Introduction
Melanoma is a highly aggressive and frequently
chemoresistant type of skin cancer. In the United States, melanoma
is the fifth and sixth most common type of skin cancer diagnosed in
males and females, respectively (1). Patients with metastatic melanoma have
a poor prognosis, with a five-year overall survival rate of 15%
(2). Despite recent therapeutic
advances in the management of melanoma, aside from early surgical
resection, no therapeutic modality has been found to have a high
likelihood of curative outcome (3). Therefore, further studies to
elucidate the molecular mechanisms underlying melanoma cell
metastasis are required to improve melanoma treatment.
The progression of melanoma from the non-metastatic
melanoma phase, also termed the radial growth phase, to the
metastatic phase, also termed the vertical growth phase, involves
various alterations in the expression of genes, including protease
activated receptor-1 (4),
glycoprotein non-metastatic melanoma protein B (5), melanoma differentiation-associated
gene-9 (6) and ocular albinism
type 1 (OA1) (7). OA1 was
originally identified as the 404 amino acid protein product of the
gene responsible for the disorder, OA1, and was isolated by a
classical positional cloning strategy from the distal short arm of
the X chromosome (8). OA1 is a
pigment cell-specific glycoprotein, which shares significant
structural and functional features with G protein-coupled receptors
(GPCRs). Previous studies have shown that OA1 ubiquitination is
required for the targeting of OA1 to the intraluminal vesicles of
multi-vesicular endosomes, thereby regulating the balance between
OA1 downregulation and OA1 melanosome delivery (9). In a previous study, OA1-knockdown
mouse melanocytes were not only found to exhibit a reduced number
of melanosomes and presence of macromelanosomes, but also an
abnormal distribution of melanosomes at the cell periphery
(10). However, the role of OA1 in
melanoma remains unclear.
The aim of the present study was to investigate the
role of OA1 in melanoma, which was achieved by knocking down OA1
using small interfering (si)RNA. In addition, the present study
aimed to provide insight into the mechanism underlying OA1-induced
melanoma.
Materials and methods
Materials
Anti-OA1, -ERK 1/2, -phosphorylated (p)-ERK1/2 and
-β-actin antibodies were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). All other chemicals and reagents
were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless
stated otherwise.
Cell culture
A375 human melanoma cells were purchased from The
Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). Cells were grown in Dulbecco’s modified
Eagle’s medium (DMEM; Invitrogen Life Technologies) supplemented
with 10% heat-inactivated fetal bovine serum (FBS) and antibiotics
(100 U/ml penicillin and 100 μg/ml streptomycin) in a humidified
atmosphere of 5% CO2 at 37°C. A375 cells were detached
using trypsin-EDTA (0.05% trypsin).
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA was extracted from melanoma cells using an
RNA Plus kit (Takara Bio Inc., Dalian, China). A total of 1 μg
total RNA per sample was used for comlementary (c)DNA synthesis
using the QuantiTect Reverse Transcription kit (Qiagen, Valencia,
CA, USA) according to the manufacturer’s instructions. The PCR
reaction contained cDNA, 0.1 nmol/l forward and reverse primer mix
and SYBR® Green I (Invitrogen Life Technologies). qPCR
analysis was performed using the 7300 RT-PCR System (Applied
Biosystems, Inc., Foster City, CA, USA). The primer sequences used
were as follows: Forward, 5′-CGG AGA TCG GCA GGACTGAGCAC-3′ and
reverse, 5′-ATA GTG GGG GAT GGC GTG GT-3′ for human OA1; and
forward, 5′-GAT CAT TGC TCC TCC TGA GC-3′ and reverse, 5′-ACT CCT
GCT TGC TGA TCC AC-3′ for β-actin. Amplification included one stage
of 2 min at 50°C and one stage of 2 min at 95°C followed by 40
cycles of 15 sec at 95°C and 30 sec at 60°C. Data were analyzed
using the 7300 RT-PCR System software and OA1 transcript levels
were adjusted relative to those of the internal control
β-actin.
