Introduction
Obesity is currently a worldwide public health
problem. In females, obesity increases mainly during the late
menopausal transition (1,2). Weight gain is a risk factor for
various comorbid conditions, including endometrial pathology
(3).
The incidence of endometrial polyps in the general
female population ranges from 7.8 to 34.9%, depending on the
population studied. Polyps are most commonly identified during
menopausal transition (4–6). The etiology and pathogenesis of
polyps remain to be fully elucidated. However, it is hypothesized
that the presence of hormone receptors is directly associated with
the pathophysiology of endometrial polyps (7). Obesity has been identified as a risk
factor for endometrial polyp development (3,8–10),
although little is known about the association between hormone
receptors in polyps and obesity.
Studies using immunohistochemical techniques have
identified high estrogen receptor (ER) and progesterone receptor
(PR) expression levels in endometrial polyp tissue compared with
the adjacent endometrium (11,12).
In addition to the effect of hormonal factors,
endometrial polyps appear to have two integrated components:
Proliferation and apoptosis. B-cell lymphoma 2 (Bcl-2) is
considered an inhibitor of apoptosis and is important in the
increased expression and consequential loss of apoptotic activity
(12).
Ki-67, a marker of cell proliferation, is found in
the proliferative phase of endometrial cells. High Ki-67
concentrations are also found in endometrial carcinomas (13). However, studies have demonstrated a
very low Ki-67 expression in endometrial polyps (12,13).
Another factor possibly associated with endometrial
polyp development is the enzyme cyclooxygenase-2 (Cox-2), which is
not produced under normal conditions. The enzyme is only produced
during inflammation, cell proliferation and differentiation
(14). Cox-2 expression has been
observed in hyperplasia and endometrial polyps, suggesting a
possible role of prostaglandins in the pathogenesis of these
lesions (15).
Increasing Cox-2 and Ki67 levels appear to be
associated with obesity. In a study by Gao et al (16) a higher leptin concentration caused
by obesity increased the production and action of Cox-2, which in
turn increased angiogenesis and cell proliferation. This mechanism
may also explain the higher frequency of endometrial polyps in
obese females (16).
Obesity appears to be a risk factor not only for the
development of polyps (9,10), but also for the possibility of
malignancy in these lesions (17,18).
The effect of body weight in the pathogenesis of polyps, its
association with estrogen/progesterone and the processes of
apoptosis, proliferation and inflammation, remain unclear and
conflicting. Knowledge of the mechanisms involved in the
development of uterine polyps may contribute to a better
understanding of endometrial polyp formation and progression in
obese females. The aim of the present study was to determine the
expression of ER, PR, Bcl-2, Cox-2 and Ki67 in benign endometrial
polyps in premenopausal and postmenopausal females and their
association with obesity.
Materials and methods
Ethics statement
A cross-sectional study was conducted at the Women’s
Hospital of Professor Dr Jose Aristodemo Pinotti, CAISM/Unicamp
School of Medicine (Campinas, Brazil) and approved by the Research
Ethics Committee of the State University of Campinas (Campinas,
Brazil), under number 092/2011.
Patients and samples
From January 1998 to December 2008, following
hysteroscopic resection of the endometrium, pathologists at the
Department of Pathology, Unicamp School of Medicine, (Campinas,
Brazil) confirmed the diagnosis of polyps in 800 females. Tamoxifen
users, current users of hormone replacement therapy and patients
presenting with premalignant or malignant polyps were excluded from
the present study. In total, 515 cases remained in the sample.
Clinical, pathological and hysteroscopic data were retrieved from
patient medical records. Age, postmenopausal bleeding, presence of
hypertension, obesity and diabetes mellitus were the clinical
features observed. Obesity was a condition defined as a body mass
index (BMI) ≥30.
A gynecologist performed surgical hysteroscopy on
the patients under spinal anesthesia. A 10 mm Karl
Storz® monopolar electrosurgical endoscope
(resectoscope) was used for the procedure and the distending medium
was a 1.5% glycine solution.
Tissue microarray (TMA)
TMA blocks were acquired by taking two 1 mm core
samples for each case. Tissue sections from the TMA blocks were 5
μm thick. Deparaffinization was performed in an incubator for 24 h
at 60°C. Subsequently, the slides were washed in xylene at 60°C for
20 min, xylene at room temperature for 20 min, 100% ethanol for 30
sec, 85% ethanol for 30 sec and then 70% ethanol for 30 sec. The
slides were washed in distilled tap water.
