Introduction
Arteriosclerotic coronary disease is now the leading
cause of mortality worldwide (1).
Percutaneous transluminal coronary angioplasty has become the most
common strategy for the treatment of severe coronary artery
disease. Restenosis is a potential limitation of percutaneous
coronary angioplasty. Although intracoronary stents reduce the
incidence of restenosis following percutaneous coronary
intervention (PCI), in-stent stenosis remains an important issue
(2). Restenosis following PCI is
predominantly caused by the proliferation of vascular smooth muscle
cells (VSMCs) (3). The
introduction of drug eluting stents (DES) has significantly
decreased the rates of restenosis by inhibiting the accumulation
and proliferation of VSMCs. Nevertheless, in-stent restenosis
following PCI remains a challenging clinical problem despite the
use of DES (4). It has been
reported that the rate of restenosis remains at 7–16% in diabetic
patients who have received DES (5). Our previous study demonstrated that
diabetes increases restenosis following rapamycin-eluting stent
placement (6), indicating that
diabetes remains an important predictor for restenosis in the DES
era. When rapamycin becomes less effective at preventing in-stent
restenosis, this condition is termed ‘rapamycin resistance’
(6). However, the underlying
mechanisms of rapamycin resistance remain to be determined.
The mammalian target of rapamycin (mTOR) is a
serine/threonine kinase that is important in mediating the
proliferation of VSMCs (7). The
best characterized downstream targets of mTOR are 4E-binding
protein 1 (4EBP1) and ribosomal S6 kinase 1 (S6K1) (8). The activation of mTOR leads to the
phosphorylation of 4EBP1 and S6K1, leading to the promotion of the
initiation and elongation phases of mRNA translation and
consequently the stimulation of VSMC proliferation. Rapamycin is a
specific inhibitor of mTOR and suppresses the proliferation of
VSMCs through inhibiting the mTOR-mediated proliferative signaling
pathway (9). Therefore, the
expressional and functional status of mTOR may affect the
antiproliferative effect of rapamycin.
The present study hypothesized that diabetes may
affect the expression of mTOR and its downstream kinases, attenuate
the action of rapamycin and cause the clinical phenomena of
rapamycin resistance. As a proof-of-concept study, the present
study assessed how diabetes affected the growth of atherosclerotic
lesions and mTOR expression in atherosclerotic plaques of
apolipoprotein E-deficient (ApoE−/−) mice.
Materials and methods
Animal care
Experimental procedures were approved by the
Hospital Animal Care and Use Committee (General Hospital of PLA
Chengdu Military Area Command (Chengdu, China). In total, 16 male
ApoE−/− mice (age, 6–8 weeks) were purchased from
Beijing Yi-Ke-Li-Hao Biotechnology Co., Ltd. (Beijing, China).
Animals were housed under a 12 h/12 h day/night cycle with access
to food and water ad libitum. The mice were fed a normal
animal diet.
Animal model
The mice were randomly divided into two groups: The
control group (n=8) and the diabetes group (n=8). The mice in the
diabetic group were intraperitoneally injected with 55 mg/kg
streptozocin (STZ; in 0.05 mol/l citrate buffer; pH 4.5; Sigma, St.
Louis, MO, USA) for five consecutive days. The mice in the control
group were injected with an equal volume of citrate buffer. Fasting
tail-blood glucose concentration (FBG) level was measured using a
glucose test strip (Roche Diagnostics GmbH, Mannheim, Germany).
Animals were considered to be diabetic if their FBG level was
>13 mmol/l. The FBG levels of all mice were monitored at
baseline and at weeks 1, 3 and 8 after injection of STZ.
Serum lipids
Fasting blood samples were obtained at the end of
the experiments. The triglycerides (TG), total cholesterol (TC),
low-density lipoprotein cholesterol (LDL-C) and high-density
lipoprotein cholesterol (HDL-C) levels were measured by
colorimetric assays using a commercially available kit (Jiancheng
Bioengineering Institute, Nanjing, China) according to our previous
study (10).
Atherosclerosis analysis
Atherosclerotic lesions in the aortic roots were
examined in cross sections of the aortic origin according to a
previous study (11). The sections
(5 μm) were stained with hematoxylin and eosin, and images were
captured on an Olympus BX41 microscope (Olympus Corp., Tokyo,
Japan). The atherosclerotic lesion area was quantified using Nikon
NIS-Elements Research software (Nikon, Tokyo, Japan).
