–16 g) were purchased from Orient Bio Inc. (Seoul, Korea)
and maintained in a room under controlled temperature (23±1°C),
humidity, lighting (12-h light/12-h dark cycle) and free access to
water. The animal experimental protocol was reviewed and approved
by the Institutional Animal Care and Use Committee of Chonbuk
National University (Jeonju-si, Korea; approval no., CBU
2011-0028). The experimental groups consisted of male and female
sham groups (n=10, each group), male and female AKI groups (n=10,
each group), castrated male and ovariectomized female AKI groups
(n=10, each group) as well as castrated males treated with
testosterone propionate and ovariectomized females treated with
17β-estradiol AKI groups (n=10, each group). The castration or
ovariectomy was performed two weeks prior to induction of renal IR
injury. From the day following the castration or ovariectomy,
testosterone propionate (100 μg/kg; Sigma-Aldrich, St. Louis, MO,
USA) or 17β-estradiol (100 μg/kg; Sigma-Aldrich) was injected
intramuscularly daily during experimental periods. Renal ischemia
was induced by bilateral clamping of the renal pedicles with a
microvascular clip for 23 min, and then circulation was restored by
removing both clips (9). Following
sacrifice of the animals by CO2 inhalation, the kidneys
were harvested to evaluate changes in the renal injury and the
degree of renal inflammation at 48 h after the IR injury.
Renal function analysis
On the final experimental day, blood was collected
from the mice by intracardiac puncture immediately after anesthesia
with ketamine (100 mg/kg; Huons, Seoul, Korea) and xylazine (10
mg/kg; Bayer Korea, Seoul, Korea). The blood urea nitrogen (BUN)
and creatinine levels were measured by an enzymatic colorimetric
method using an automatic analyzer (Hitachi 7180; Hitachi, Ltd.,
Tokyo, Japan).
Histological examination
The kidneys were fixed in 4% paraformaldehyde and
embedded in paraffin. The tissue was cut into 5-μm sections and
stained with periodic acid-Schiff. The renal tubular injury was
assessed as previously described (9). The tubular injury was scored by
estimating the percentage of tubules in the cortex or the outer
medullar that exhibited epithelial necrosis or luminal necrotic
debris and tubular dilatation in a high-power field under a light
microscope (Zeiss Z1 microscope; Carl Zeiss, Göttingen, Germany),
with the scores as follows: 0, none; 0.5, <10%; 1, 10–25%; 2,
25–50%; 3, 50–75%; and 4, >75%. All evaluations were made on 10
non-overlapping fields per section and 10 sections per kidney were
analyzed. The morphometric examinations were performed in a blinded
manner by two independent investigators.
Immunofluorescence staining
Immunofluorescence staining was performed as
described previously (10).
Briefly, freshly frozen renal tissues were fixed with 4%
paraformaldehyde, permeabilized in 1% Triton X-100 and then
incubated with a blocking buffer. Subsequently, the samples were
incubated with rat anti-mouse F4/80 antibody (eBioscience, Inc.,
San Diego, CA, USA). The slides were exposed to Cy3-labeled
secondary antibody (Chemicon, Temecula, CA, USA). The nuclear
staining was performed using DAPI. The digital images were captured
with a Zeiss Z1 microscope (Carl Zeiss AG, Göttingen, Germany) in
10 randomly chosen, non-overlapping fields at a magnification of
×400.
Quantitative polymerase chain reaction
(qPCR)
qPCR was performed as described previously (11). Briefly, total RNA was extracted
from the kidney homogenates using TRIzol® reagent
(Invitrogen, Carlsbad, CA, USA). A Transcriptor First Strand cDNA
Synthesis Kit (Roche Diagnostics, Mannheim, Germany) was used to
synthesize cDNA from the total RNA according to the manufacturer’s
instructions. Specific primers for each gene (Table I) were designed using Primer
Express software, v3.0 (Applied Biosystems, Inc., Foster City, CA,
USA). qPCR was performed in a 7900HT Fast Real-Time PCR system
(Applied Biosystems, Inc.). A 10-fold dilution of each cDNA
transcript was amplified in a 10-μl volume, using SYBR®
Green PCR Master Mix (Applied Biosystems, Inc.), with a 200 nmol/l
final concentration of each primer. To confirm the use of equal
amounts of RNA in each reaction, all samples were examined in
parallel for glyceraldehyde 3-phosphate dehydrogenase mRNA
expression.
 | Table ISequences and accession numbers of the
forward and reverse primers used in the quantitative polymerase
chain reaction. |
Table I
Sequences and accession numbers of the
forward and reverse primers used in the quantitative polymerase
chain reaction.