Western blot analysis
Total protein extracts were prepared using
radio-immunoprecipitation assay lysis buffer (Beyotime, Nantong,
China) according to the manufacturer’s instructions. The protein
concentration in the cell lysates was assessed using a
bicinchoninic acid protein assay kit (Beyotime). For western blot
analysis, proteins lysates (30 μg/lane) were separated using
SDS-PAGE and transferred onto polyvinylidene difluoride membranes
(Whatman PLC, Brentford, UK). The membranes were blocked for 20 min
at room temperature using the SuperBlock® T20 (TBS)
Blocking Buffer. Subsequent to blocking, target proteins were
probed with anti-OA1, -ERK1/2, -p-ERK1/2 and -β-actin primary
antibodies overnight at 4°C. The membranes were washed and
incubated with horseradish peroxidase-conjugated secondary
antibodies. Following washing, the sites of antibody binding were
visualized via chemiluminescence (Boehringer Mannheim GmbH,
Mannheim, Germany) according to the manufacturer’s instructions and
the expression of each protein relative to β-actin expression was
analyzed.
Recombinant lentivirus construction and
OA1 siRNA transfection
The OA1 lentivirus was constructed and amplified
according to the manufacturer’s instructions (BD Biosciences, San
Jose, CA, USA). The sense strand for the OA1 double-stranded siRNA
was synthesized using the following sequence: 5′-GGA TAT GAA CCA
CAC GGA A-3′. The OA1 non-targeting control siRNA sequence was as
follows: 5′-AAT TCT CCG AAC GTG TCA CGT-3′. In vitro
co-transfection was performed using Lipofectamine™ 2000 according
to the manufacturer’s instructions.
Transwell migration assay
Migration assays were performed using melanoma cells
transfected with siRNA targeting OA1 or scrambled siRNAs. Cells
were analyzed using Transwell® cell culture chambers
(Abcam PLC, Cambridge, UK). The lower face of the polycarbonate
membrane (8-μm pore size) was coated with 0.25 mg fibronectin
overnight. Following trypsin treatment, melanoma cells were washed
three times with phosphate-buffered saline and were resuspended in
DMEM supplemented with 4% FBS. Melanoma cells (2×105/ml
in 100 μl medium) were added to the upper chamber and 600 μl DMEM
was added to the lower chamber. Subsequent to incubation in a
humidified atmosphere of 5% CO2 at 37°C for 12 h, all
non-migrated cells were removed from the upper chamber using a
cotton swab. Migrated cells were fixed using methanol for 10 min at
4°C and stained with 0.5% crystal violet solution for 30 min. The
number of cells per four high power fields was counted using a
phase-contrast microscope (Leica, Wetzlar, Germany) in order to
determine the average number of cells that had migrated. The number
of cells was considered to represent migration activity.
Statistical analysis
Data are presented as the mean ± standard deviation.
Student’s t-tests were performed for the comparison of two groups
and one-way analysis of variance was used for multiple comparisons.
P<0.05 was considered to indicate a statistically significant
difference.
Results
OA1 expression in melanoma cells
To assess the functional role of OA1, OA1 was
overexpressed using lentivirus-mediated OA1-cDNA transfection in
melanoma cells. As shown in Fig.
1, qPCR and western blot analyses revealed a significant
upregulation of OA1 expression in melanoma cells following
transfection with OA1-cDNA. OA1-cDNA transfection was observed to
increase OA1 mRNA and protein levels to 162.3±3.9 and 158.1±2.3% of
the control, respectively (P<0.05). In addition, melanoma cells
transfected with siRNA-OA1 showed a significant downregulation of
OA1 expression, with siRNA-OA1 reducing OA1 mRNA and protein levels
to 31.3±0.9 and 21.1±2.3% of the control, respectively
(P<0.05).
OA1 promotes melanoma cell migration
In the present study, OA1 was found to be expressed
in melanoma cells and it was hypothesized that the migration of
melanoma cells was associated with the development of melanoma.
