Introduction
Saikosaponins (SSs) are triterpene saponins isolated
from the root of Bupleurum falcatum L. (Umbelliferae)
(1). As all SSs have a common
steroid-like structure, they were expected and also confirmed to
exert a number of steroid-associated pharmacological activities
(2,3). SSs are also regarded as the major
effective components of xiao-chai-hu-tang, one of the most popular
Chinese medicinal formulae that has been widely used for its
various pharmacological effects, including anti-inflammatory,
antioxidant and antihepatic fibrosis properties (4–6).
Thus far, at least 10 types of SS have been identified, and among
which saikosaponin-d (SSd) is considered to be the most active
component (1,7). Studies have reported that SS may
potently inhibit the proliferation of hepatocellular carcinoma
(8), cervical cancer (9), lung adenocarcinoma (10), colon carcinoma (11), breast cancer (12) and melanoma (13) cells. However, the effect of SS on
human prostate cancer cell lines remains to be elucidated.
Prostate cancer is one of the most commonly
diagnosed cancers among males, and is the second most common cause
of cancer mortalities in developed countries (14). With the developments in diagnosis
and therapy, the mortality rate of prostate cancer has decreased
significantly (15–18). Although prostate cancers are
initially treatable, the androgen-insensitive or hormone-refractory
recurrent cases of prostate cancer are not responsive to current
therapies. Therefore, there is an urgent requirement for novel
therapeutic agents.
In the present study, the anti-proliferative effects
and associated mechanisms of SSd on the DU145 human prostate cancer
cell line were investigated for the first time, to the best of our
knowledge.
Materials and methods
Reagents
SSd was obtained from the National Institute for the
Control of Pharmaceutical and Biological Products (Beijing, China).
Dimethyl sulfoxide (DMSO) was purchased from Sangon Biotech
(Shanghai) Co., Ltd. (Shanghai, China). Rabbit anti-human
cleaved-caspase-3, Bcl-2-associated X protein (Bax), p21, p53,
cytochrome-c, mouse anti-human B-cell lymphoma 2 (Bcl-2) and
β-actin primary antibodies were purchased from Cell Signaling
Technology, Inc. (Shanghai, China). Horseradish
peroxidase-conjugated secondary antibodies (anti-mouse and
anti-rabbit) were purchased from Santa Cruz Biotechnology, Inc.
(Beijing, China). Trypsin, Hoechst 33258, Rhodamine 123 (Rho-123),
penicillin and streptomycin were purchased from Sigma-Aldrich
(Beijing, China).
Cell culture
The DU145 human prostate cancer cell line (ATCC
HTB-81) was obtained from the American Type Culture Collection
(Manassas, VA, USA), and cultured in Dulbecco’s modified Eagle’s
medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum
(HyClone Laboratories, Inc., Logan, UT, USA), 100 U/ml penicillin
and 100 μg/ml streptomycin in a CO2 incubator (37°C, 5%
CO2, 95% humidity).
MTT assay
Cell viability was determined by an MTT assay as
previously described (19).
Briefly, 100 μl cell suspension (1×105 cells) was seeded
in 96-well plates and incubated for 12 h, and then the cells were
exposed to SSd (0, 1, 2.5, 5, 10, 20 or 50 μM) for 24 h.
Following treatment, 20 μl MTT (5 mg/ml) was added and the cells
were incubated at 37°C for 4 h. The culture medium was removed and
150 μl DMSO was added to each well to dissolve the formazan
crystals. The absorbance (570 nm) was measured using a microplate
reader (Varioskan Flash; Thermo Fisher Scientific, Waltham, MA,
USA). The percentage of viable cells was determined using the
following formula: Cell viability (%) = (A570 treated / A570
control) × 100; and the IC50-values were calculated
using GraphPad Prism, version 5 (GraphPad Software, Inc., La Jolla,
CA, USA).
Cellular morphological changes
DU145 cells were incubated with different
concentrations (3, 9 or 15 μM) of SSd for 24 h.
Morphological changes of the cells were visualized under a phase
contrast microscope (1×71; Olympus Corporation, Tokyo, Japan),
recorded with a charge-coupled device (CCD) camera (DP72; Olympus
Corporation) and analyzed using DP2-BSW software, version 2.2
(Olympus Corporation). For mouse splenocyte morphological study,
the cells were analyzed by trypan blue (0.4%) staining prior to the
microscopic visualization.
Flow cytometric analysis of
apoptosis
Cell apoptosis was detected by flow cytometry using
an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis Detection
kit (Beyotime Institute of Biotechnology, Shanghai, China).
Briefly, DU145 cells cultured in six-well tissue culture plates
were treated with different concentrations of SSd for 12 h.