| Gene | Accession no. | Forward primer
sequence | Reverse primer
sequence |
|---|
| TNF-α | (NM 013693) |
AGGGTCTGGGCCATAGAACT |
CCACCACGCTCTTCTGTCTAC |
| MCP-1 | (NM 011333) |
ATTGGGATCATCTTGCTGGT |
CCTGCTGTTCACAGTTGCC |
| IFN-γ | (NM 008337) |
TGAGCTCATTGAATGCTTGG |
ACAGCAAGGCGAAAAAGGAT |
| CCL-17 | (NM 011332) |
ATAGGAATGGCCCCTTTGAA |
TGCTTCTGGGGACTTTTCTG |
| IL-10 | (NM 010548) |
TGTCAAATTCATTCATGGCCT |
ATCGATTTCTCCCCTGTGAA |
| IL-4 | (NM 021283) |
CGAGCTCACTCTCTGTGGTG |
TGAACGAGGTCACAGGAGAA |
| GAPDH | |
TTGAGGTCAATGAAGGGGTC |
TCGTCCCGTAGACAAAATGG |
Western blot analysis
Western blot analysis was performed as described
previously (12). The kidney
tissues were homogenized in phosphate-buffered saline with a
protease inhibitor cocktail (Calbiochem, San Diego, CA, USA) and
the protein concentration was quantified using the Bradford protein
assay. The samples (40 μg protein per lane) were mixed with buffer,
boiled for 6 min, separated by 8% SDS-PAGE and electroblotted onto
a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). The membrane was blocked with 5% nonfat dry milk in
Tris-buffered saline with Tween-20 buffer (25 mmol/l Tris, pH 7.5,
150 mmol/l NaCl and 0.1% Tween-20) for 1 h and then incubated
overnight at 4°C with goat anti-mouse intercellular adhesion
molecule (ICAM)-1 monoclonal antibody (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA). The blots were washed with
Tris-buffered saline and Tween 20 buffer and incubated with
horseradish peroxidase-conjugated donkey anti-goat IgG (Santa Cruz
Biotechnology, Inc.). Signals were visualized with a
chemiluminescent detection kit according to the manufacturer’s
instructions (Amersham Pharmacia Biotech, London, UK). The
membranes were then reprobed with a rabbit anti-mouse actin
antibody to verify equal loadings of protein in each lane. All
signals were analyzed by densitometric scanning (LAS-3000;