Citrate buffer solution (10 mM; pH 6.0) was heated
to the boil in a pressure cooker (Eterna®; Nigro,
Araraquara, São Paulo, Brazil) without sealing the lid. The slides
were immersed into boiling retrieval buffer and the cooker lid was
sealed with the safety valve open. Following the release of
saturated vapor, the safety valve was lowered until total
pressurization. The lid of the cooker containing the slides was
lifted and the slides were washed in distilled tap water.
Endogenous peroxidase activity was quenched by 3%
hydrogen peroxide (H2O2; 10 volumes) and
washed three times for 10 min each. The slides were rinsed in
distilled tap water and 10 mM phosphate-buffered saline (PBS) for 5
min (pH 7.4).
The slides were incubated with primary antibody
diluted in a predefined titer in 1% PBS containing bovine serum
albumin (A9647; Sigma-Aldrich, St Louis, MO, USA) and 0.1% sodium
azide for 18 h in a humidity chamber at 4°C. Primary monoclonal
antibodies against ER (dilution, 1:250; no. M7047; clone 1D5; Dako,
Carpinteria, CA, USA), PR (dilution, 1:500, no. M3569; clone PgR
636; Dako), Bcl-2 (dilution, 1:200; no. M887; Biogen Idec, Weston,
MA, USA) and Ki-67 (dilution 1:1,000; no. M7240; clone MIB 1; Dako,
Glostrup, Denmark) were used in the procedure. Polyclonal Cox-2
antibody (dilution, 1:200; Abcam, Cambridge, MA, USA) was used.
The slides were washed in PBS three times for 3 min
each and incubated at 37°C for 30 min with Advance™ HRP
Link (no. K4068; Dako). The slides were then washed with PBS buffer
three times for 3 min each and incubated with Advance™
HRP-enzyme at 37°C for 30 min. The slides were washed in PBS three
times for 3 min each and incubated in substrate solution containing
100 mg of 3,3′-diaminobenzidine tetrahydrochloride (no. D-5637;
Sigma-Aldrich), 1 ml dimethylsulfoxide, 1 ml
H2O2 6% (20 volumes) and 100 ml PBS at 37°C
for 5 min under protection from light. The slides were washed in
distilled tap water for 3 min. Counterstaining was performed with
Harris’ hematoxylin for 1 min and the slides were rinsed in
distilled tap water. The slides were immersed twice in ammonia
(0.5% ammonium hydroxide in water) and then rinsed in distilled tap
water. The slides were dehydrated in 80% ethanol for 30 sec, 95%
ethanol for 30 sec, 100% ethanol twice for 30 sec each and xylene
four times for 30 sec each. The slides were mounted with Entellan