Immunohistochemistry
The protein expression of smooth muscle (SM) α-actin
and mTOR in atherosclerotic plaques was detected by
immunohistochemical staining (12). Aortic roots were fixed in 4%
paraformaldehyde for 12 h, embedded in paraffin and then cut into
sections (5 μm). The sections were incubated with anti-SM α-actin
(BM002) and mTOR (Ab-2448) antibodies (diluted 1:50). Specific
binding was detected using complexes of biotinylated goat
anti-rabbit IgG secondary antibody and horseradish peroxidase using
ABC kits (Boster Bioengineering Co., Wuhan, China). The
antigen-antibody complex was subsequently visualized with DAB
solution (Boster Bioengineering Co.). The sections were viewed
under a light microscope. For the detection of positive staining,
the portion of the color spectrum representing the positive signal
for each stain was defined using the color cube-based method in
Image-Pro plus 6.0 (Media Cybernetics, Inc., Bethesda, MD, USA).
This color definition was then applied uniformly to all digitized
images for that stain to determine the total positive area within
the plaque and this positive area measurement was then normalized
to the total area of the plaque. Additionally, the mean light
density of the positive area was calculated.
Western blot analysis
Protein lysates were obtained by homogenizing aortic
tissues with lysis buffer containing 1% Triton X-100, 150 mmol/l
NaCl, 1 mmol/l EDTA, 2.5 mmol/l sodium pyrophosphate, 1 mmol/l
β-glycerophosphate, 1 mmol/l Na3VO4, 1 μg/ml
leupeptin, 1 μg/ml aprotinin and 20 mmol/l Tris (pH 7.5) (13). The protein concentration was
determined using Bio-Rad protein assay reagent (Bio-Rad, Hercules,
CA, USA). Equal quantities of protein from the heart extracts were
separated by SDS-PAGE (12%). The samples were then electroblotted
onto a nitrocellulose membrane (Boehringer Mannheim Corporation,
Indianapolis, IN, USA) and probed with antibodies against mTOR
(Ab-2448, rabbit polyclonal), phospho-mTOR-Ser2448 (p-mTOR;
Ab-11221, rabbit polyclonal), phospho-4EBP1-Ser 65/Thr 70 (p-4EBP1;
sc-12884, goat polyclonal), phospho-S6K1 (p-S6K1; eptomics-1175-1,
rabbit monoclonal) and glyceraldehyde 3 phosphate dehydrogenase
(GAPDH; sc-365062, mouse monoclonal) overnight. Following washing,
the membrane was incubated with a horseradish peroxidase-conjugated
secondary antibody (1:1,000 dilution; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) and bound antibody was visualized using
a colored reaction. The relative band densities were quantified by
densitometry using the Multi-Analyst software package (Bio-Rad).
The equal loading of protein was confirmed by measuring GAPDH
expression.
Statistical analysis
Continuous data are presented as the mean ± standard
error of the mean. Comparisons between the two groups were
determined by the independent samples t-test (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Characteristics of diabetic
ApoE−/− mice
The body weight of control ApoE−/− mice
significantly increased 8 weeks after the start of the experiment.
However, the body weight of diabetic ApoE−/− mice
decreased marginally following STZ administration and was
significantly lower than that of the control mice at the end of the
experiment (P<0.01; Fig. 1A).
No significant changes in the fasting blood glucose level in the
control mice were observed. However, the fasting glucose
concentration of diabetic mice increased to a level >20 mmol/l 3
weeks after STZ injection and maintained the hyperglycemic state
until the end of the experiment (Fig.
1B). The plasma TG, TC, LDL-C and HDL-C concentrations in the
diabetic mice were significantly higher than those of the control
mice (Fig. 1C; all P<0.01).