Following treatment, the cells were collected and washed twice with
ice-chilled phosphate-buffered saline (PBS). The cell pellets were
stained with Annexin V-FITC and propidium iodide (PI) according to
the manufacturer’s instructions. Late apoptosis was defined as
Annexin V-positive/PI-positive and early apoptosis was defined as
Annexin V-positive/PI-negative as determined by flow cytometry
(Epics XL; Beckman Coulter, Miami, FL, USA).
Nuclear staining
Following exposure to the test compound for 12 h,
the DU145 cells were harvested and washed twice with PBS. The cells
were then stained with Hoechst 33258 (50 μg/ml) at 37°C for 10 min
in the dark. After the staining, the cells were washed twice with
PBS and analyzed using a fluorescence microscope (1×71; Olympus
Corporation) installed with a CCD camera (DP72; Olympus
Corporation) and analyzed using DP2-BSW software (Olympus
Corporation). Apoptotic cells were defined as cells exhibiting
nuclear shrinkage and chromatin condensation.
Flow cytometric analysis of the cell
cycle
DU145 cells were treated with different
concentrations (3, 9 and 15 μM) of SSd for 12 h, trypsinized,
washed twice with PBS and fixed in 70% ice-cold ethanol overnight.
The fixed cells were rinsed twice with PBS and then stained with 50
μg/ml PI (containing 100 μg/ml RNase A) using a Cell Cycle and
Apoptosis Analysis kit (Beyotime Institute of Biotechnology)
according to the manufacturer’s instructions. Cell cycle phase
distributions of nuclear DNA were assayed using flow cytometry
(Epics XL; Beckman Coulter) and CellQuest software (BD Biosciences,
Franklin Lakes, NJ, USA).
Reactive oxygen species (ROS) generation
detection
DU145 cells cultured in six-well tissue culture
plates were treated with different concentrations of SSd. The cells
were then stained with 10 μmol/l
2′,7′-dichlorofluorescein-diacetate using a ROS Assay kit (Beyotime
Institute of Biotechnology) according to the manufacturer’s
instructions. The cells were then collected, washed three times
with PBS and assayed using flow cytometry (Epics XL; Beckman
Coulter) as described previously (20).
Mitochondrial membrane potential (MMP)
determination
DU145 cells were treated with different
concentrations of SSd for 12 h. The cells were trypsinized,
collected in a centrifuge tube, and then stained with Rho-123 (10
μg/ml) at 37°C for 30 min in the dark. Following staining, the
cells were washed three times with PBS and assayed using flow
cytometry (Epics XL; Beckman Coulter).
Western blot analysis
DU145 cells were treated with different
concentrations of SSd for 12 h and then cell extracts were prepared
using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology). The cell lysates (containing 40 μg protein) were
subjected to SDS-PAGE and analyzed by western blotting using
various antibodies according to standard protocols. The proteins
were visualized using an Enhanced Chemiluminescence Plus kit
(Millipore Corporation, Billerica, MA, USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean from at least three independent experiments.
Statistical analysis was performed using Student’s t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Antiproliferative effect of SSd on DU145
human prostate carcinoma cells
The inhibitory effects of SSd (chemical structure is
shown in Fig. 1A) on the
proliferation of DU145 human prostate carcinoma cells were
determined using an MTT assay. As shown in Fig. 1B, SSd treatment induced
concentration-dependent proliferation inhibition of the DU145
cells. At 24 h, maximal inhibition was achieved with 50 μM SSd,
which inhibited 80% of the DU145 cells proliferation, and the
IC50-value of the inhibition was ~10 μM. Morphological
changes of the DU145 cells treated with SSd were visualized under a
phase contrast microscope, which revealed a reduced number of
adherent cells accompanying an increased number of floating cells
in the culture medium compared with those in the culture medium
containing the untreated cells (Fig.
1C). Furthermore, the effect of SSd on primarily cultured mouse
splenocytes was investigated and trypan blue analysis indicated
that SSd had little toxicity on the cells compared with the control
cells (Fig. 1D).