Neu (no. 1.07961; Merck, Darmstadt, Germany). The final reaction
product observed by microscopy was a golden brown precipitate,
which varied depending on the marker.
TMA reading was performed manually by a single
experienced pathologist using conventional optical microscopy. The
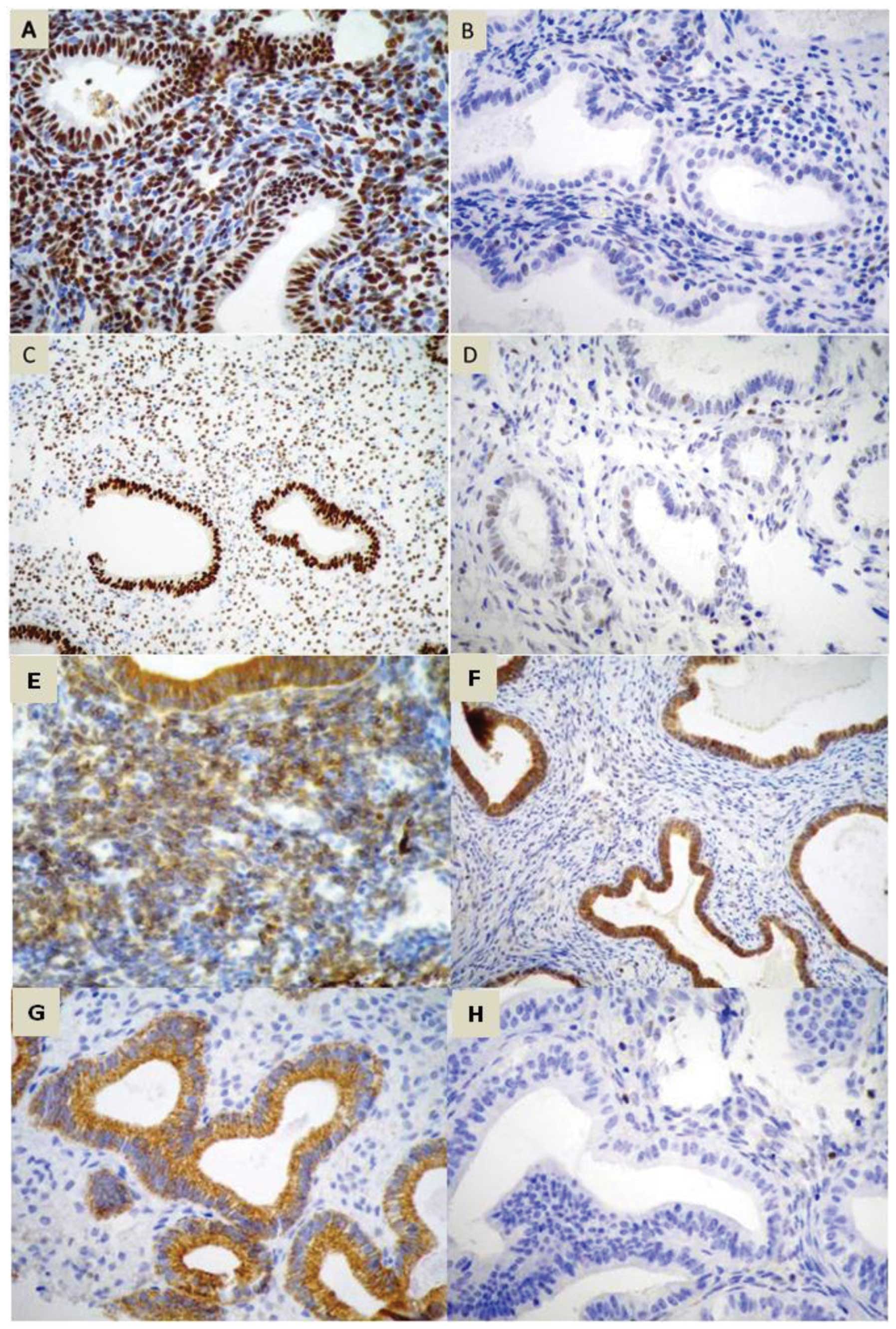
expression of ER and PR, apoptotic (Bcl-2), Cox-2 (Fig. 1) and a proliferation marker (Ki67)
were evaluated in the stromal and glandular epithelial tissue.
Receptor expression was evaluated by a semi-quantitative method of
nuclear reaction for ER, PR and Ki67 and a cytoplasmic reaction for
Cox-2 and Bcl-2, analyzing the percentage of stained cells, nuclear
staining intensity and final score (19).
The percentage of stained cells was estimated
visually. The classification was as follows: Grade 0, no cells
stained; grade 1, <1%; grade 2, 1–10%; grade 3, 11–33%; grade 4,
34–66% and grade 5, >66% of cells stained. The staining
intensity was also assessed and graded according to the following
scale: Grade 0, negative; grade 1, weak; grade 2, moderate; grade
3, severe reaction (19). The sum
of positivity and intensity resulted in the final score, ranging
from 0–8 (excluding value 1). Ki67 expression was evaluated by
immunohistochemistry on a scale from 0 to 3+ and categorized as
follows: 0, <10%; 1+, 11–50%; 2+, 51–80%; 3+, >80% of cells
showing positivity. The samples containing >10% Ki67 positive
cell nuclei were scored as Ki67-positive, those with ≤10% were
Ki67-negative (20,21). Appropriate positive and negative
controls were used.
Statistical analysis
Clinical characteristics were compared using the
χ2 test, Fisher’s exact test or the nonparametric
Mann-Whitney test. For comparison of the median ER, PR, Bcl-2,
Cox-2 and Ki67 in the glandular and stromal components, the
nonparametric Mann-Whitney test was used. Data were analyzed
separately for postmenopausal and premenopausal females. The
Statistical Analysis System, version 9.2 (SAS Institute Inc., Cary,
NC, USA), was used for calculations. P<0.05 was considered to
indicate a statistically significant difference.
Results
Overview
Polyps removed from 515 females were analyzed,
including 258 from obese (BMI ≥30) and 257 from non-obese (BMI
<30) females. The mean age of non-obese females was 53.8 yrs,
while obese females was 58.7 yrs on average (P<0.0001).
Postmenopausal females were also more prevalent in the obese group.
