Introduction
Dilated cardiomyopathy (DCM), the most common of the
cardiomyopathies and is characterized by the progressive
deterioration of myocardial contractile function and ventricular
dilation (1,2). The five-year survival rate following
diagnosis is 50%, as patients often develop progressive congestive
heart failure and complications, including thromboembolic
conditions and arrhythmias (2,3).
Although it has been described that the dysregulation of T-cells,
including T helper (Th) 1 cells, Th2, Th17 and regulatory T-cells,
have a critical role in the autoimmune response of DCM (4–7),
with the discovery of the anti-heart antibodies, including the
autoantibody against the adenine nucleotide translocator (ANT)
(8), the pathophysiology of DCM
remains to be fully elucidated.
Th22 cells are a novel subset of T cells clearly
separated from other known Th cells. Th22 is distinct from
interleukin (IL)-17-secreting and retinoic acid receptor-related
orphan receptor γt-regulated Th17, interferon (IFN)-γ-secreting and
T-bet-regulated Th1 or IL-4-secreting and trans-acting
T-cell-specific transcription factor GATA-3-regulated Th2 cells.
Th22 is characterized by producing abundant cytokine IL-22 and is
regulated by the key transcription factor aryl hydrocarbon receptor
(AHR) and other cytokines, including tumor necrosis factor-α and
IL-6 (9,10). Excluding the chemokine receptor C-C
chemokine receptor (CCR) type 10, Th22 shares common surface
markers with Th17 cells, including CCR6 and CCR4. In previous
years, studies have focused on the participation of Th22 cells in
various human inflammatory disorders and immune-mediated tissue
injury, including rheumatoid arthritis, Crohn’s disease and
systemic sclerosis. The elevation of circulating Th22 cells was
positively correlated with the levels of autoimmune antibodies,
Th17, Th1 cells and the severity of several diseases (11–15).
Up to date, the involvement of IL-22 in DCM has remained to be
elucidated and no data exist with regard to Th22 cells and their
association with Th17 or Th1 in patients with DCM.
In the present study, the percentages of peripheral
Th22, Th17 and Th1, the expression of AHR in peripheral blood
mononuclear cells (PBMCs) as well as the levels of IL-22, ANT,
brain natriuretic peptide (BNP), erythrocyte sedimentation rate
(ESR) and C-reactive protein (CRP) in the peripheral blood of
patients with DCM were measured, and their relevance was
evaluated.
Materials and methods
Patients and controls
The study included 60 individuals, comprising 30
cases with primary DCM and 30 normal individuals (controls) and was
conducted between December 2010 and February 2013. Primary DCM was
diagnosed based on the guidelines of the World Health Organization
(1). Accordingly, various
secondary causes of heart failure, including coronary artery
disease and valvular diseases, were excluded in order to diminish
the potential confounding effects of etiological heterogeneity of
heart failure. A control group of normal individuals, defined as
asymptomatic age- and gender-matched individuals with normal
electrocardiograms and echocardiograms, was included. A normal
echocardiogram excluded the diagnosis of DCM in the control
individuals. Patients who were receiving anti-inflammatory or
immunosuppressive medications, recently treated with antibiotics
and other underlying acute or chronic diseases, were all excluded
from the present study. To reduce the potential confounding effects
of diabetes mellitus, those who had this disease were excluded. The
present study was performed in accordance with protocols approved
by the Guangxi Medical University Ethics Committee (Guangxi, China)
and the participants gave informed consent. The clinical data,
including ESR and CRP were also collected. The characteristics of
the study subjects are summarized in Table I.
 | Table ICharacteristics of patients with DCM
and control (mean ± standard deviation). |
Table I
Characteristics of patients with DCM
and control (mean ± standard deviation).
| Characteristics | DCM | Control |
|---|
| Patients, n | 30 | 30 |
| Age, years | 51.6±7.1 | 49.8±6.7 |
| Male/female (n) | 8/22 | 8/22 |
| Heart rate,
beats/min | 74.3±12.6 | 77.1±14.1 |
| Systolic BP,
mmHg | 115.2±14.6 | 126.1±17.5 |
| Diastolic BP,
mmHg | 68.2±8.4 | 74.7±11.3 |
| Functional class
(NYHA) |
| I | 4 | 30 |
| II | 6 | 0 |
| III | 9 | 0 |
| IV | 11 | 0 |
| LVEF, % | 23.2±7.2 | 62.5±8.9 |
| LVEDD, mm | 63.8±12.1 | 46.7±6.8 |
| LVESD, mm | 52.1±12.1 | 33.4±6.4 |
| Medications |
| β-blockers | 32 | 0 |
| ACE
inhibitors | 21 | 1 |
| ATR blockers | 3 | 0 |
| Aldosterone
receptor blockers | 10 | 0 |
| Furosemide | 21 | 0 |
| Digoxin | 12 | 0 |
Isolation and stimulation of PBMCs
Peripheral blood was harvested with 50 U/ml heparin
and diluted with an equal volume of phosphate-buffered saline.