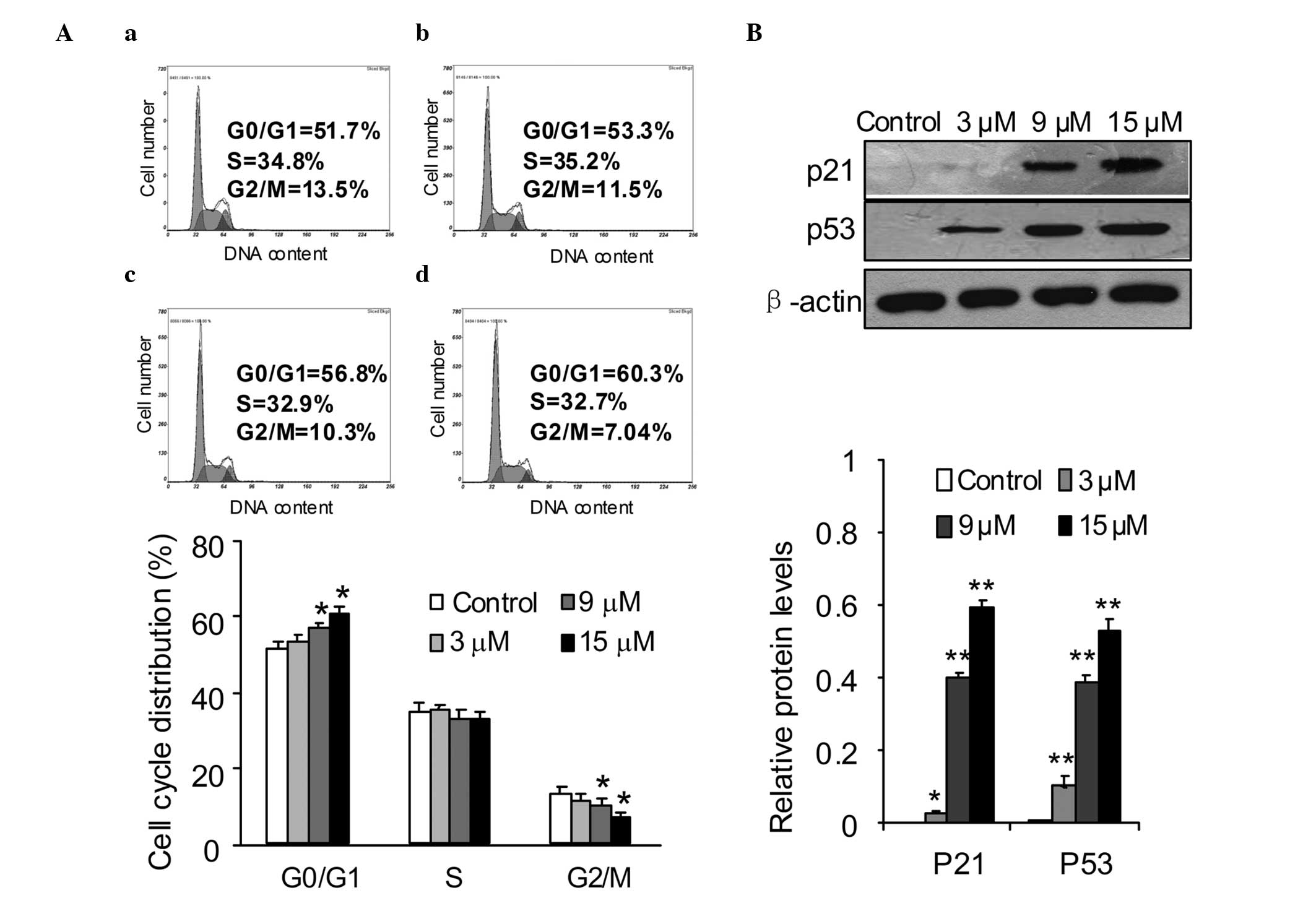
Effects of SSd on cell cycle
distribution
Cell cycle arrest and apoptosis are major causes of
cell proliferation inhibition (20–22).
To investigate the mechanisms responsible for SSd-induced
inhibition of DU145 cell proliferation, the cell cycle distribution
affected by SSd was measured. DU145 cells were exposed to different
concentrations of SSd for 12 h and then the cell cycle
distributions were determined using PI staining and flow cytometric
analysis. The data indicated that SSd caused a significant
accumulation of DU145 cells in G0/G1 phase compared with that of
the control cells. Compared with that of the DMSO control, the
proportions of cells in G0/G1 phase were increased from 51.7 to
53.3, 56.8 and 60.3% following treatment with 3, 9 and 15 μM SSd,
respectively (Fig. 2A). To
elucidate the molecular mechanism underlying the G0/G1 phase arrest
induced by SSd, several key proteins involved in the G1 phase
transition were investigated in DU145 cells. The cells were treated
with 3, 9 and 15 μM SSd for 12 h and the expression levels of p53
and p21 proteins were analyzed by western blotting. The data showed
that SSd treatment significantly increased the expression levels of
p53 and p21 compared with those of the control cells (Fig. 2B).
Effects of SSd on apoptosis
induction
Subsequently, the effect of SSd on the induction of
apoptosis of DU145 cells was investigated. Cell apoptosis, a type
of programmed cell death, is characterized by nuclear condensation,
cell shrinkage, membrane blebbing and DNA fragmentation (23), with nuclear condensation being a
key characteristic (24).
Morphological changes of the cell nuclei were observed using
Hoechst 33258 staining. The results revealed that treatment of
DU145 cells with SSd resulted in significant levels of nuclear
condensation: 3, 9 and 15 μM SSd treatment increased the percentage
of cleaved nuclei from 5.13±0.84 (in the DMSO control group) to
10.01±1.71, 24.50±1.82 and 51.44±2.39%, respectively (Fig. 3A).
 | Figure 3Induction of apoptosis of DU145 cells
by SSd. (A) Cells were treated with (a) control, (b) 3 μM, (c) 9 μM
and (d) 15 μM SSd for 2 h, and stained with Hoechst 33258. The
stained cells were observed under a fluorescence microscope. Arrows
indicate the condensed and fragmented nuclei. Scale bar, 20 μm.