The history of hormonal replacement therapy prior to and following
menopausal bleeding did not differ between the two groups of obese
and non-obese females. Obese females had a higher incidence of
hypertension and diabetes mellitus (DM; Table I).
 | Table IClinical characteristics of obese and
non-obese females undergoing hysteroscopic polypectomy (n=515). |
Table I
Clinical characteristics of obese and
non-obese females undergoing hysteroscopic polypectomy (n=515).
| Obesity status | |
|---|
|
| |
|---|
| BMI ≥30 | BMI <30 | |
|---|
|
|
| |
|---|
| Clinical
characteristic | n | % | n | % | P-valuea |
|---|
| Menopausal status
(n=515) | | | | | <0.0001 |
| Menopause | 204 | 79.10 | 150 | 58.40 | |
| Premenopause | 54 | 20.90 | 107 | 41.60 | |
| HRT (n=511) | | | | | 0.294 |
| Past users | 15 | 5.90 | 21 | 8.20 | |
| Never used | 241 | 94.10 | 234 | 91.80 | |
| Postmenopausal
bleeding (n=354)b | | | | | 0.108 |
| Yes | 88 | 44.2 | 52 | 35.6 | |
| No | 111 | 55.8 | 94 | 64.4 | |
| Hypertension
(n=514) | | | | | <0.0001 |
| Yes | 191 | 74.00 | 107 | 41.80 | |
| No | 67 | 26.00 | 149 | 58.20 | |
| Diabetes mellitus
(n=513) | | | | | 0.0002 |
| Yes | 71 | 27.60 | 36 | 14.10 | |
| No | 186 | 72.40 | 220 | 85.90 | |
Estrogen and progesterone receptors
A high median PR final score in the stromal and
glandular compartments of obese postmenopausal females was
identified (median glandular PR: 8 in obese and 7 in non-obese
females; P=0.0057; median stromal PR: 6 in obese and 5 in non-obese
females; P<0.0001). However, no differences in ER expression
were identified between obese and non-obese females (median
glandular ER: 7 in obese and non-obese females; P=0.139; median
stromal ER: 7 in obese and 6 in non-obese females; P=0.328). Among
premenopausal females, no difference in ER and PR expression was
identified between the obese and non-obese groups. The median rate
of ER and PR expression in the premenopausal and postmenopausal
females was 7 (Table II).
 | Table IIMedian final score of ER and PR in
the glandular and stromal compartment of endometrial polyps in
postmenopausal and premenopausal females in obese and non-obese
groups. |
Table II
Median final score of ER and PR in
the glandular and stromal compartment of endometrial polyps in
postmenopausal and premenopausal females in obese and non-obese
groups.
| Obesity status | |
|---|
|
| |
|---|
| BMI ≥30 | BMI <30 | |
|---|
|
|
| |
|---|
| Final score | Na | Median | Standard
deviation | Median | Na | Median | Standard
deviation | Median | P-value |
|---|
| Postmenopausal |
| Glandular ER | 199 | 6.1 | 2.6 | 7.0 | 147 | 5.7 | 2.8 | 7.0 | 0.1394 |
| Stromal ER | 199 | 5.6 | 2.7 | 7.0 | 150 | 5.2 | 2.9 | 6.0 | 0.3286 |
| Glandular PR | 201 | 7.0 | 1.7 | 8.0 | 143 | 6.3 | 2.5 | 7.0 | 0.0057 |
| Stromal PR | 201 | 5.7 | 2.0 | 6.0 | 146 | 4.8 | 2.3 | 5.0 |
<0.0001 |
| Premenopausal |
| Glandular ER | 53 | 6.0 | 2.6 | 7.0 | 107 | 6.3 | 2.4 | 7.0 | 0.9970 |
| Stromal ER | 54 | 5.6 | 2.6 | 7.0 | 107 | 5.7 | 2.4 | 7.0 | 0.9694 |
| Glandular PR | 53 | 6.3 | 2.2 | 7.0 | 106 | 6.1 | 2.6 | 7.0 | 0.9620 |
| Stromal PR | 54 | 6.6 | 1.3 | 7.0 | 107 | 6.6 | 1.8 | 7.0 | 0.4095 |
Cox-2 and Bcl-2
Among postmenopausal females, obese females
demonstrated increased Cox-2 and Bcl-2 expression levels in the
glandular epithelium compared with non-obese females (median Cox-2
expression in the glandular compartment was 6 in obese and 5 in
non-obese females; P=0.0187; glandular Bcl-2 was 5 in obese and 3
in non-obese females; P<0.0001). Among premenopausal females,
obese females demonstrated only an increase in Bcl-2 expression in
the glandular epithelium (median expression was 4 in obese and 0 in
non-obese females; P=0.010). Cox-2 or Bcl-2 expression was null in
the stromal compartment in premenopausal or postmenopausal, obese
or non-obese females. No other differences were identified in
relation to the endometrial stroma (Table III).
 | Table IIIMedian final score of Cox-2 and Bcl-2
in the glandular and stromal compartments of endometrial polyps in
postmenopausal and premenopausal females in obese and non-obese
groups. |
Table III
Median final score of Cox-2 and Bcl-2
in the glandular and stromal compartments of endometrial polyps in
postmenopausal and premenopausal females in obese and non-obese
groups.