PBMCs were isolated using Ficoll-Plaque (Solarbio Science &
Technology, Beijing, China) by gradient centrifugation at 300 × g
and cells were resuspended with a final concentration of
2×106 in complete RPMI-1640 (Solarbio Science &
Technology) supplemented with 100 U/ml penicillin and 100 μg/ml
streptomycin, followed by incubation at 37°C with 5% CO2
for 5 h in the presence of 25 ng/ml phorbol myristate acetate (PMA)
1 μg/ml ionomycin (both from Sigma-Aldrich, St. Louis, MO, USA) and
Golgi Plug (1 μl/106 cells; BD Biosciences, San Diego,
CA, USA).
Flow cytometric analysis
The expression of markers on T cells from blood were
determined by flow cytometry following surface or intracellular
staining with anti-human-specific antibodies. Briefly, following
incubation, the PBMCs were stained with fluorescein
isothyocyanate-conjugated anti-CD4 monoclonal antibodies (cat no.
555346; BD Biosciences) at room temperature in the dark for 30 min.
Next, the cells were stained with allophycocianin-conjugated
anti-IFN-γ (cat no. 554702; BD Biosciences), peridinin
chlorophyll-CY5.5-conjugated anti-IL-17A (cat no. 560799; BD
Biosciences) and phycoerythrin-conjugated anti-IL-22 monoclonal
antibodies (cat no. 12-7229-42; eBioscience, San Diego, CA, USA)
following fixation and permeabilization. Isotype controls (BD
Bioscience) were given to enable correct compensation and confirm
the antibody specificity. Stained cells were analyzed by flow
cytometric analysis using a FACScan cytometer equipped with
CellQuest software (BD Biosciences). Pure Th22 cells were defined
as IL-22+IL-17−
IFN-γ−CD4+. The Th17 cells were identified as
those that were CD4+IL-17+, and Th1 cells
were CD4+IFN-γ+.
AHR mRNA expression by
quantitative-polymerase chain reaction (qPCR)
TRIzol reagent (Invitrogen, Carlsbad, CA, USA) was
used to isolate the total RNA of PBMCs. The RNA was transcribed
into cDNA with a reverse transcription kit (Ferma, Mountain View,
CA, USA) according to the manufacturer’s instructions. qPCR was
performed for AHR and the housekeeping gene β-actin on an ABI 7500
Real-Time PCR System (Applied Biosystems, Grand Island, NY, USA)
using SYBR® Green. The PCR reactions were cycled 40
times following initial denaturation at 94°C for 3 min with the
following parameters: Denaturation at 94°C for 30 sec, annealing at
60°C for 30 sec and extension at 72°C for 60 sec. The primers used
were as follows: AHR forward, 5′-ACT CCA CTT CAG CCA CCA TC-3′ and
reverse, 3′-ATG GGA CTC GGC ACA ATA AA-5′; β-actin forward, 5′-GCA
AGC AGG AGT ATG ACG AG-3′ and reverse, 3′-CAA ATA AAG CCA TGC CAA
TC-5′. The relative gene expression of AHR was normalized to the
level of β-actin transcripts and quantified by the ΔΔCt method
using 7500 System Sequence Detection software (Applied Biosystems,
Foster City, CA, USA). All experiments were conducted in
triplicate.
IL-22 and BNP assessment by ELISA
Serum was obtained from all subjects by
centrifugation at 200 × g and stored at −80°C for the determination
of cytokine levels. Serum IL-22 levels were determined with a
quantitative sandwich enzyme immunoassay technique following the
manufacturer’s instructions (cat no. BMS2047; eBioscience). The
levels of BNP were measured using ELISA (cat no. CSB-E07970h;
Cusabio, Wuhan, China). The minimum detectable concentration was 5
pg/ml for IL-22 and 23 pg/ml for BNP, respectively. All the samples
were assayed in duplicate.
ANT assessment by ELISA
For the qualitative determination of human ANT
autoantibody concentrations in serum, an ANT autoantibody ELISA kit
was used according to manufacturer’s instructions (cat no.
CSB-EQ027458HU; Cusabio, Wuhan, China). In order to calculate the
valence of the human ANT autoantibody, the sample well was compared
with the control. A cutoff value was defined as the average
negative control value plus 0.2. An optical density (OD) sample
< the cutoff value was negative, while an OD sample ≥ the cut
off value was positive.