Fujifilm, Tokyo, Japan).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Multiple comparisons were examined for significant differences
using analysis of variance, followed by individual comparison with
the Tukey’s post-hoc test, with P<0.05 considered to indicate a
statistically significant difference.
Results
Effect of gender differences on renal
function and histological changes following IR injury
The male mice exhibited significantly elevated BUN
and creatinine levels following IR injury compared with those of
the male control group. Following castration, renal function was
preserved despite the ischemic injury. However, testosterone
replacement in the castrated male mice aggravated renal function
following IR injury compared with that of the castrated male AKI
group (Fig. 1A). In the female
mice, IR injury induced a minimal significant increase in the
levels of BUN compared with those of the control female mice. The
levels of creatinine did not change significantly in the female
mice with AKI compared with those of the control female mice.
However, the ovariectomized female mice exhibited significantly
increased BUN and creatinine levels compared with those of the mice
in the female AKI group. Replacement of estrogen in the
ovariectomized female mice attenuated the IR-induced increase in
the BUN and creatinine levels compared with those of the
ovariectomized female mice (Fig.
1B).
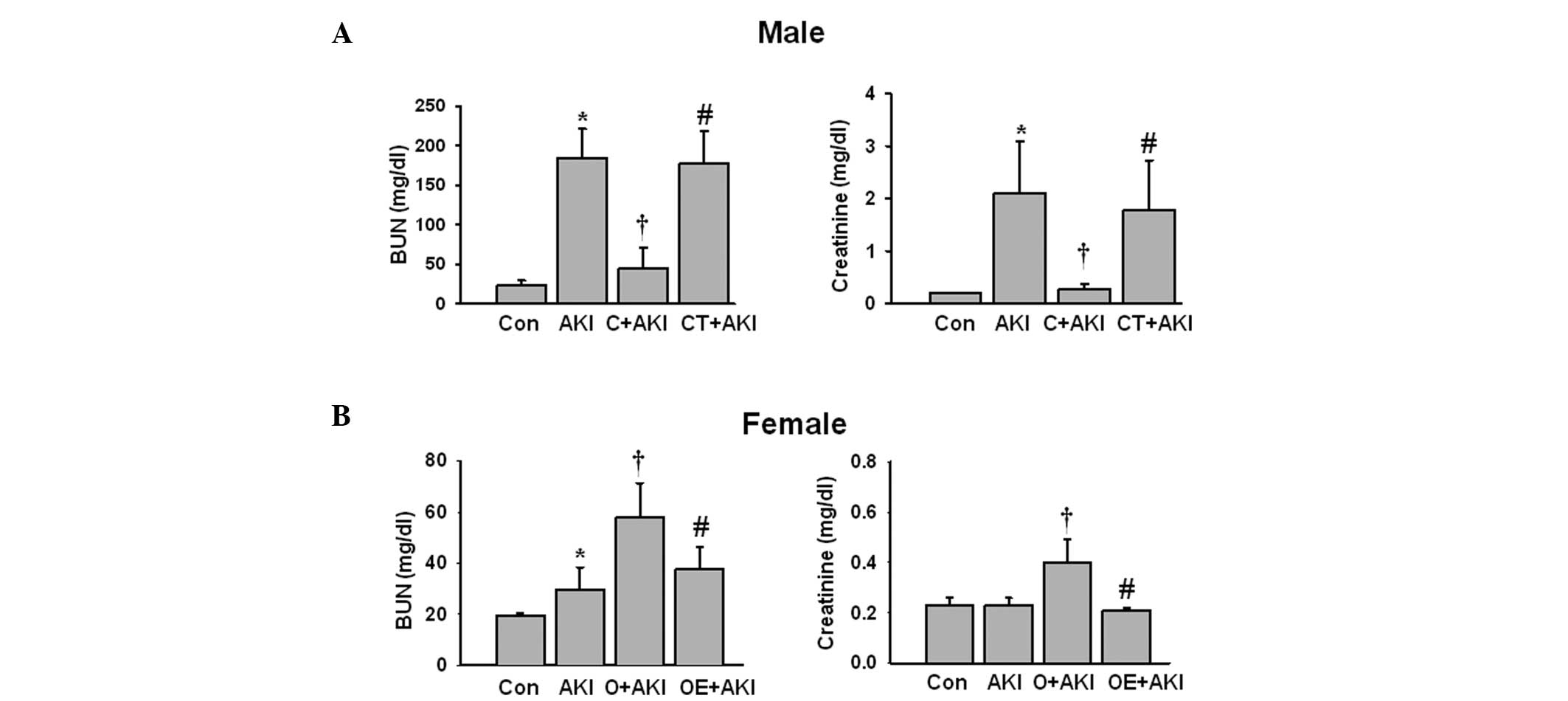 | Figure 1Effect of sex hormones on renal
function following IR injury. AKI was induced by bilateral renal
ischemia for 23 min. Blood samples were collected 48 h after IR
injury from the normal male mice (Con), normal male mice with AKI
(AKI), castrated male mice with AKI (C+AKI), testosterone-replaced
castrated male mice with AKI (CT+AKI), as well as normal female
mice (Con), normal female mice with AKI (AKI), ovariectomized
female mice with AKI (O+AKI), and estrogen-replaced ovariectomized