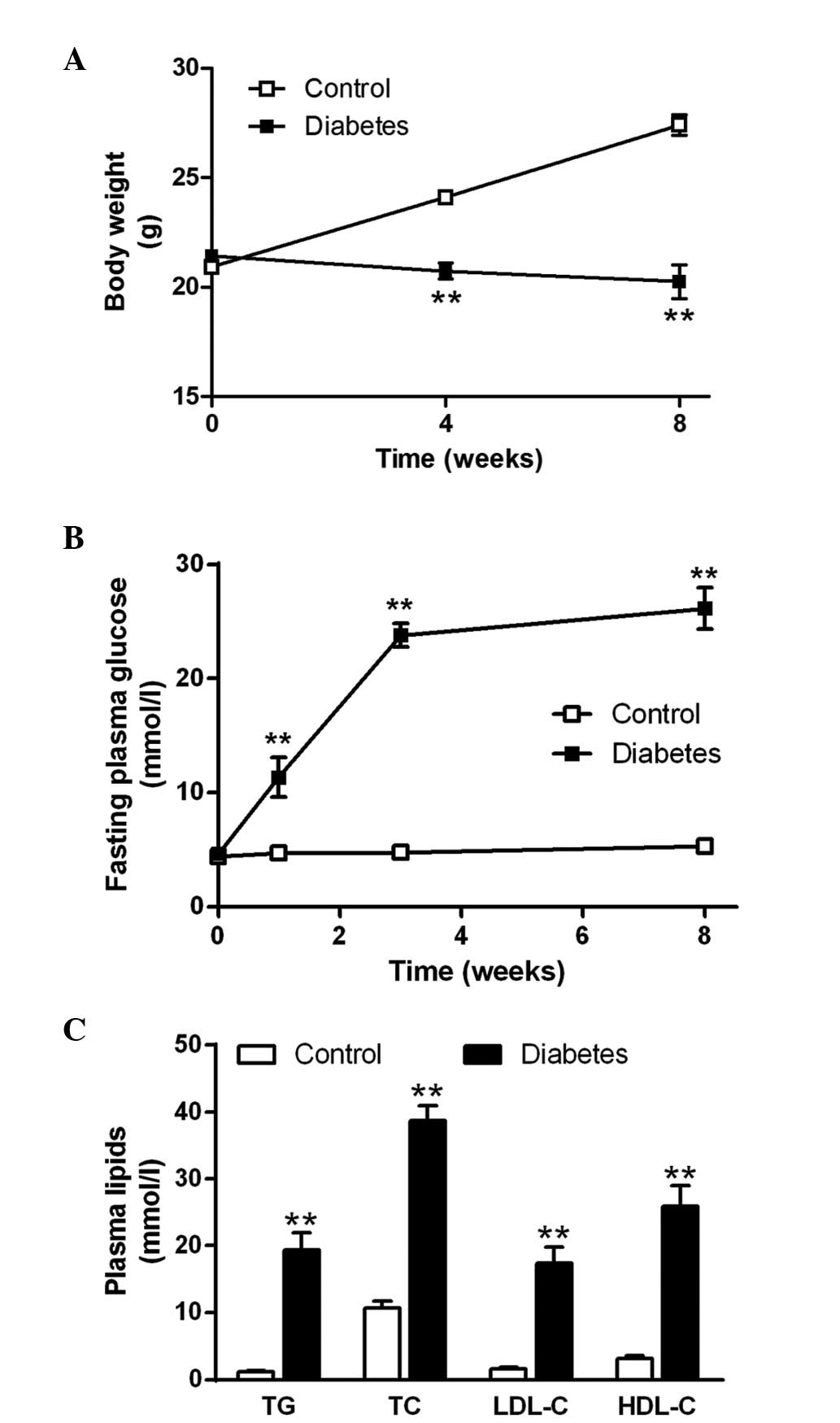 | Figure 1Characteristics of diabetic
ApoE−/− mice. (A) Body weight of mice from the control
and diabetic groups at baseline, week 4 and week 8 after STZ
administration. (B) Fasting plasma glucose levels of mice from the
control and diabetic groups at baseline, week 1, week 3 and week 8
after STZ administration. (C) TG, TC, LDL-C and HDL-C
concentrations in ApoE−/− mice in the control (open
bars) and diabetic (solid bars) groups at week 8 after STZ
treatment. The data are expressed as the mean ± standard error of