| Obesity status | |
|---|
|
| |
|---|
| BMI ≥30 | BMI <30 | |
|---|
|
|
| |
|---|
| Final score | Na | Median | Standard
deviation | Median | Na | Median | Standard
deviation | Median | P-value |
|---|
| Postmenopausal |
| Glandular
Cox-2 | 201 | 5.4 | 2.2 | 6.0 | 145 | 5.0 | 2.3 | 5.0 | 0.0187 |
| Stromal Cox-2 | 201 | 1.2 | 2.1 | 0.0 | 146 | 1.1 | 2.0 | 0.0 | 0.5886 |
| Glandular
Bcl-2 | 201 | 4.2 | 2.5 | 5.0 | 142 | 3.0 | 2.5 | 3.0 |
<0.0001 |
| Stromal Bcl-2 | 201 | 1.0 | 1.9 | 0.0 | 144 | 1.4 | 2.2 | 0.0 | 0.1975 |
| Premenopausal |
| Glandular
Cox-2 | 54 | 5.5 | 1.9 | 6.0 | 105 | 6.0 | 1.7 | 6.0 | 0.0807 |
| Stromal Cox-2 | 54 | 1.8 | 2.5 | 0.0 | 105 | 2.3 | 2.8 | 0.0 | 0.2379 |
| Glandular
Bcl-2 | 53 | 3.3 | 2.4 | 4.0 | 103 | 2.2 | 2.5 | 0.0 | 0.0104 |
| Stromal Bcl-2 | 53 | 1.8 | 2.4 | 0.0 | 103 | 1.4 | 2.1 | 0.0 | 0.2594 |
Ki67
Regarding Ki67 expression, no statistically
significant differences were identified among obese and non-obese
postmenopausal females (positive glandular expression was 26% in
the obese group vs. 17% in the non-obese group; P=0.057; positive
stromal expression was 2.5% in the obese group vs. 2.7% in the
non-obese group; P=1.0) or premenopausal females (positive
glandular expression was 26% in the obese group vs. 39% in the
non-obese group; P=0.09; positive stromal expression was 13% in the
obese group vs. 16% in the non-obese group; P=0.52; data not
shown).
Obesity as a major factor
Analysis of the effects of diabetes and hypertension
on study results revealed that alterations in the immunoexpression
of the markers were only due to obesity and were not associated
with the presence of these comorbid conditions (data not
shown).
Discussion
The pathogenesis of endometrial polyps remains
poorly understood and few studies have investigated their
association with obesity. The present study was conducted to
evaluate the effect of obesity on the expression of hormone
receptors, Ki67, Bcl-2 and Cox-2 in benign endometrial polyps.
Obesity was demonstrated to be associated with PR and Cox-2/Bcl-2
expression in the glandular endometrial compartments of
postmenopausal females. In premenopausal females, obesity was
associated with Bcl-2 expression in the glandular compartment.
Obesity, which is characterized by increased
peripheral aromatization of androgens to estrogens in adipose
tissue, appears to be associated with estrogen-induced endometrial
abnormalities. Obesity may be important in the pathogenesis of
endometrial polyps (9). To the
best of our knowledge, no studies on hormonal receptors in
endometrial polyps in obese females have been reported to date.
This appears to be a highly complex association. In the present
study, the high ER expression levels detected in polyps were
similar to those observed in previous studies (11,22).
However, in the present study, when the expression of the ER was
compared, there were no differences in the results between obese
and non-obese, premenopausal and postmenopausal females.
This finding is in agreement with a study by
Belisário et al (11) based
on 35 cases of polyps and atrophic endometrial tissue samples from
postmenopausal females. The authors also failed to find any
association between the BMI and ER expression in polyps. In
atrophic endometrial glands, there was an inverse association
between BMI and ER expression. No difference was identified between
ER and PR expression in endometrial polyps in obese and non-obese
females (11). The authors
suggested that increased serum estrogen levels observed in obese
females were able to reduce ER expression in the atrophic
endometrium; however, not in endometrial polyps (11).
The present study demonstrated an increase in PR
expression levels in endometrial polyps, in the stromal and
glandular tissues of obese postmenopausal females. A study by Gul
et al (23) reported an
increase in PR expression in the stromal compartment associated
with increased plasma estrogen levels. This reflected the greater
effect of estrogen levels in obese females, since estrogens
stimulate the formation of the PR. By contrast, when PR expression
and its association with the BMI was directly interrogated, no
differences between obese and non-obese females were observed.