female mice with AKI (OE+AKI). The BUN and creatinine levels from
(A) male and (B) female mice were measured. Data are expressed as
the mean ± standard deviation (n=10 mice per group).
*P<0.05 vs. Con; †P<0.05 vs. AKI;
#P<0.05 vs. castrated or ovariectomized AKI. BUN,
blood urea nitrogen; IR, ischemia-reperfusion; AKI, acute kidney
injury. |
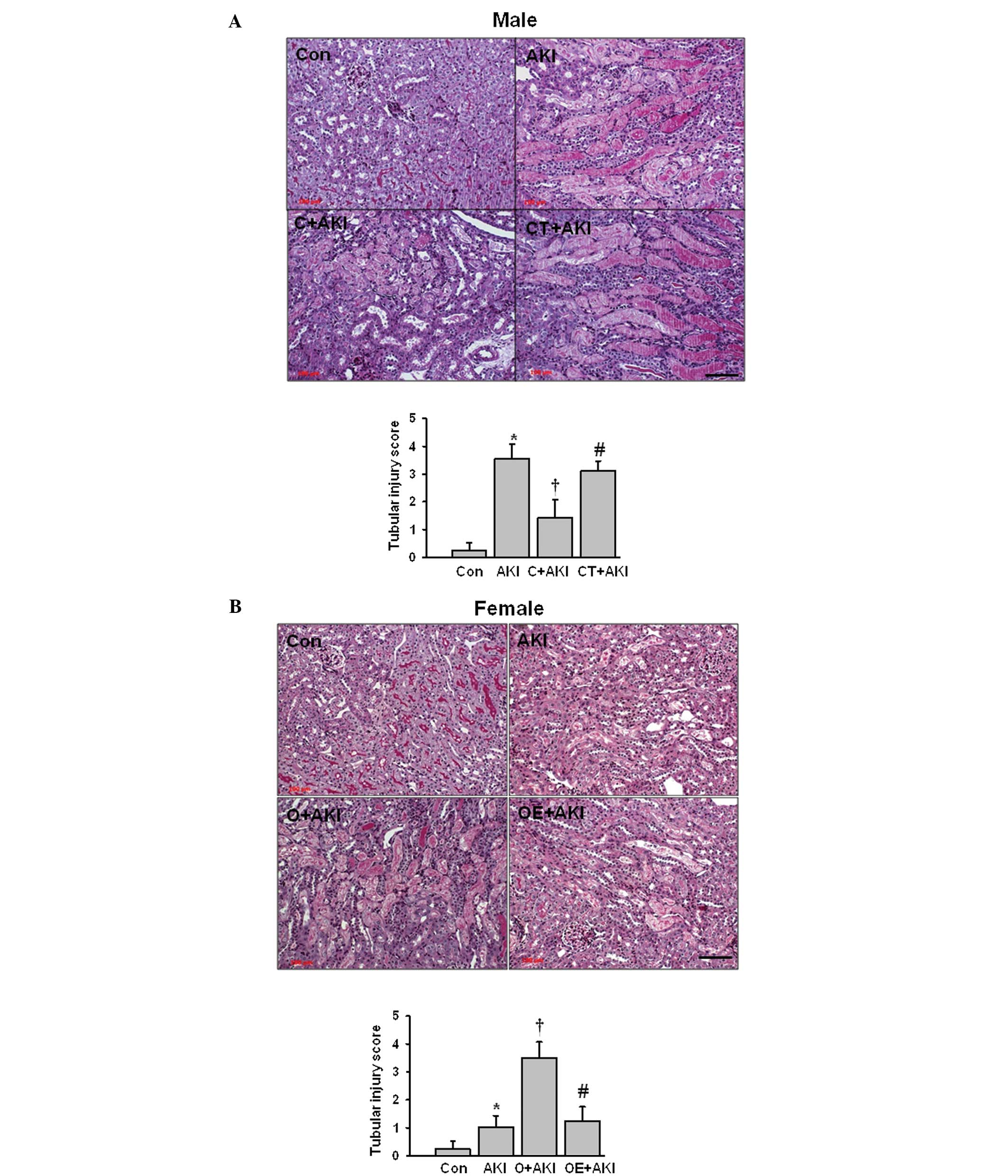
Tubular injury score increases
significantly in the male AKI group compared with that of the
control group
Following castration, the tubular injury score
decreased significantly when compared with that of the male AKI
group. However, replacement of testosterone in the castrated male
mice aggravated the tubular injury compared with that in the mice
in the castrated male AKI group (Fig.
2A). The ovariectomized female mice had significantly increased
levels of tubular injury compared with those of the female AKI
group. Estrogen replacement in the ovariectomized female mice
significantly attenuated the IR-induced tubular damage compared
with that in the ovariectomized female AKI group (Fig. 2B).
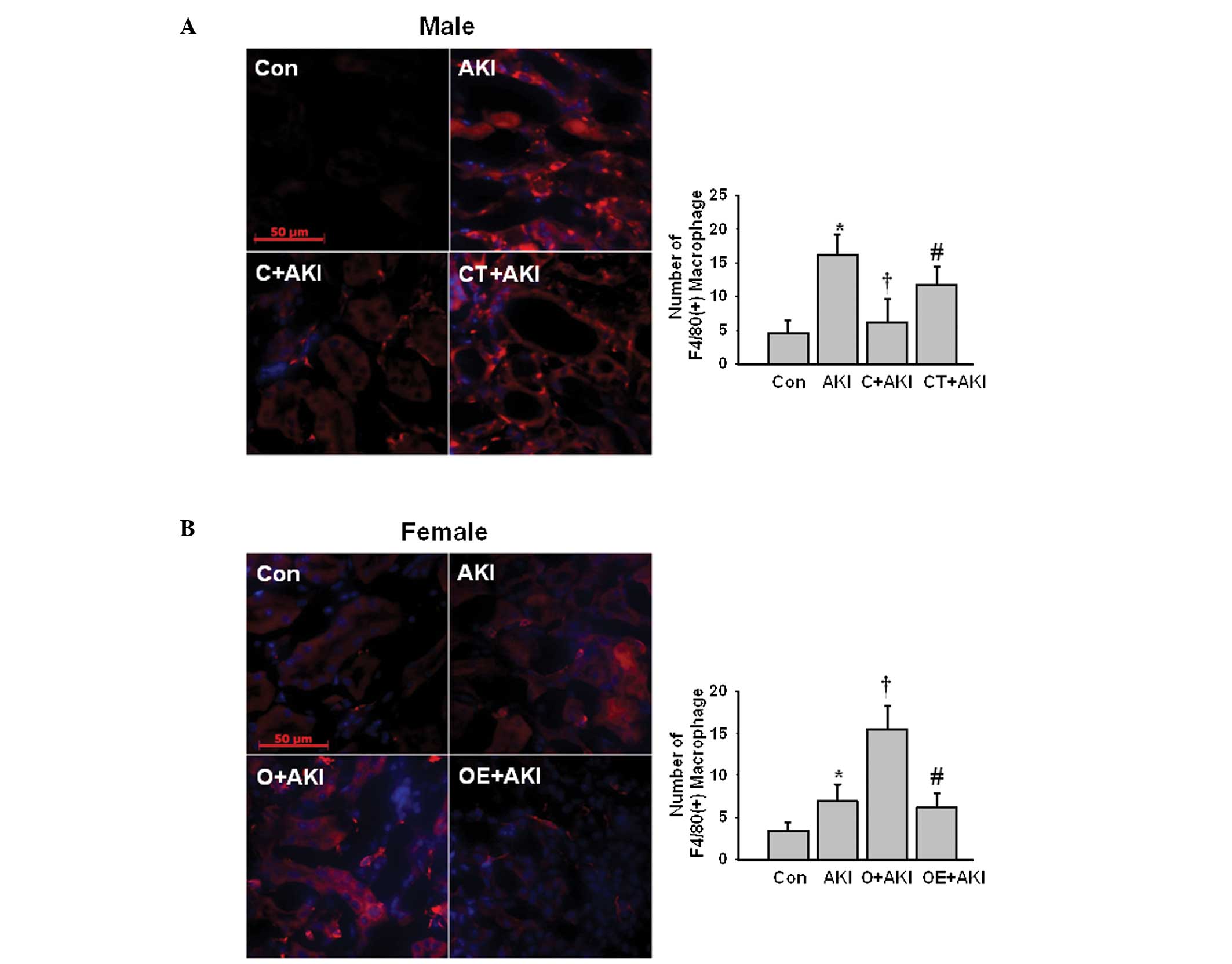
Effect of gender differences on the
levels of macrophage infiltration following IR injury
The F4/80(+) macrophage infiltration was evaluated
following IR injury in the male and female mice. The number of
F4/80(+) macrophages increased significantly following IR injury in
the male AKI group compared with that in the male control group.
Following castration, the levels of F/80(+) macrophage infiltration
were significantly reduced compared with those in the male AKI
group. However, replacement of testosterone in the castrated male
mice significantly increased the levels of F4/80(+) macrophage
infiltration following IR injury compared with those in the
castrated male mice (Fig. 3A). In
the ovariectomized female mice, the number of F4/80(+) macrophages
increased following IR injury compared with that in the female AKI