the mean; n=8 mice in the control group and n=6 in the diabetic
group. **P<0.01 compared with the control group. STZ,
streptozocin; TG, plasma triglyceride; TC, total cholesterol;
LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density
lipoprotein cholesterol; ApoE−/−, apolipoprotein
E-deficient. |
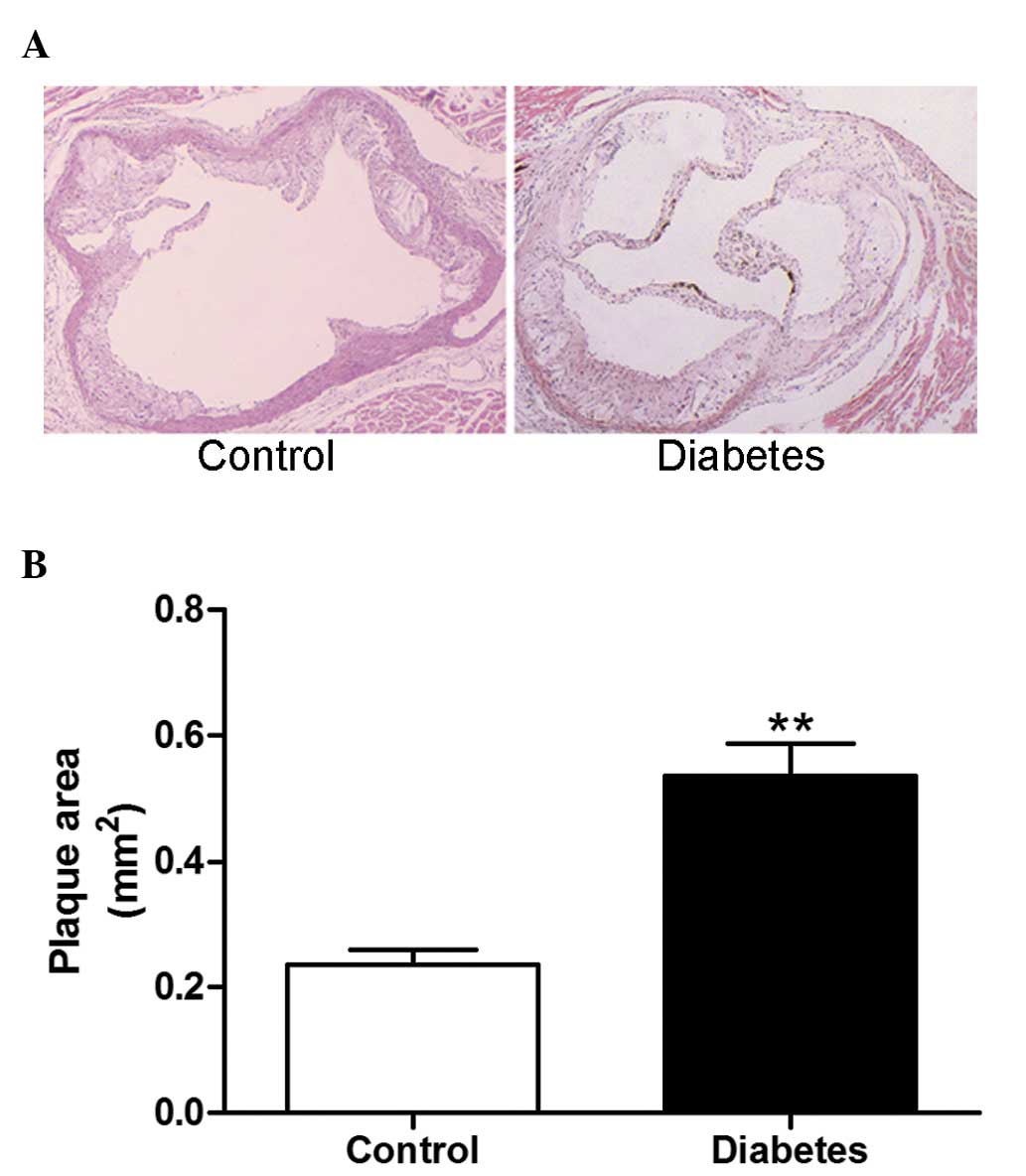
Diabetes enhances atherosclerotic
lesions
At week 8 after STZ administration, the
atherosclerotic plaque area of the aortic root was significantly
increased in diabetic mice compared with the control group
(P<0.01; Fig. 2A and B).