Histogram shows the percentage of cleaved nuclei counted
microscopically from 100 nuclei. Data are expressed as the mean ±
standard error of three independent experiments with the similar
results. Magnification, ×400. (B) DU145 cells treated with (a)
control, or (b) 3 μM, (c) 9 μM and (d) 15 μM SSd for 12 h. The
cells were then stained with FITC-conjugated Annexin V and PI for
flow cytometric analysis. The x-axis and y-axis represent Annexin
V-FITC staining and PI, respectively. The cell populations shown in
the lower right quadrant (Annexin V+/PI−)
represent apoptotic cells, and those in the quadrant upper right
(Annexin V+/PI+) represent necrotic cells.
*P<0.05, **P<0.01 compared with the
control. SSd, saikosaponin-d; FITC, fluorescein isothiocyanate; PI,
propidium iodide. |
To further quantify the SSd-induced apoptotic
effect, the treated cells were stained with Annexin V-FITC/PI and
assayed using flow cytometry. A concentration-dependent increase in
the percentages of necrotic (Annexin V-positive, PI-positive) and
apoptotic (Annexin V-positive, PI-negative) cells was observed.
Treatment of the DU145 cells with 3, 9 and 15 μM SSd for 12 h
increased the rate of apoptosis from 7.37±2.39 to 27.26±2.68,
46.43±4.43 and 75.77±3.01%, respectively, with <2% of cells
being necrotic (Fig. 3B). Notably,
~75% of the cells were apoptotic in the 15 μM SSd treatment group
after 12 h, with a concomitant <10% increase in the G1 phase
proportion (Fig. 2A). G1 phase
arrest may only minimally account for the antiproliferative effect