group. The replacement of estrogen attenuated the IR-induced
increase in the levels of F4/80(+) macrophage infiltration compared
with those in the ovariectomized female AKI group (Fig. 3B).
Effect of gender differences on renal
cytokine expression levels following IR injury
Subsequently, the cytokine and chemokine expression
levels were evaluated following IR injury using qPCR in the male
and female mice. Tumor necrosis factor (TNF)-α, monocyte
chemotactic protein (MCP)-1, interferon (IFN)-γ and chemokine (C-C
motif) ligand (CCL)-17 mRNA levels were significantly increased
following IR injury in the male AKI group compared with those in
the male control group. Depletion of testosterone by castration
reduced the expression levels of TNF-α, MCP-1, IFN-γ and CCL-17
mRNA compared with those in the male AKI group. Following
replacement of testosterone in the castrated male mice, the
expression levels of the proinflammatory cytokines reversed
compared with those in the castrated male mice. The expression
levels of interleukin (IL)-10 and IL-4 mRNA following renal IR
injury did not change significantly between the male groups despite
hormonal modulation in the male mice (Fig. 4A). In female mice, the TNF-α mRNA
levels were significantly increased following IR injury compared
with those in the control female group. Following estrogen
depletion via ovariectomy, the expression levels of TNF-α, MCP-1
and CCL-17 mRNA increased significantly compared with those in the
female AKI group. However, following the replacement of estrogen in
the ovariectomized female mice, the expression levels of the
proinflammatory cytokines, with the exception of IFN-γ, decreased
significantly compared with those in the ovariectomized AKI group.
The expression levels of IL-10 and IL-4 mRNA did not change
significantly following AKI in the female mice compared with those
in the control female group (Fig.
4B).