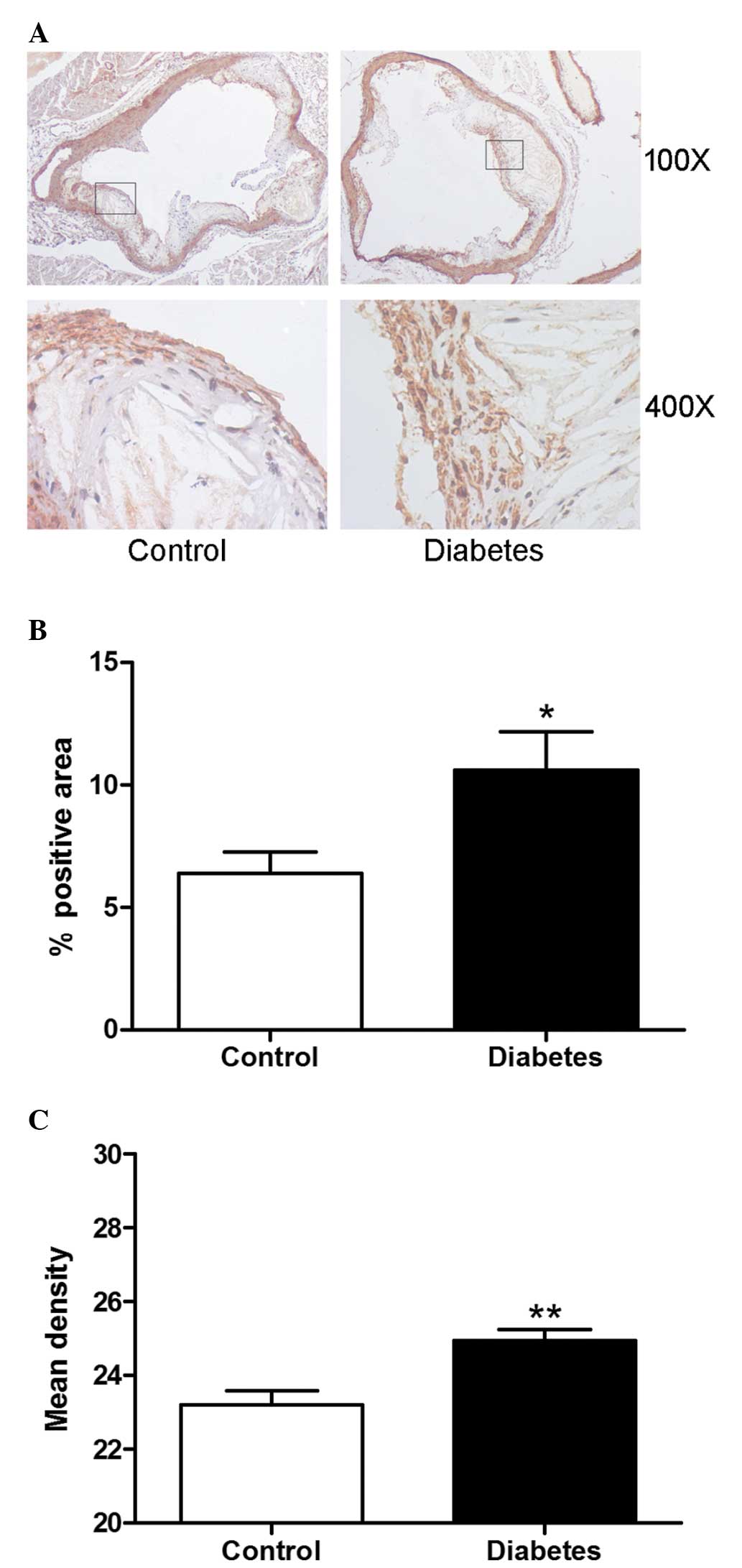
Diabetes increases VSMC content in
atherosclerotic plaques
The proliferation of VSMCs in the atherosclerotic
plaque and the vascular wall contributes to the development of
restenosis. The VSMCs in atherosclerotic plaques were detected by
immunohistochemistry staining. The content of VSMCs in diabetic
mice expressed as the positive area or mean density was
significantly higher than that in the control group (P<0.01;
Fig 3A, B and C).