Statistical analysis
Quantitative variables were expressed as the mean ±
standard deviation. Differences between two groups were determined
using the Newman-Keuls multiple comparison test (q-test). The
Pearson’s or Spearman’s correlation test was used for correlation
analysis depending on the data distribution. Qualitative variables
of ANT were expressed as percentages and differences in ANT
distribution between groups were obtained using the χ2
test. Analysis was performed using SPSS version 17.0 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Elevated circulating Th22, Th17 and Th1
frequencies in DCM
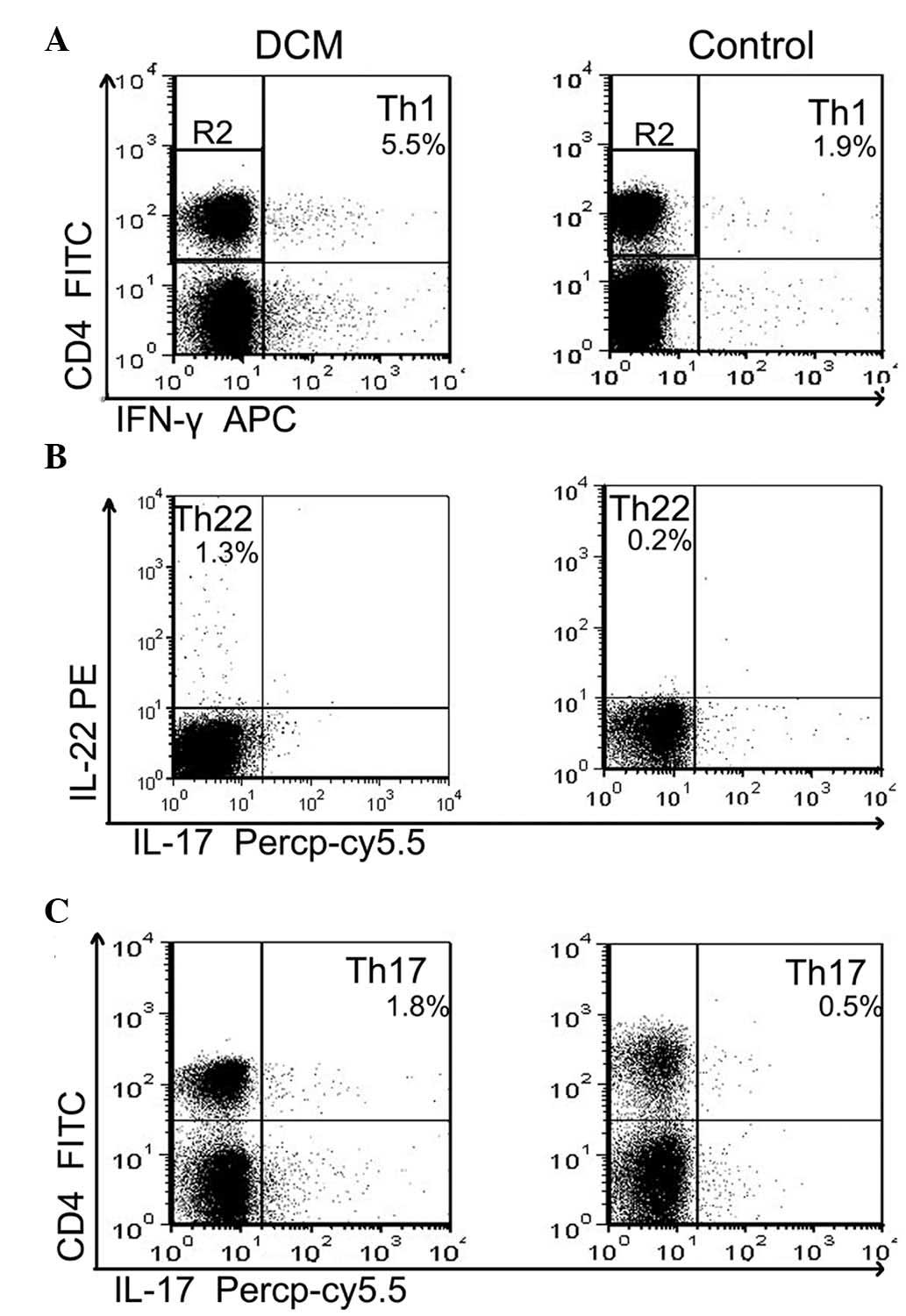
The frequencies of Th22, Th17 and Th1 based on
cytokine patterns following in vitro activation by phorbol
myristate acetate, ionomycin and monensin were analyzed in
short-term cultures. Pure Th22 was defined as
CD4+IFN-γ−IL-17−IL-22+
T cells to exclude Th1 or Th17 cells. Th17 and Th1 cells were
identified as CD4+IL-17+ T cells and
IFN-γ+CD4+ T cells, respectively.