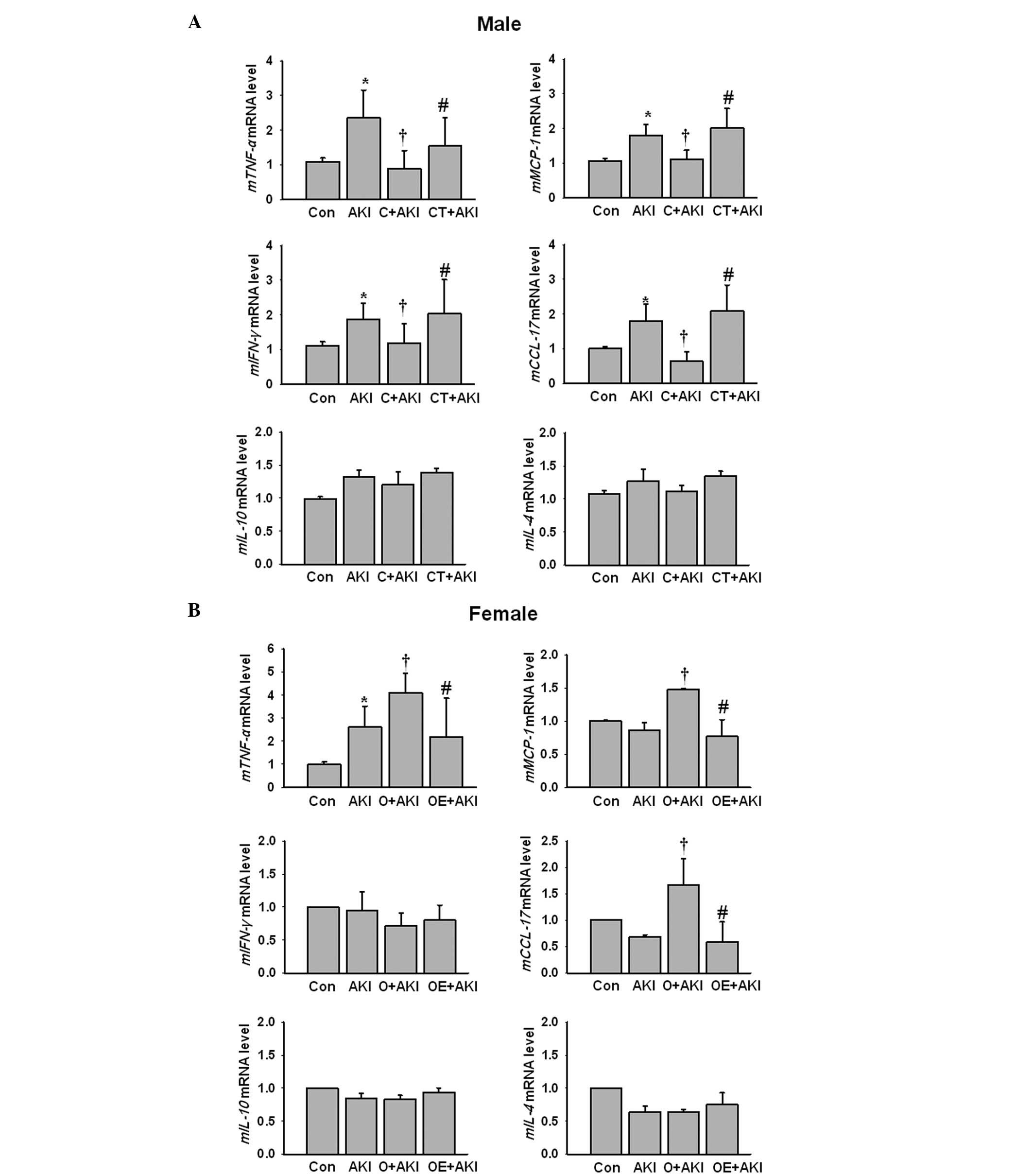 | Figure 4Effect of sex hormones on cytokine
expression levels following IR injury. Kidneys from (A) normal male
mice (Con), normal male mice with AKI (AKI), castrated male mice
with AKI (C+AKI) and testosterone-replaced castrated male mice with
AKI (CT+AKI) as well as (B) normal female mice (Con), normal female
mice with AKI (AKI), ovariectomized female mice with AKI (O+AKI)
and estrogen replaced ovariectomized female mice with AKI (OE+AKI)
were evaluated for the levels of TNF-α, MCP-1, IFN-γ, CCL-17,
IL-10, and IL-4 mRNA expression by quantitative polymerase chain
reaction. Data are presented as the relative expression levels to
those of the control after normalization with GADPH. Bars present
the mean ± standard deviation (n=10 for each experimental group).
*P<0.05 vs. Con; †P<0.05 vs. AKI;
#P<0.05 vs. castrated or ovariectomized AKI. TNF,
tumor necrosis factor; MCP, monocyte chemotactic protein; IFN,
interferon; CCL, chemokine (C-C motif) ligand; IL, interleukin; IR,
ischemia-reperfusion; AKI, acute kidney injury. |
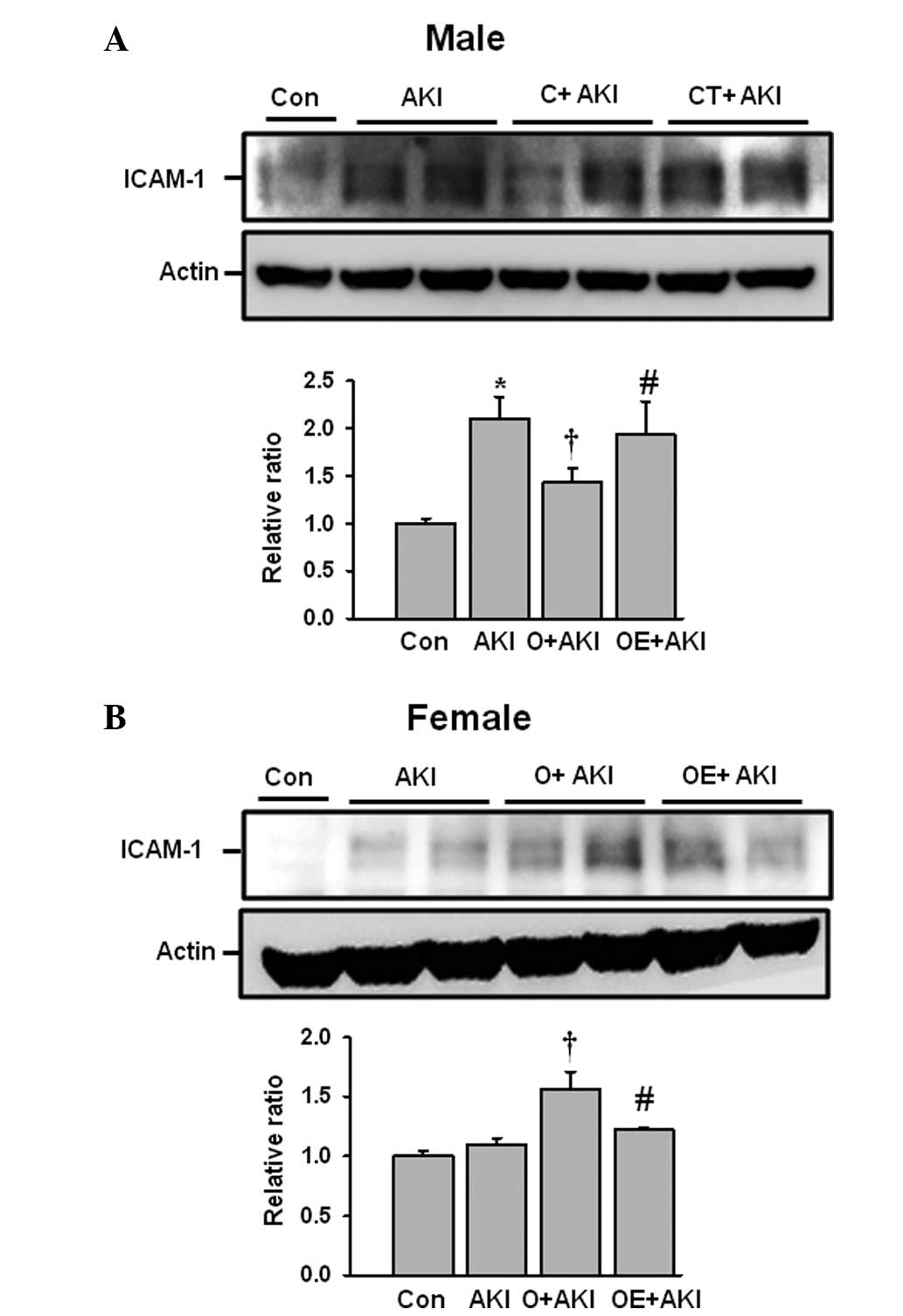
Effect of gender differences on renal
intercellular adhesion molecule (ICAM)-1 expression levels
following IR injury
The expression levels of ICAM-1 were also evaluated
following IR injury in the male and female mice. The male mice in
the AKI group exhibited significantly increased ICAM-1 expression
levels compared with those in the control group. Following
castration, the ICAM-1 levels decreased compared with those in the
AKI group. Replacement of testosterone in the castrated male mice
significantly increased the levels of ICAM-1 expression compared
with those in the mice in the castrated male AKI group (Fig. 5A). The ovariectomized female group
had significantly increased ICAM-1 expression levels following IR
injury compared with those in the female AKI group. However,
estrogen replacement reduced the ICAM-1 expression levels following
IR injury (Fig. 5B).
Discussion
The present study demonstrated that male and female