Diabetes inhibits the mTOR signaling
pathway
The mTOR signaling pathway and its downstream
kinases have been viewed as the major regulators of the
proliferation of VSMCs. The protein expression of mTOR in
atherosclerotic plaques from diabetic mice was significantly
decreased compared with that of the control animals (P<0.05;
Fig. 4A and C). The protein
expression of mTOR, p-mTOR and its major downstream kinases p-4EBP1
and p-S6K1 in aortic tissue were significantly downregulated in the
diabetic mice compared with the control group (all P<0.01;
Fig. 4B and D).
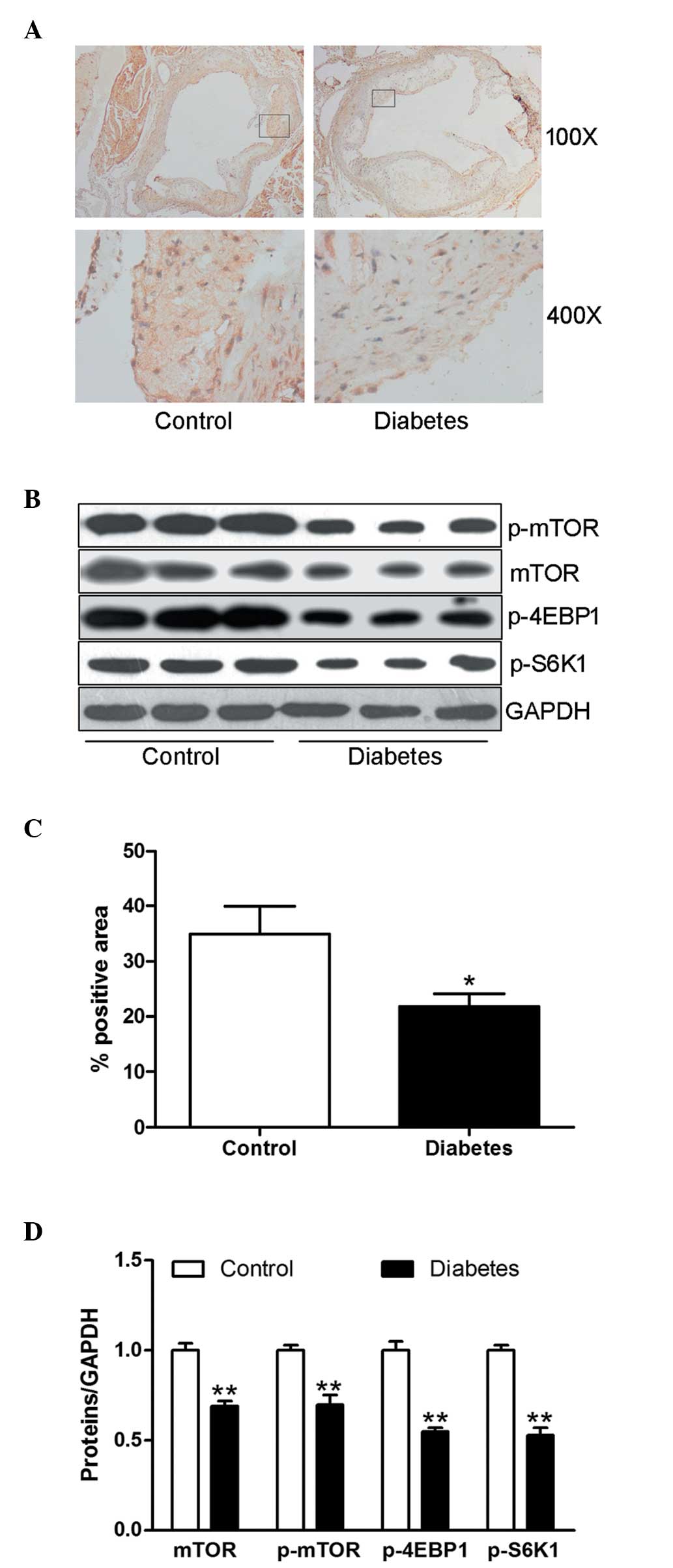 | Figure 4Diabetes blunts the mTOR signaling
pathway. (A) Representative images of immunohistochemical staining
demonstrating the expression of mTOR in the aortic atherosclerotic
plaque. Upper panel, magnification, ×100; lower panel,
magnification, ×400. (B) Protein expression of mTOR, p-mTOR,
p-4EBP1 and p-S6K1 in aortic tissues was determined by western
blotting using specific antibodies. Equal protein loading was
confirmed using the GAPDH antibody. (C) Expression level of mTOR
was determined by the positively stained area (brown) percentage.