Representative plots of the proportion of Th22, Th17 and Th1 cells
in a representative patient with DCM and a healthy control are
shown in Fig. 1A–C. As shown in
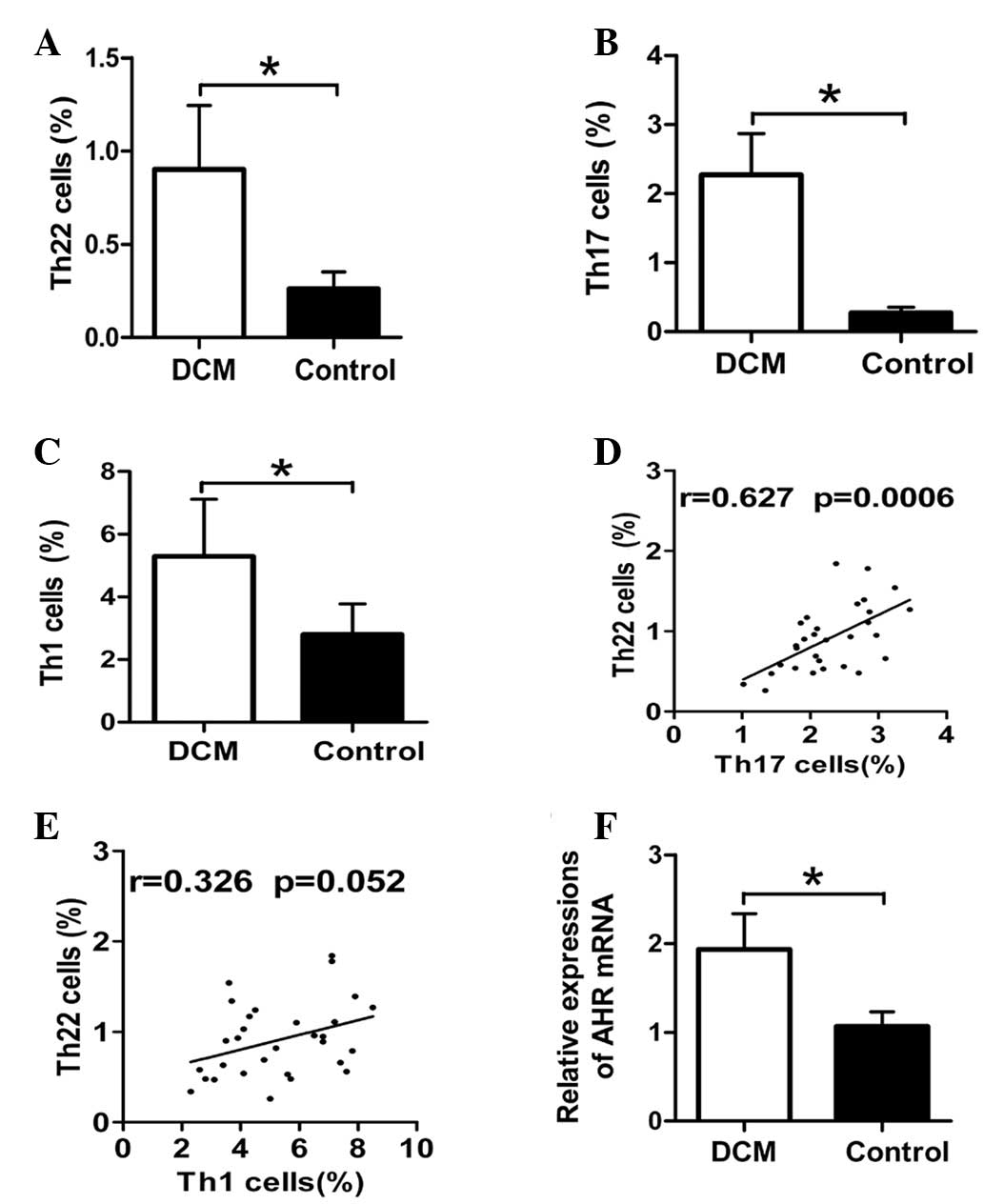
Fig. 2A, the frequencies of Th1
cells were significantly elevated in patients with DCM as compared
with the healthy donors (5.29±1.82 vs. 2.81±0.97%; P<0.01). The
proportions of the peripheral Th22 cells were significant higher in
the DCM group (0.91±0.34%) in comparison with the control group
(0.26±0.08%; P<0.01) (Fig. 2B).
Similarly, the percentages of the Th17 cells were profoundly
increased in the DCM group (2.27±0.59%) as compared with the
control group (0.27±0.07%; P<0.01) (Fig. 2C).