of SSd observed in DU145 cells.
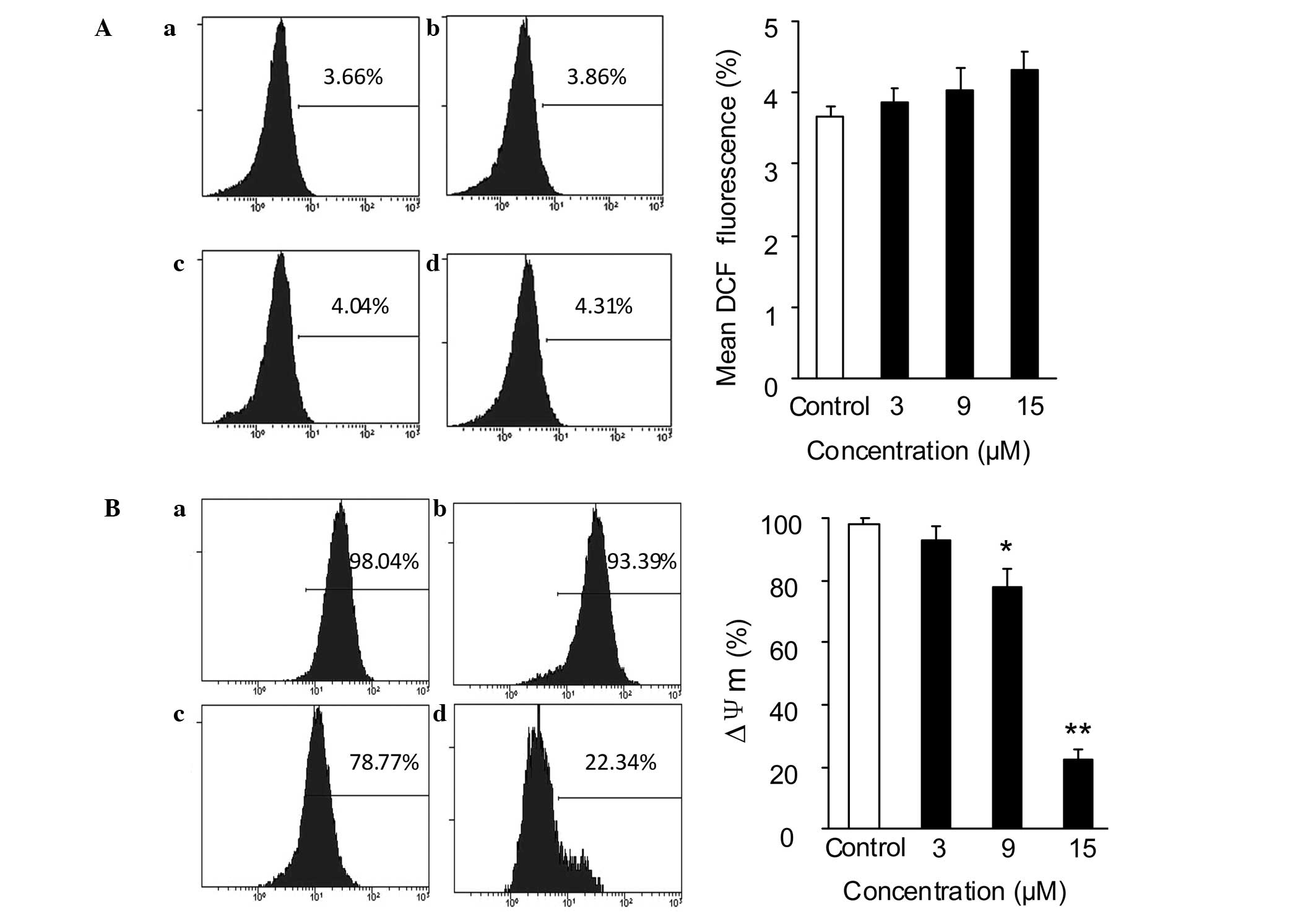
Effect of SSd on ROS generation and
MMP
The mitochondria-mediated intracellular signaling
pathway is characterized by increased ROS generation, MMP
dissipation and release of cytochrome c from the
mitochondria (25). A study
indicated that SSd induced cellular ROS accumulation in cervical
(HeLa and Siha), ovarian (SKOV3) and non-small cell lung (A549)
cancer cell lines (9). Thus, the
present study investigated the effect of SSd on the mitochondrial
signaling pathway. ROS generation in DU145 cells was detected using
a ROS Assay kit. As shown in Fig.
4A, following incubation with 3, 9 or 15 μM SSd for 30 min, the
ROS levels in the SSd-treated group remained almost unchanged
compared with those in the control group.
As depolarization of the MMP is a characteristic
feature of apoptosis (26,27), the MMP in DU145 cells was
subsequently determined using Rho-123 staining and flow cytometry
assay. The DU145 cells were exposed to different concentrations of
SSd (3, 9 and 15 μM) for 12 h prior to Rho-123 staining. The
results showed that SSd reduced the MMP in a
concentration-dependent manner, from 98.15±1.84 (in the DMSO
control group) to 93.17±3.91, 78.01±5.87 and 22.21±3.41%,
respectively (Fig. 4B).
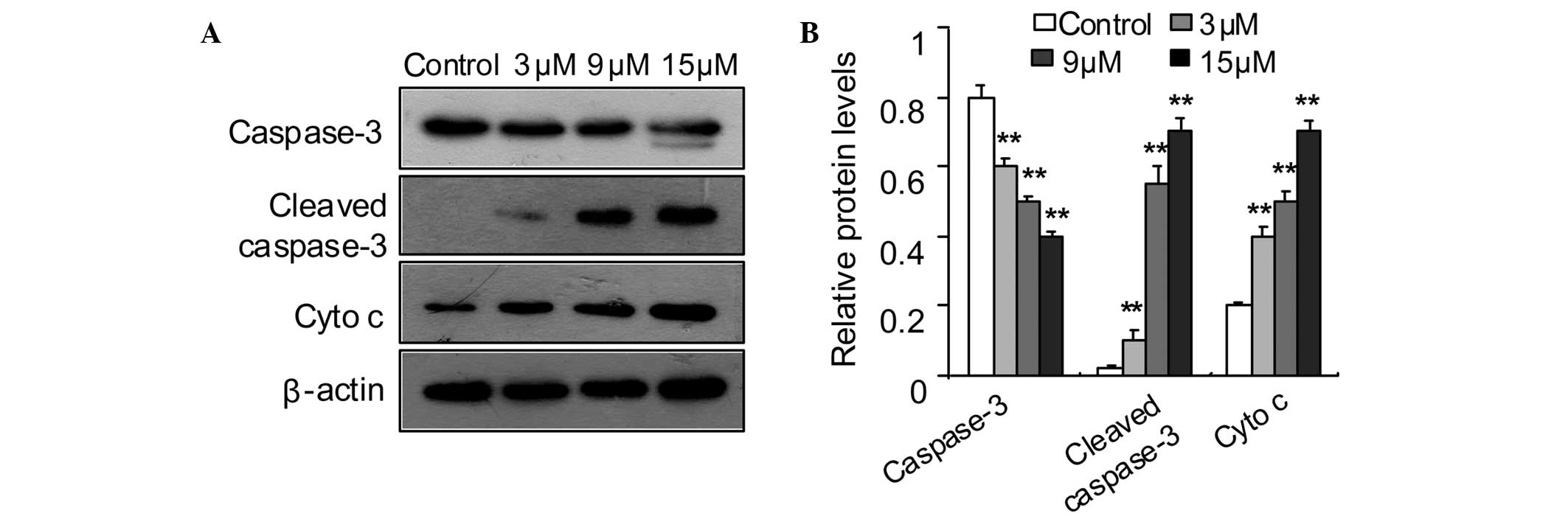
Effect of SSd on cytochrome c
translocation and caspase-3 activation
Disruption of the MMP may lead to the release of
cytochrome c from the intermembrane space to the cytosol,
which leads to the activation of procaspase-3 (28–30).