(D) Ratios of mTOR, p-mTOR, p-4EBP1 and p-S6K1 to GAPDH are shown
in the bar graph. The data are expressed as the mean ± standard
error of the mean; n=8 mice in the control group and six mice in
the diabetic group. *P<0.05 and
**P<0.01, compared with the control group. mTOR,
mammalian target of rapamycin; p-mTOR, phosphor-mTOR; p-4EBP1,
phospho-4E-binding protein 1; p-S6K1, phospho-ribosomal S6 kinase
1; GAPDH, glyceraldehyde-3-phosphate dehydrogenase. |
Discussion
Our previous clinical follow-up study demonstrated
that diabetes remains an independent risk factor for restenosis
following DES in coronary arteries (6). There is also evidence that the
efficacy of DES in the diabetic state varied with the type of stent
(everolimus eluting stents and sirolimus eluting stents >
paclitaxel eluting stents and zotarolimus eluting stents) (14). These results suggest that diabetic
patients have a distinct expressional model of the target of eluted
drug. Therefore, the present study hypothesized that rapamycin
resistance in diabetes is attributed to alterations in the
expression of the rapamycin target mTOR and its downstream
signaling pathway. The present study provides compelling evidence
supporting the concept that diabetes not only significantly
enhances the growth of atherosclerotic plaques and the
proliferation of VSMCs, but also downregulates the expression of
mTOR, 4EBP1 and S6K1. Therefore, alterations in the expression of
the mTOR signaling pathway may mediate the clinical phenomenon of
rapamycin resistance.
Although the macrophage-derived foam cell is
important in the development of atherosclerosis, the migration and
proliferation of VSMCs in the arterial intima are also key events
in the formation of atherosclerotic lesions (15). The proliferation of VSMCs is not
only involved in the formation of plaques, but also contributes to
the process of in-stent restenosis. Growth factors, including
platelet-derived growth factor, basic fibroblast growth factor and
insulin-like growth factor-1, stimulate VSMCs to enter the cell
cycle and proliferate (16).
Currently, DESs delivering rapamycin, rapamycin analogues or
paclitaxel were used to prevent in-stent restenosis through
inhibiting the proliferation of VSMCs (17). The specific target of rapamycin is
mTOR. The mTOR pathway regulates VSMC growth through effectors,
including 4EBP1 and S6K1. Diabetic patients remain at an increased
risk of developing in-stent restenosis despite the use of rapamycin
eluting stents, which was observed in the present study and in
other studies (6,18). The phenomenon of rapamycin
resistance may result from two distinct issues. One is that the
mTOR signaling pathway is impaired in diabetes and the other is
that the mTOR signaling pathway is over-activated by diabetes. A
previous in vitro experiment demonstrated that increased
leptin levels, which mimicked hyperleptinemia in diabetes and
metabolic syndrome, may activate the mTOR signaling pathway in
primary cultured VSMCs (19),
suggesting that a higher dose of rapamycin may be more effective in
limiting in-stent restenosis in the setting of hyperleptinemia.
However, the present in vivo study demonstrated that the
mTOR signaling pathway is downregulated in the setting of
hyperglycemia. This result suggests that a higher dose of rapamycin
is not an effective therapeutic strategy to limit in-stent
restenosis.
Based on the current available evidence, the
critical mechanism mediating in-stent restenosis in the setting of
diabetes has not been elucidated. As the mTOR signaling pathway has
been demonstrated to be inhibited by diabetes, certain other
signaling pathways may exhibit compensatory upregulation or
activation. The underlying mechanisms controlling the proliferation
of VSMCs in the setting of hyperglycemia remain to be revealed in
future studies. The in-stent restenosis in diabetes may be resolved
by using two or multiple DES.