Positive correlation between Th22 and
Th17 cells in DCM
Correlations between the frequencies of Th22, Th17
and Th1 cells in patients with DCM were examined. There was a
significant positive correlation between numbers of Th22 and Th17
cells, as shown in Fig. 2D
(r=0.676 and P<0.001). However, the correlation analysis between
the percentages of Th1 and Th22 cells showed no significant
association in patients with DCM (r=0.326 and P=0.052) (Fig. 2E).
Enhanced mRNA expression of peripheral
AHR in DCM
The mRNA expression of AHR, the key transcription
factor directing Th22 lineage commitment, was determined by qPCR to
further confirm the results of the present study obtained from flow
cytometric analysis. Consistent with the higher proportion of Th22
cells in patients with DCM, the data demonstrated that levels of
the relative AHR transcript were notably higher in the PBMCs of
patients with DCM as compared with those in the healthy control
(1.93±0.4 vs. 1.06±0.16; P<0.01) (Fig. 2F).
Plasma levels of IL-22
Plasma levels of IL-22 were evaluated by ELISA. Of
note, the expression of IL-22 did not differ between patients with
DCM and healthy controls (19.78±5.9 vs. 21.3±5.4 pg/ml; P=0.303)
(Fig. 3A). Furthermore, no
significant correlation was identified between Th22 cells and IL-22
levels (r=0.345 and P=0.061; Fig.
3B), nor between Th17 cells and IL-22 (r=0.235 and P=0.211;
Fig. 3C). As shown in Fig. 3D, the peripheral Th1 cells did not
show a statistical correlation with circulating levels of IL-22
(r=0.221 and P=0.241).
Th22 cells but not IL-22 levels are
increased in ANT antibody-positive patients with DCM
The percentages of ANT-positive individuals among
patients with DCM and healthy controls were 40 and 6.7%,
respectively (P<0.05). It was investigated whether the levels of
Th22 cells and IL-22 in the DCM group (n=30) demonstrated a
difference between ANT-positive and -negative patients. Compared
with ANT-negative patients with DCM, levels of circulating Th22
cells were increased in ANT-positive patients with DCM (1.04±0.32
vs. 0.69±0.26%; P=0.005; Fig. 4A).
However, the levels of plasma IL-22 did not differ between ANT
antibody-positive and −negative individuals (21.11±5.76 vs.
17.78±5.76 pg/ml; P=0.132; Fig.
4B).
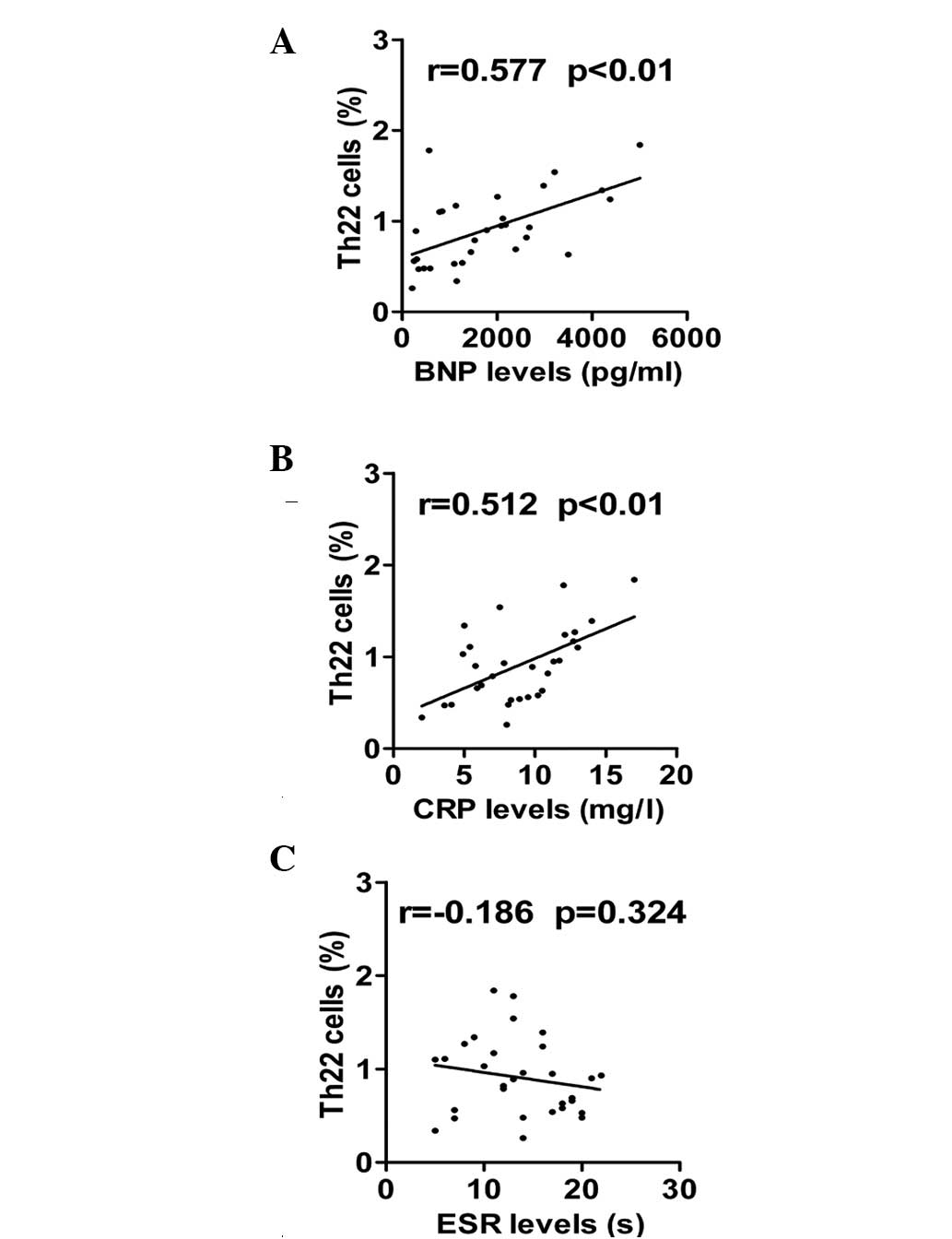
Positive correlation between the
frequencies of Th22 cells with BNP and CRP in patients with
DCM
Finally, the correlation between the percentages of
Th22 cells with the levels of laboratory parameters, including BNP,
CRP and ESR, were explored. Significantly higher levels of BNP were
observed in the DCM group in comparison with the control group
(1781.9±1329.8 vs. 78.6±21.4; P<0.05). As shown in Fig. 5A, there was a significant positive
correlation between proportion of Th22 cells and BNP levels in
patients with DCM (r=0.567 and P<0.01). Additionally, a positive
correlation was found between the frequencies of Th22 cells and the