mice exhibit different inflammatory responses following IR injury.
Testosterone depletion in the male mice attenuated the renal
IR-induced inflammatory response compared with that in the male
mice with AKI; however, estrogen depletion in the female mice
aggravated the inflammatory response compared with that in the
female mice with AKI. The IR-induced renal inflammatory responses
were more prominent in the castrated male mice treated with
testosterone replacement compared with those in the castrated male
mice without hormone replacement. Estrogen replacement in the
ovariectomized female mice recovered the attenuated IR-induced
renal inflammatory response compared with that in the female mice
in an estrogen-depleted state.
The renal inflammatory response is responsible for
the pathogenesis of acute and chronic kidney injury. Inflammatory
cytokines or chemokines are produced due to the renal IR injury and
have an important role in the recruitment of inflammatory cells,
including neutrophils and monocytes/macrophages (13). Several studies suggest that gender-
or sex hormone-mediated inflammatory processes are responsible for
ischemic injury processes in the liver (14), brain (15) and heart (16). Similar findings have been
demonstrated in renal IR injury (5,6).
Consistent with the findings of previous studies, the present study
identified that female mice were more resistant to renal IR injury
compared with male mice. However, replacement of estrogen in the
ovariectomized female mice did not result in a completely
protective effect following IR injury compared with that in the
mice with AKI. The female mice exhibited an increase in the TNF-α
mRNA expression levels following renal IR injury, despite no change
in the levels of MCP-1, IFN-γ and CCL17 mRNA expression following
IR injury compared with those in the control female mice. These
data suggest that estrogen exerts a partially protective effect on
the renal IR injury-induced inflammatory response. In contrast to
that of female sex hormones, testosterone depletion is more
protective in renal IR injury.