To further define the apoptotic pathway, the levels of cytosolic
cytochrome c and activated caspase-3 in DU145 cells were
determined. The western blotting results indicated that SSd
increased the cytochrome c levels in the cytosol and induced
cleavage of caspase-3 (Fig.
5).
Effect of SSd on the expression levels of
Bcl-2 and Bax
The Bcl-2 family proteins regulate apoptosis by
controlling mitochondrial membrane stability (31). The Bcl-2 family contains
anti-apoptotic, e.g. Bcl-2-associated agonist of cell death (Bad),
BH3 interacting domain death agonist (Bid), Bax and Bcl-2-like 11
(apoptosis facilitator) (Bim), and pro-apoptotic, e.g. Bcl-2 and
B-cell lymphoma-extra large (Bcl-xL), proteins, and the ratios of
anti-apoptotic and pro-apoptotic proteins determine the fate of
cells (32,33). The present study investigated the
effect of SSd on the expression levels of Bcl-2 and Bax. As shown
in Fig. 6, SSd induced elevated
levels of Bax, while it reduced levels of Bcl-2 in the DU145 cells
compared with those in the control cells.
Discussion
Previous studies have reported that SSd inhibited
proliferation of several cancer cell lines (8–11),
while its effect on human prostate cancer cells remained under
investigation. The present study revealed, for the first time to
the best of our knowledge, that SSd induced apoptosis in the DU145
human prostate cancer cell line. The present study also provides
insights into the mechanisms involved in SSd-induced apoptosis of
DU145 cells.
The results of the present study demonstrated that
SSd inhibited the proliferation of DU145 cells in a
concentration-dependent manner. Nuclear fragmentation and
chromosomal condensation are landmarks of apoptosis (34). The chromosomal condensation in the
present study was confirmed by Hoechst 33258 staining. At the early
stages of apoptosis, phosphatidyl serine (PS) is translocated from
the inner face of the plasma membrane to the cell surface, while
the cell membrane integrity decreases at late stages of apoptosis
(35). Apoptotic and necrotic
cells are discriminated using the PS-binding dye Annexin V-FITC and
the DNA-binding dye PI. The Annexin V-FITC/PI double staining
results of the present study revealed that SSd treatment induced
apoptosis of DU145 cells in a concentration-dependent manner and
<2% of the treated cells were necrotic. Thus, the results
suggested that the reduced cell viability in the MTT assay was due
to apoptosis rather than necrosis.
Apoptosis is induced through two main pathways: The
death receptor pathway and the intrinsic or mitochondrial pathway
(29). A previous study showed
that SS induced apoptosis via mitochondria-dependent and
-independent pathways (11). As
involvement of a mitochondria-mediated pathway in apoptosis is of
notable value in the treatment of cancer (29), the effect of SSd on the
mitochondrial apoptotic pathway was investigated in the present
study. Depolarization of the MMP is a typical characteristic of
apoptosis (25,29); therefore, the MMP in DU145 cells
was examined in the present study. The results revealed that the
MMP was significantly dissipated following SSd treatment, which
suggests the mitochondrial apoptotic pathway was induced by
SSd.
The Bcl-2 family proteins, including anti-apoptotic
(e.g. Bcl-2 and Bcl-xL) and proapoptotic (e.g. Bad, Bid, Bax and
Bim) members, are key regulators in the mitochondrial apoptotic
pathway (36). A slight change in
the levels of these proteins may result in apoptosis (36). In the present study, SSd reduced
the expression levels of Bcl-2 and increased the expression levels
of Bax in a concentration-dependent manner, which resulted in an
increased Bax/Bcl-2 ratio and thus may have triggered the
mitochondrial pathway of apoptosis. Bcl-2 and Bax have roles in
regulating cytochrome c release (31,37).
Upon release from the mitochondria, cytochrome c activates
the caspase cascade through the mitochondrial transition pore
(32). The data of the present
study revealed that SSd induced the release of cytochrome c
and activation of caspase-3. These results further support that
SSd-induced apoptosis in DU145 cells via regulation of the Bcl-2
family proteins.
Cytochrome c release from mitochondria may
result from overproduction of ROS (38,39);
thus, the effect of SSd on ROS production in DU145 cells was
examined in the present study. The results showed that the ROS
levels in SSd-treated DU145 cells remained unchanged compared with
those in the control cells. SS compounds have long been used as
antioxidants, and a previous study also confirmed the antioxidant
activity of SSd in normal hepatocytes (40). Wang et al (9) reported that SSd induced cellular ROS
accumulation in several types of cell line. The reason for the
differences between the results of these studies remains to be
determined, but may in part be due to the different cell lines used
in the studies.