expression of CRP (r=0.556 and P<0.01; Fig. 5B). By contrast, Th22 cells did not
show a statistically significant correlation with ESR levels
(r=−0.186 and P=0.324; Fig.
5C).
Discussion
The identification of Th17 and Th22 cells as
distinct subsets of CD4+ T cells has extended the
Th1/Th2 paradigm in autoimmune and inflammatory diseases (11–15).
However, to date, no studies have directly assessed the presence of
circulating Th22 cells in patients with DCM. By applying a
multi-parameter cytofluorimetric analysis, the present study
revealed that there were significantly increased frequencies of
Th22 cells in patients with DCM as compared with healthy controls.
In parallel, an elevated expression of AHR, the key transcription
factor for Th22-cell differentiation, was observed in the DCM
group. These data indicated that an enhanced Th22-cell response may
participate in the pathogenesis of DCM. Of note, a previous study
by our group reported elevated levels of Th22 cells in viral
myocarditis (VMC) (16), a disease
which may evolve into DCM (2,3,17).
Thus, whether higher levels of Th22 cells are involved in the
evolvement from VMC to DCM, is an interesting unsettled question.
It should be mentioned that the levels of Th22 cells identified in
the present study appear to differ from those in other reports
(13,15). It is hypothesized that these
discrepancies may be due to different conjugated
anti-human-specific antibodies used in the flow cytometric
analysis. Furthermore, the enrichment of Th22 cells was closely
associated with elevated levels of Th17 cells, indicating a
polyfunctional cytokine profile and a potentially synergistic
function of CD4+ T cells in patients with DCM.
ANT is an autoantibody against the adenosine
diphosphate/adenosine triphosphate carrier. A higher frequency of
Th22 cells was found in ANT antibody-positive patients with DCM as
compared with those in negative subjects. Given that ANT production
may have been induced by a loss of tolerance to cardiac
self-antigens and is capable of cross-reacting with subunits of the
calcium channel on the cardiac cell membrane, which results in
myocardial cytotoxic injury (18,19),
the findings of the present study indicated that the Th22 response
may be involved in organ-specific autoimmunity and myocardial
injury in patients with DCM. In addition, a positive correlation
was identified between the proportions of Th22 cells and the values
of CRP, a significant inflammatory marker and independent predictor
of future cardiovascular events (20,21).
Furthermore, there was a positive correlation between the
percentages of Th22 cells and the levels of BNPs, which are
predominantly secreted by cardiac myocytes in response to
stretching forces (22,23). BNPs have been demonstrated to be
powerful predictors of mortality and major adverse cardiovascular
events in patients with DCM (22–25).