Suppression of the inflammatory response following
renal IR injury preserves renal function and prevents progression
to chronic kidney disease. The present study identified that the
sex hormone status may be important for initiation of the
inflammatory response following renal IR injury. In the male mice,
the proinflammatory cytokine expression levels increased
significantly following renal IR injury compared with those in the
control mice. However, depletion of testosterone reduced the
expression levels of the proinflammatory cytokines compared with
those in the mice with AKI. Furthermore, testosterone replacement
in the castrated male mice aggravated the IR-induced renal
inflammatory response compared with that in the castrated mice
without hormone replacement. In the female mice, these inflammatory
responses were less prominent even following estrogen depletion or
replacement of estrogen in the ovariectomized female mice. These
data suggest that testosterone may have more important roles than
estrogen in renal IR injury via enhancing the renal inflammatory
response.
In conclusion, the results of the present study
suggested that the male gender confers greater susceptibility to
renal IR injury via the enhancement the inflammatory response by
testosterone. Further studies are required to address the
underlying mechanism of the gender differences in AKI.
Acknowledgements
This study was supported by the Fund of the Research
Institute of Clinical Medicine, Chonbuk National University
Hospital.
References
|
1
|
Ferenbach D, Kluth DC and Hughes J:
Inflammatory cells in renal injury and repair. Semin Nephrol.
27:250–259. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Akcay A, Nguyen Q and Edelstein CL:
Mediators of inflammation in acute kidney injury. Mediators
Inflamm. 2009:1370722009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kher A, Meldrum KK, Wang M, Tsai BM,
Pitcher JM and Meldrum DR: Cellular and molecular mechanisms of sex
differences in renal ischemia-reperfusion injury. Cardiovasc Res.
67:594–603. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neugarten J, Acharya A and Silbiger SR:
Effect of gender on the progression of nondiabetic renal disease: a
meta-analysis. J Am Soc Nephrol. 11:319–329. 2000.PubMed/NCBI
|
|
5
|
Park KM, Kim JI, Ahn Y, Bonventre AJ and
Bonventre JV: Testosterone is responsible for enhanced
susceptibility of males to ischemic renal injury. J Biol Chem.
279:52282–52292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim J, Kil IS, Seok YM, et al: Orchiectomy
attenuates post-ischemic oxidative stress and ischemia/reperfusion
injury in mice. A role for manganese superoxide dismutase. J Biol
Chem. 281:20349–20356. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takaoka M, Yuba M, Fujii T, Ohkita M and
Matsumura Y: Oestrogen protects against ischaemic acute renal
failure in rats by suppressing renal endothelin-1 overproduction.
Clin Sci (Lond). 103(Suppl 48): 434S–437S. 2002.PubMed/NCBI
|
|
8
|
Satake A, Takaoka M, Nishikawa M, et al:
Protective effect of 17beta-estradiol on ischemic acute renal
failure through the PI3K/Akt/eNOS pathway. Kidney Int. 73:308–317.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jung YJ, Kim DH, Lee AS, et al:
Peritubular capillary preservation with COMP-angiopoietin-1
decreases ischemia-reperfusion-induced acute kidney injury. Am J
Physiol Renal Physiol. 297:F952–F960. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang KP, Kim DH, Jung YJ, et al:
Alpha-lipoic acid attenuates cisplatin-induced acute kidney injury
in mice by suppressing renal inflammation. Nephrol Dial Transplant.
24:3012–3020. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee AS, Lee JE, Jung YJ, et al: Vascular
endothelial growth factor-C and -D are involved in
lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney
Int. 83:50–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang KP, Park SK, Kim DH, et al: Luteolin
ameliorates cisplatin-induced acute kidney injury in mice by
regulation of p53-dependent renal tubular apoptosis. Nephrol Dial
Transplant. 26:814–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chung AC and Lan HY: Chemokines in renal
injury. J Am Soc Nephrol. 22:802–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen SQ, Zhang Y and Xiong CL: The
protective effects of 17beta-estradiol on hepatic
ischemia-reperfusion injury in rat model, associated with
regulation of heat-shock protein expression. J Surg Res. 140:67–76.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yong Y, Xie HJ, Zhang YF, et al:
17beta-estradiol potentiates ischemia-reperfusion injury in
diabetic ovariectomized female rats. Brain Res. 1054:192–199. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Squadrito F, Altavilla D, Squadrito G, et
al: 17Beta-oestradiol reduces cardiac leukocyte accumulation in
myocardial ischaemia reperfusion injury in rat. Eur J Pharmacol.
335:185–192. 1997. View Article : Google Scholar : PubMed/NCBI
|