Inhibition of cell proliferation is implemented by
cell cycle arrest. Although the data of the present study indicated
that SSd treatment caused DU145 cell cycle arrest at G1 phase, the
results suggest that apoptosis was the major reason for the
inhibition of cell proliferation.
p21 (Cip1/Waf1), a major transcriptional target of
p53 protein, has key roles in the transition from G1 phase into S
phase (41,42). p21 is a broad-specificity inhibitor
of cyclin/cyclin-dependent kinase complexes; upregulation of p21
may lead to cell cycle arrest and inhibition of proliferation
(43). Thus, the present study
examined the expression levels of p21 and p53. The data showed that
SSd significantly increased the levels of these two proteins
compared with those in the control cells, which further supports
the conclusion that SSd induces G1 arrest in DU145 cells.
In conclusion, the present study revealed that
SSd-induced mitochondrial dysfunction is the major reason for the
induction of apoptotic cell death in DU145 human prostate cancer
cells treated with SSd. The induction of apoptosis was associated
with dissipation of the MMP, release of cytochrome c,
activation of caspase-3 and modulation of Bcl-2 family proteins.
G0/G1 phase arrest is a minor reason for the SSd-induced inhibition
of cell proliferation; this effect was associated with upregulation
of the levels of p53 and p21. Further in-depth studies are required
to examine SSd-induced apoptosis and cell cycle perturbation in
DU145 cells. SSd may be developed into a leading candidate drug for
prostate cancer therapy.
Acknowledgements
This study was supported by the National Clinical
Key Specialty Construction Project (no. 2011-873).
References
|
1
|
Lee J, Yang DH, Suh JH, Kim U, Eom HY, Kim
J, Lee MY, Kim J and Han SB: Species discrimination of Radix
Bupleuri through the simultaneous determination of ten
saikosaponins by high performance liquid chromatography with
evaporative light scattering detection and electrospray ionization
mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci.
879:3887–3895. 2011. View Article : Google Scholar
|
|
2
|
Kato M, Pu MY, Isobe K, Iwamoto T, Nagase
F, Lwin T, Zhang YH, Hattori T, Yanagita N and Nakashima I:
Characterization of the immunoregulatory action of saikosaponin-d.
Cell Immunol. 159:15–25. 1994. View Article : Google Scholar
|
|
3
|
Kato M, Pu MY, Isobe K, Hattori T,
Yanagita N and Nakashima I: Cell type-oriented differential
modulatory actions of saikosaponin-d on growth responses and DNA
fragmentation of lymphocytes triggered by receptor-mediated and
receptor-bypassed pathways. Immunopharmacology. 29:207–213. 1995.
View Article : Google Scholar
|
|
4
|
Chang JS, Wang KC, Liu HW, Chen MC, Chiang
LC and Lin CC: Sho-saiko-to (Xiao-Chai-Hu-Tang) and crude
saikosaponins inhibit hepatitis B virus in a stable HBV-producing
cell line. Am J Chin Med. 35:341–351. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohtake N, Nakai Y, Yamamoto M, Sakakibara
I, Takeda S, Amagaya S and Aburada M: Separation and isolation
methods for analysis of the active principles of Sho-saiko-to (SST)
oriental medicine. J Chromatogr B Analyt Technol Biomed Life Sci.
812:135–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bao Y, Li C, Shen H and Nan F:
Determination of saikosaponin derivatives in Radix bupleuri and in
pharmaceuticals of the chinese multiherb remedy xiaochaihu-tang
using liquid chromatographic tandem mass spectrometry. Anal Chem.
76:4208–4216. 2004. View Article : Google Scholar
|
|
7
|
Wu GC, Wu H, Fan LY and Pan HF:
Saikosaponins: a potential treatment option for systemic lupus
erythematosus. Ir J Med Sci. 180:259–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu YL, Kuo PL, Chiang LC and Lin CC:
Involvement of p53, nuclear factor kappaB and Fas/Fas ligand in
induction of apoptosis and cell cycle arrest by saikosaponin d in
human hepatoma cell lines. Cancer Lett. 213:213–221. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Zheng XL, Yang L, Shi F, Gao LB,
Zhong YJ, Sun H, He F, Lin Y and Wang X: Reactive oxygen
species-mediated apoptosis contributes to chemosensitization effect
of saikosaponins on cisplatin-induced cytotoxicity in cancer cells.
J Exp Clin Cancer Res. 29:1592010. View Article : Google Scholar
|
|
10
|
Hsu YL, Kuo PL and Lin CC: The
proliferative inhibition and apoptotic mechanism of Saikosaponin D
in human non-small cell lung cancer A549 cells. Life Sci.