In conclusion, decreases in the expression and
phosphorylation of mTOR and its downstream kinases may be one of
the molecular mechanisms underlying rapamycin resistance. Future
investigation is required to identify potential targets involved in
in-stent restenosis under diabetic conditions.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81070191 and
81100232).
References
|
1
|
Ohira T and Iso H: Cardiovascular disease
epidemiology in Asia: an overview. Circ J. 77:1646–1652. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Indermuehle A, Bahl R, Lansky AJ, et al:
Drug-eluting balloon angioplasty for in-stent restenosis: a
systematic review and meta-analysis of randomised controlled
trials. Heart. 99:327–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chaabane C, Otsuka F, Virmani R and
Bochaton-Piallat ML: Biological responses in stented arteries.
Cardiovasc Res. 99:353–363. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Caterina AR, Cuculi F and Banning AP:
Incidence, predictors and management of left main coronary artery
stent restenosis: a comprehensive review in the era of drug-eluting
stents. EuroIntervention. 8:1326–1334. 2013.PubMed/NCBI
|
|
5
|
Pate GE, Lee M, Humphries K, et al:
Characterizing the spectrum of in-stent restenosis: implications
for contemporary treatment. Can J Cardiol. 22:1223–1229. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ma S, Yang D, Zhang X, et al: Comparison
of restenosis rate with sirolimus-eluting stent in STEMI patients
with and without diabetes at 6-month angiographic follow-up. Acta
Cardiol. 66:603–606. 2011.PubMed/NCBI
|
|
7
|
Trovati M, Doronzo G, Barale C, Vaccheris
C, Russo I and Cavalot F: Leptin and vascular smooth muscle cells.
Curr Pharm Des. May 14–2013.(Epub ahead of print).
|
|
8
|
Chong ZZ and Maiese K: Mammalian target of
rapamycin signaling in diabetic cardiovascular disease. Cardiovasc
Diabetol. 11:452012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li W, Li Q, Qin L, et al: Rapamycin
inhibits smooth muscle cell proliferation and obstructive
arteriopathy attributable to elastin deficiency. Arterioscler
Thromb Vasc Biol. 33:1028–1035. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ma S, Yang D, Li D, Tang B and Yang Y:
Oleic acid induces smooth muscle foam cell formation and enhances
atherosclerotic lesion development via CD36. Lipids Health Dis.
10:532011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin F, Jiang S, Yang D, et al: Acipimox
attenuates atherosclerosis and enhances plaque stability in
ApoE-deficient mice fed a palmitate-rich diet. Biochem Biophys Res
Commun. 428:86–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang S, Jin F, Li D, et al: Intermittent
hypobaric hypoxia promotes atherosclerotic plaque instability in
ApoE-deficient mice. High Alt Med Biol. 14:175–180. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ma S, Yang D, Li D, Tang B, Sun M and Yang
Y: Cardiac extracellular matrix tenascin-C deposition during
fibronectin degradation. Biochem Biophys Res Commun. 409:321–327.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bangalore S, Kumar S, Fusaro M, et al:
Outcomes with various drug eluting or bare metal stents in patients
with diabetes mellitus: mixed treatment comparison analysis of
22,844 patient years of follow-up from randomised trials. BMJ.
345:e51702012.
|
|
15
|
Koga J and Aikawa M: Crosstalk between
macrophages and smooth muscle cells in atherosclerotic vascular
diseases. Vascul Pharmacol. 57:24–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jung F, Haendeler J, Goebel C, Zeiher AM
and Dimmeler S: Growth factor-induced phosphoinositide 3-OH
kinase/Akt phosphorylation in smooth muscle cells: induction of
cell proliferation and inhibition of cell death. Cardiovasc Res.
48:148–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marx SO, Totary-Jain H and Marks AR:
Vascular smooth muscle cell proliferation in restenosis. Circ
Cardiovasc Interv. 4:104–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scheen AJ, Warzée F and Legrand VM:
Drug-eluting stents: meta-analysis in diabetic patients. Eur Heart
J. 25:2167–2168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shan J, Nguyen TB, Totary-Jain H, Dansky
H, Marx SO and Marks AR: Leptin-enhanced neointimal hyperplasia is
reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci USA.
105:19006–19011. 2008. View Article : Google Scholar : PubMed/NCBI
|