Thus, these observations implied that Th22 cells may be one
predictor for cardiac events in DCM, but further research with
larger subjects and a long-term follow-up are warranted.
IL-22 is the most significant functional cytokine of
Th22 cells and positive correlations have been observed between
elevated levels of IL-22 and Th22 cells in various types of
autoimmune diseases (11–15,26,27).
The present study observed higher levels of Th22 cells in patients
with DCM, and unexpectedly, the plasma levels of IL-22 were
comparable between the DCM and control group. Furthermore, IL-22
levels did not show a statistically significant difference between
ANT-positive and −negative patients with DCM. In addition, plasma
concentrations of IL-22 did not show a statistically significant
correlation with the percentage of Th22 cells, nor did the Th17 and
Th1 cells. The results of the present study were discrepant with
previous observations (11–15,26,27),
which may be due to different biological characteristics among
different autoimmune disorders, indicating that Th22 cells are not
the major producer of IL-22 in DCM. IL-22 may be produced by
various cell types in the microenvironment of DCM, including innate
lymphoid cells, natural killer and γδ T cells, but production is
not limited to Th22, Th1 and Th17 cells (28–30).
Principal limitations of the present study are the
small sample size, observations made at only one time-point and
single blood sampling. Furthermore, due to the limited number of
subjects with DCM, a definitive causal correlation between the Th22
cells and New York Heart Association (NYHA) functional impairment
was not identified. In addition, the subjects of the present study
were primarily comprised of men; thus, these results may not
necessarily apply to women. Further prospective studies enrolling
larger cohorts of patients are required to further elucidate the
pathophysiological function and prognostic role of Th22 cells in
patients with DCM.
In conclusion, the present study demonstrated an
elevation of Th22 cells in patients with DCM, particularly in ANT
autoantibody-positive subjects. These novel findings imply that the
Th22 response may participate in the pathogenesis of DCM. Further
studies in a larger sized population are required to validate these
findings and to determine the role of Th22 in the
immunopathogenesis of DCM.
Acknowledgements
The present study was supported by the National
Natural Science Foundations of China (nos. 81260045 and 81160032).
The authors would like to thank Dr Jiao Lan for technical
assistance.
Abbreviations:
|
qPCR
|
quantitative polymerase chain
reaction
|
|
DCM
|
dilated cardiomyopathy
|
|
IL-22
|
interleukin-22
|
|
ANT
|
adenine nucleotide translocator
|
|
BNP
|
brain natriuretic peptide
|
|
ESR
|
erythrocyte sedimentation rate
|
|
CRP
|
C-reactive protein
|
|
VMC
|
viral myocarditis
|
References
|
1
|
Richardson P, McKenna W, Bristow M, et al:
Report of the 1995 World Health Organisation/International Society
and Federation of Cardiology Task Force on the Definition and
Classification of Cardiomyopathies. Circulation. 93:841–842. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luk A, Ahn E, Soor GS and Butany J:
Dilated cardiomyopathy: a review. J Clin Pathol. 62:219–225. 2009.
View Article : Google Scholar
|
|
3
|
Grogan M, Redfield MM, Bailey KR, et al:
Long-term outcome of patients with biopsy-proven myocarditis:
comparison with idiopathic dilated cardiomyopathy. J Am Coll
Cardiol. 26:80–84. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan J, Cao AL, Yu M, et al: Th17 cells
facilitate the humoral immune response in patients with acute viral
myocarditis. J Clin Immunol. 30:226–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guedes PM, Gutierrez FR, Silva GK, et al:
Deficient regulatory T cell activity and low frequency of
IL-17-producing T cells correlate with the extent of cardiomyopathy
in human Chagas’ disease. PLoS Negl Trop Dis.
6:e16302012.PubMed/NCBI
|
|
6
|
Li J, Wang L, Wang S, et al: The Treg/Th17
imbalance in patients with idiopathic dilated cardiomyopathy. Scand
J Immunol. 71:298–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yi A, Jian L, Xiaojing H and Hui X: The
prevalence of Th17 cells in patients with dilated cardiomyopathy.
Clin Invest Med. 32:E144–E150. 2009.PubMed/NCBI
|
|
8
|
Okazaki T and Honjo T: Pathogenic roles of
cardiac autoantibodies in dilated cardiomyopathy. Trends Mol Med.
11:322–326. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duhen T, Geiger R, Jarrossay D,
Lanzavecchia A and Sallusto F: Production of interleukin 22 but not
interleukin 17 by a subset of human skin-homing memory T cells. Nat
Immunol. 10:857–863. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang N, Pan HF and Ye DQ: Th22 in
inflammatory and autoimmune disease: prospects for therapeutic
intervention. Mol Cell Biochem. 353:41–46. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tian T, Yu S and Ma D: Th22 and related
cytokines in inflammatory and autoimmune diseases. Expert Opin Ther
Targets. 17:113–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen H, Goodall JC and Hill Gaston JS:
Frequency and phenotype of peripheral blood Th17 cells in
ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum.