75:1231–1242. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim BM and Hong SH: Sequential caspase-2
and caspase-8 activation is essential for saikosaponin a-induced
apoptosis of human colon carcinoma cell lines. Apoptosis.
16:184–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JC, Chang NW, Chung JG and Chen KC:
Saikosaponin-A induces apoptotic mechanism in human breast
MDA-MB-231 and MCF-7 cancer cells. Am J Chin Med. 31:363–377. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zong Z, Fujikawa-Yamamoto K, Tanino M,
Teraoka K, Yamagishi H and Odashima S: Saikosaponin b2-induced
apoptosis of cultured B16 melanoma cell line through
down-regulation of PKC activity. Biochem Biophys Res Commun.
219:480–485. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
American Cancer Society. [http://www.cancer.org/cancer/prostatecancer/overviewguide/prostate-cancer-overview-key-statistics].
|
|
15
|
Marta GN, Hanna SA, Fernandes da Silva JL
and de Carvalho HA: Screening for prostate cancer: an updated
review. Expert Rev Anticancer Ther. 13:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kollmeier MA and Zelefsky MJ: How to
select the optimal therapy for early-stage prostate cancer. Crit
Rev Oncol Hematol. 84(Suppl 1): e6–e15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Drudge-Coates L and Turner B: Prostate
cancer overview. Part 1: non-metastatic disease. Br J Nurs.
21:S23–S28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Drudge-Coates L and Turner B: Prostate
cancer overview. Part 2: metastatic prostate cancer. Br J Nurs.
21:S23–S24. S26–S28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Twentyman PR and Luscombe M: A study of
some variables in a tetrazolium dye (MTT) based assay for cell
growth and chemosensitivity. Br J Cancer. 56:279–285. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuchs Y and Steller H: Programmed cell
death in animal development and disease. Cell. 147:742–758. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rhind N and Russell P: Signaling pathways
that regulate cell division. Cold Spring Harb Perspect Biol.
4:a0059422012. View Article : Google Scholar
|
|
22
|
Dash BC and El-Deiry WS: Cell cycle
checkpoint control mechanisms that can be disrupted in cancer.
Methods Mol Biol. 280:99–161. 2004.PubMed/NCBI
|
|
23
|
Kitazumi I and Tsukahara M: Regulation of
DNA fragmentation: the role of caspases and phosphorylation. FEBS
J. 278:427–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zimmermann KC, Bonzon C and Green DR: The
machinery of programmed cell death. Pharmacol Ther. 92:57–70. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mignotte B and Vayssiere JL: Mitochondria
and apoptosis. Eur J Biochem. 252:1–15. 1998. View Article : Google Scholar
|
|
26
|
Armstrong JS: Mitochondria: a target for
cancer therapy. Br J Pharmacol. 147:239–248. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bras M, Queenan B and Susin SA: Programmed
cell death via mitochondria: different modes of dying. Biochemistry
(Mosc). 70:231–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar
|
|
29
|
Chalah A and Khosravi-Far R: The
mitochondrial death pathway. Adv Exp Med Biol. 615:25–45. 2008.
View Article : Google Scholar
|
|
30
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ola MS, Nawaz M and Ahsan H: Role of Bcl-2
family proteins and caspases in the regulation of apoptosis. Mol
Cell Biochem. 351:41–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
García-Sáez AJ: The secrets of the Bcl-2
family. Cell Death Differ. 19:1733–1740. 2012.
|
|
33
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar
|
|
34
|
Robertson JD, Orrenius S and Zhivotovsky
B: Review: nuclear events in apoptosis. J Struct Biol. 129:346–358.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wlodkowic D, Skommer J and Darzynkiewicz
Z: Cytometry of apoptosis. Historical perspective and new advances.
Exp Oncol. 34:255–262. 2012.PubMed/NCBI
|
|
36
|
Zinkel S, Gross A and Yang E: BCL2 family
in DNA damage and cell cycle control. Cell Death Differ.
13:1351–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burlacu A: Regulation of apoptosis by
Bcl-2 family proteins. J Cell Mol Med. 7:249–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schultz DR and Harrington WJ Jr:
Apoptosis: programmed cell death at a molecular level. Semin
Arthritis Rheum. 32:345–369. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gottlieb RA: Mitochondria and apoptosis.
Biol Signals Recept. 10:147–161. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan J, Li X, Li P, Li N, Wang T, Shen H,
Siow Y, Choy P and Gong Y: Saikosaponin-d attenuates the
development of liver fibrosis by preventing hepatocyte injury.
Biochem Cell Biol. 85:189–195. 2007.PubMed/NCBI
|
|
41
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Junttila MR and Evan GI: p53 - a Jack of
all trades but master of none. Nat Rev Cancer. 9:821–829. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Abbas T and Dutta A: p21 in cancer:
intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|