These results were similar to those obtained by other studies
(11,23).
In the present study, Ki67 expression was low and
did not show any difference between obese and non-obese females. In
2010, Villavicencio et al (24) studied the presence of this marker
in normal endometrial tissue and in endometrial cancer. The study
showed that Ki67 expression in the endometrium was 9.9-fold higher
in overweight females and 12-fold higher in obese females, in
comparison with females with a normal BMI (24). In the present study, a higher Ki67
concentration in polyps from obese females was expected, and this
would have explained why obese females are more likely to develop
endometrial pathology, including endometrial polyps. However, a low
Ki67 expression was observed in endometrial polyps, with no
differences in expression between obese and non-obese females in
the present study. To the best of our knowledge, there are no other
studies directly assessing the presence of Ki67 in endometrial
polyps and its correlation with the BMI.
In postmenopausal females, Bcl-2 expression levels
in endometrial polyps are increased compared with those in adjacent
atrophic endometrial tissue, suggesting that the inhibition of
apoptosis is an important mechanism in polyp development (25). Higher Bcl-2 expression levels were
observed in the glandular epithelium of polyps from premenopausal
and postmenopausal obese females. No differences in the stromal
tissue were identified within this group. These results are in
agreement with those reported by other studies in which polyps
demonstrated low Ki67 expression levels and increased Bcl-2
expression levels (26,27). This corroborates the hypothesis
that polyp development is more closely associated with decreased
apoptosis than with increased cell division (26,27).
Other studies have also demonstrated an increase in
Bcl-2 expression in the glandular epithelium of endometrial
lesions, particularly in hyperplasia and endometrial cancer
(28). Bcl-2 expression in
endometrial glands is likely to have an important role in the early
stages of endometrial carcinogenesis (28). It may also be associated with
obesity, which is considered a risk factor for endometrial
cancer.
The present study evaluated polyps in premenopausal
and postmenopausal females. PR expression was highest in the
glandular and stromal compartments. The expression of Cox-2/Bcl-2
was highest in the glandular compartments of polyps from obese
postmenopausal females. Hormone receptor expression did not differ
between premenopausal obese and non-obese females, with the
exception of Bcl-2 expression in glandular tissue. Furthermore,
McGurgan et al (26)
compared endometrial polyps in premenopausal and postmenopausal
females, reporting that ER expression in the stromal tissue and PR
expression in glandular tissue were higher in postmenopausal
females. In addition, Ki67 expression was higher in the stromal
compartment of premenopausal females. Bcl-2 expression did not
differ between premenopausal and postmenopausal females. The
authors observed that these findings further strengthen the
hypothesis that endometrial polyps do not develop due to increased
cell proliferation, but as a result of decreased cell death through
apoptosis. However, the exact mechanism of polyp formation remains
unknown (26). Gul et al
reported an increase in PR expression in the stromal compartment of
polyps from premenopausal patients, however, ER did not differ
statistically (25). The results
were discordant from those obtained in the present study, where
polyps from postmenopausal females demonstrated the highest
expression.
By comparing the levels of Cox-2 in polyps of
premenopausal and postmenopausal females, Erdemoglu et al
(29) discovered an increase in
Cox-2 expression in the stromal compartments of polyps from
premenopausal females with no difference in the glandular
compartments of the polyps (29).
This finding is conflicts with the results of the present study,
which demonstrated an increase in Cox-2 expression in the glandular
epithelium of postmenopausal polyps.
Similarly to other studies, a high Cox-2 expression
was observed in endometrial polyps (29). The present study revealed a higher
Cox-2 expression in the glandular compartments of polyps from
postmenopausal obese females compared with those from non-obese
females. This difference was not identified in the stromal
compartments or tissue samples from premenopausal females. One
possible explanation for these results is an increase in leptin, a
hormone secreted by adipocytes. It increases the production and
activity of Cox-2, resulting in increased cell proliferation and
angiogenesis (17). To the best of
our knowledge, there are no studies specifically assessing Cox-2
expression in polyps and its association with obesity. However, the
association between increased leptin, obesity and increased
endometrial Cox-2 expression has been confirmed in studies on
females with endometrial cancer (17). Adipose tissue is now considered an
active endocrine organ, producing several humoral factors that
promote the release of proinflammatory cytokines (adipokines). In
obesity, these cytokines contribute to the systemic inflammatory
process observed in metabolic syndrome (30).
Clinical evaluation of the patients revealed that
hypertension and diabetes were more prevalent in obese females,
suggesting that inflammatory factors may have an effect on these
patients. This may potentially explain the higher Cox-2 expression
in polyps of obese postmenopausal females found in the present
study.