60:1647–1656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Truchetet ME, Brembilla NC, Montanari E,
Allanore Y and Chizzolini C: Increased frequency of circulating
Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis:
association with interstitial lung disease. Arthritis Res Ther.
13:R1662011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baba N, Rubio M, Kenins L, et al: The aryl
hydrocarbon receptor (AhR) ligand VAF347 selectively acts on
monocytes and naïve CD4(+) Th cells to promote the development of
IL-22-secreting Th cells. Hum Immunol. 73:795–800. 2012.PubMed/NCBI
|
|
15
|
Hu Y, Li H, Zhang L, et al: Elevated
profiles of Th22 cells and correlations with Th17 cells in patients
with immune thrombocytopenia. Hum Immunol. 73:629–635. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kong Q, Wu W, Yang F, et al: Increased
expressions of IL-22 and Th22 cells in the coxsackievirus
B3-induced mice acute viral myocarditis. Virol J. 9:2322012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maron BJ, Towbin JA, Thiene G, et al:
Contemporary definitions and classification of the
Cardiomyopathies: an American Heart Association Scientific
Statement From the Council on Clinical Cardiology, Heart Failure
and Transplantation Committee; Quality of Care and Outcomes
Research and Functional Genomics and Translational Biology
Interdisciplinary Working Groups; and Council on Epidemiology and
Prevention. Circulation. 113:1807–1816. 2006.
|
|
18
|
Liao YH: Functional analysis of
autoantibodies against ADP/ATP carrier from dilated cardiomyopathy.
Int J Cardiol. 54:165–169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schulze K, Witzenbichler B, Christmann C
and Schultheiss HP: Disturbance of myocardial energy metabolism in
experimental virus myocarditis by antibodies against the adenine
nucleotide translocator. Cardiovasc Res. 44:91–100. 1999.
View Article : Google Scholar
|
|
20
|
De Gennaro L, Brunetti ND, Cuculo A,
Pellegrino PL and Di Biase M: Systemic inflammation in nonischemic
dilated cardiomyopathy. Heart Vessels. 23:445–450. 2008.PubMed/NCBI
|
|
21
|
Kones R: Rosuvastatin, inflammation,
C-reactive protein, JUPITER, and primary prevention of
cardiovascular disease - a perspective. Drug Des Devel Ther.
4:383–413. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang F, Wu Y, Tang L, et al: Brain
natriuretic peptide for prediction of mortality in patients with
sepsis: a systematic review and meta-analysis. Crit Care.
16:R742012. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goetze JP, Christoffersen C, Perko M,
Arendrup H, Rehfeld JF, Kastrup J and Nielsen LB: Increased cardiac
BNP expression associated with myocardial ischemia. FASEB J.
17:1105–1107. 2003.PubMed/NCBI
|
|
24
|
Nishii M, Inomata T, Takehana H, et al:
Prognostic utility of B-type natriuretic peptide assessment in
stable low-risk outpatients with nonischemic cardiomyopathy after
decompensated heart failure. J Am Coll Cardiol. 51:2329–2335. 2008.
View Article : Google Scholar
|
|
25
|
Shimazu S, Hirashiki A, Okumura T, et al:
Association between indoxyl sulfate and cardiac dysfunction and
prognosis in patients with dilated cardiomyopathy. Circ J.
77:390–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peng D, Xu B, Wang Y, Guo H and Jiang Y: A
high frequency of circulating Th22 and Th17 cells in patients with
new onset graves’ disease. PLoS One. 8:e684462013.PubMed/NCBI
|
|
27
|
Yang XY, Wang HY, Zhao XY, Wang LJ, Lv QH
and Wang QQ: Th22, but not Th17 might be a good index to predict
the tissue involvement of systemic lupus erythematosus. J Clin
Immunol. 33:767–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolk K, Kunz S, Asadullah K and Sabat R:
Cutting edge: immune cells as sources and targets of the IL-10
family members? J Immunol. 168:5397–5402. 2002.PubMed/NCBI
|
|
29
|
Wolk K, Witte E, Wallace E, et al: IL-22
regulates the expression of genes responsible for antimicrobial
defense, cellular differentiation, and mobility in keratinocytes: a
potential role in psoriasis. Eur J Immunol. 36:1309–1323. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Simonian PL, Wehrmann F, Roark CL, Born
WK, O’Brien RL and Fontenot AP: gammadelta T cells protect against
lung fibrosis via IL-22. J Exp Med. 207:2239–2253. 2010. View Article : Google Scholar : PubMed/NCBI
|