Studies have evaluated the presence of inflammatory
markers, including C-reactive protein, TNF-α and interleukin-6 in
patients with obesity and an increased risk of developing
endometrial pathology, including endometrial cancer. Weight loss
was demonstrated to reduce the level of these inflammatory markers
and the risk of cancer. Furthermore, the presence of markers was
important in the development of cancer (31).
To the best of our knowledge, no other studies
considered the role of obesity concomitant with the parameters the
present study investigated in benign endometrial polyps.
Differences between the results of several immunohistochemical
studies of endometrial polyps may be explained by differences in
populations evaluated (7,13,11,27).
The present study was unique in that it explicitly investigated
polyps in obese and non-obese females. Furthermore, most earlier
studies were based on a small sample, while the present study
covered a significantly larger number of cases. The
semi-quantitative nature of the present study contributed to the
different results obtained between studies, which were dependent on
the criteria used.
In conclusion, the present study suggested that the
pathogenesis of endometrial polyps in obese females was able to
explain the differences in certain aspects of polyp development in
general, including a greater effect of hormone receptors (mainly
PRs), inhibition of apoptosis (evidenced by Bcl-2), and
inflammation (associated with pro-inflammatory effect of obesity;
COX-2), but is poorly correlated with a proliferation marker
(Ki67).
Abbreviations:
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
References
|
1
|
World Health Organization (WHO). Obesity
and overweight. http://www.who.int/mediacentre/factsheets.
Accessed 22 May, 2012
|
|
2
|
Utian WH, Archer DF, Bachmann GA,
Gallagher C, Grodstein F, Heiman JR, et al: Estrogen and
progestogen use in postmenopausal women: July 2008 position
statement of The North American Menopause Society. Menopause.
15:584–602. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gouveia DA, Bahamondes L, Aldrighi JM,
Tamanaha S, Ribeiro AL and Aoki T: Prevalence of endometrial injury
in asymptomatic obese women. Rev Assoc Med Bras. 53:344–348.
2007.(In Portuguese).
|
|
4
|
Dreisler E, Stampe Sorensen S, Ibsen PH
and Lose G: Prevalence of endometrial polyps and abnormal uterine
bleeding in a Danish population aged 20–74 years. Ultrasound Obstet
Gynecol. 33:102–108. 2009.
|
|
5
|
Baiocchi G, Manci N, Pazzaglia M, Giannone
L, Burnelli L, Giannone E, et al: Malignancy in endometrial polyps:
A 12-year experience. Am J Obstet Gynecol. 201:462.e1–4.
2009.PubMed/NCBI
|
|
6
|
Lieng M, Istre O, Sandvik L and Qvigstad
E: Prevalence, 1-year regression rate, and clinical significance of
asymptomatic endometrial polyps: cross-sectional study. J Minim
Invasive Gynecol. 16:465–471. 2009.PubMed/NCBI
|
|
7
|
Sant’Ana de Almeida EC, Nogueira AA,
Candido dos Reis FJ, Zambelli Ramalho LN and Zucoloto S:
Immunohistochemical expression of estrogen and progesterone
receptors in endometrial polyps and adjacent endometrium in
postmenopausal women. Maturitas. 49:229–233. 2004.
|
|
8
|
Soliman PT, Wu D, Tortolero-Luna G,
Schmeler KM, Slomovitz BM, Bray MS, Gerhenson DM and Lu KH:
Association between adiponectin, insulin resistance, and
endometrial cancer. Cancer. 106:2376–2381. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oguz S, Sargin A, Kelekci S, Aytan H,
Tapisiz OL and Mollamahmutoglu L: The role of hormone replacement
therapy in endometrial polyp formation. Maturitas. 50:231–236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onalan R, Onalan G, Tonguc E, Ozdener T,
Dogan M and Mollamahmutoglu L: Body mass index is an independent
risk factor for the development of endometrial polyps in patients
undergoing in vitro fertilization. Fertil Steril. 91:1056–1060.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Belisário MS, Vassallo J, Andrade L,
Alvarenga M, Pinto GA and Monteiro IM: The expression of the
hormone receptors in the endometrium and endometrial polyps in
postmenopausal women and its relationship to body mass index.
Maturitas. 53:114–118. 2006.PubMed/NCBI
|
|
12
|
McGurgan P, Taylor LJ, Duffy SR and
O’Donovan PJ: An immunohistochemical comparison of endometrial
polyps from postmenopausal women exposed and not exposed to HRT.
Maturitas. 53:454–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Risberg B, Karlsson K, Abeler V, Lagrelius
A, Davidson B and Karlsson MG: Dissociated expression of Bcl-2 and
Ki-67 in endometrial lesions: diagnostic and histogenic
implications. Int J Gynecol Pathol. 21:155–160. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tokyol C, Aktepe F, Dilek FH, Sahin O and
Arioz DT: Expression of cyclooxygenase-2 and matrix
metalloproteinase-2 in adenomyosis and endometrial polyps and its
correlation with angiogenesis. Int J Gynecol Pathol. 28:148–156.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maia H Jr, Pimentel K, Silva TM, Freitas
LA, Zausner B, Athayde C, et al: Aromatase and cyclooxygenase-2
expression in endometrial polyps during the menstrual cycle.
Gynecol Endocrinol. 22:219–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao J, Tian J, Lv Y, Shi F, Kong F, Shi H,
et al: Leptin induces functional activation of cyclooxygenase-2
through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human
endometrial cancer cells. Cancer Sci. 100:389–395. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregoriou O, Konidaris S, Vrachnis N,
Bakalianou K, Salakos N, Papadias K, Kondi-Pafiti A and Creatsas G:
Clinical parameters linked with malignancy in endometrial polyps.
Climacteric. 12:454–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Costa-Paiva L, Godoy CE Jr, Antunes A Jr,
Caseiro JD, Arthuso M and Pinto-Neto AM: Risk of malignancy in
endometrial polyps in premenopausal and postmenopausal women
according to clinicopathologic characteristics. Menopause.
18:1278–1282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harvey JM, Clark GM, Osborne CK and Allred
DC: Estrogen receptor status by immunohistochemistry is superior to
the ligand-binding assay for predicting response to adjuvant
endocrine therapy in breast cancer. J Clin Oncol. 17:1474–1481.
1999.PubMed/NCBI
|
|
20
|
Stuart-Harris R, Caldas C, Pinder SE and
Pharoah P: Proliferation markers and survival in early breast
cancer: a systematic review and meta-analysis of 85 studies in
32,825 patients. Breast. 17:323–334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dowsett M, Nielsen TO, A’Hern R, Bartlett
J, Coombes RC, Cuzick J, et al: Assessment of Ki67 in breast
cancer: recommendation from the International Ki67 in Breast Cancer
working group. J Natl Cancer Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lopes RG, Baracat EC, de Albuquerque Neto
LC, Ramos JF, Yatabe S, Depesr DB, et al: Analysis of estrogen- and
progesterone-receptor expression in endometrial polyps. J Minim
Invasive Gynecol. 14:300–303. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gul A, Ugur M, Iskender C, Zulfikaroglu E
and Ozaksit G: Immunohistochemical expression of estrogen and
progesterone receptors in endometrial polyps and its relationship
to clinical parameters. Arch Gynecol Obstet. 281:479–483. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Villavicencio A, Aguilar G, Argüello G,
Dünner C, Gabler F, Soto E, et al: The effect of overweight and
obesity on proliferation and activation of AKT and ERK in human
endometria. Gynecol Oncol. 117:96–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inceboz US, Nese N, Uyar Y, Ozcakir HT,
Kurtul O, Baytur YB, et al: Hormone receptor expressions and
proliferation markers in postmenopausal endometrial polyps. Gynecol
Obstet Invest. 61:24–28. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McGurgan P, Taylor LJ, Duffy SR and
O’Donovan PJ: Are endometrial polyps from pre-menopausal women
similar to postmenopausal women? An immunohistochemical comparison
of endometrial polyps from pre- and post-menopausal women.
Maturitas. 54:277–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Taylor LJ, Jackson TL, Reid JG and Duffy
SR: The differential expression of oestrogen receptors,
progesterone receptors, Bcl-2 and Ki67 in endometrial polyps. BJOG.
110:794–798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amalinei C, Cianga C, Balan R, Cianga P,
et al: Immunohistochemical analysis of steroid receptors,
proliferation markers, apoptosis related molecules, and gelatinases
in non-neoplastic and neoplastic endometrium. Ann Anat. 193:43–55.
2011. View Article : Google Scholar
|
|
29
|
Erdemoglu E, Güney M, Karahan N and Mungan
T: Expression of cyclooxygenase-2, matrix metalloproteinase-2 and
matrix metalloproteinase-9 in premenopausal and postmenopausal
endometrial polyps. Maturitas. 59:268–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nishimura S, Manabe I and Nagai R: Adipose
tissue in obesity and metabolic syndrome. Discov Med. 8:55–60.
2009.
|
|
31
|
Byers T and Sedjo RL: Does intentional
weight loss reduce cancer risk? Diabetes Obes Metab. 13:1063–1072.
2011. View Article : Google Scholar : PubMed/NCBI
|