Therefore, the effect of OA1 on human metastatic melanoma cell
motility was assessed using the Transwell migration assay. As shown
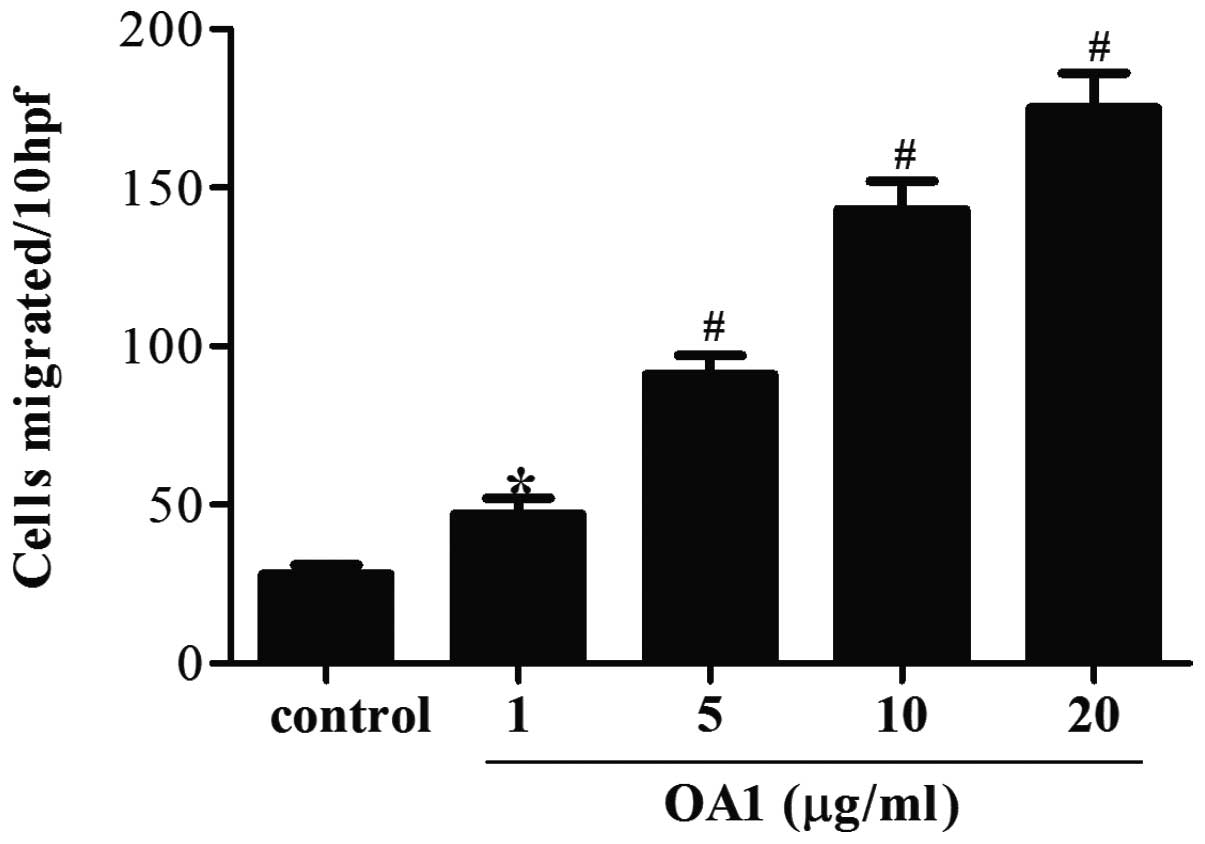
in Fig. 2, Transwell migration
assay revealed a dose-dependent increase in melanoma cell migration
by OA1. The number of migrated melanoma cells was observed to
increase with increasing OA1 concentration compared with the
control group. Significant increases in cell migration were found
to begin at 1 μg/ml OA1 (P<0.05) and peak at 20 μg/ml OA1
(P<0.01). These findings indicate that OA1 may promote melanoma
cell migration.
siRNA-induced OA1 knockdown reduces
melanoma cell migration
To further determine the function of OA1 in
promoting melanoma cell migration, a specific siRNA targeting OA1
was generated. As shown in Fig. 3,
Transwell migration assay revealed that transfection with siRNA
targeting OA1 significantly reduced the number of migrated cells,
compared with transfection with scramble siRNA (P<0.05). Thus,
OA1 may be involved in the regulation of melanoma cell
migration.
OA1-induced melanoma cell migration is
mediated by the RAS/RAF/MEK/ERK signaling pathway
The RAS/RAF/MEK/ERK signaling pathway is important
in the regulation of various cellular processes, including gene
expression, proliferation, migration and survival (11–13).
Therefore, the present study aimed to investigate whether OA1 was
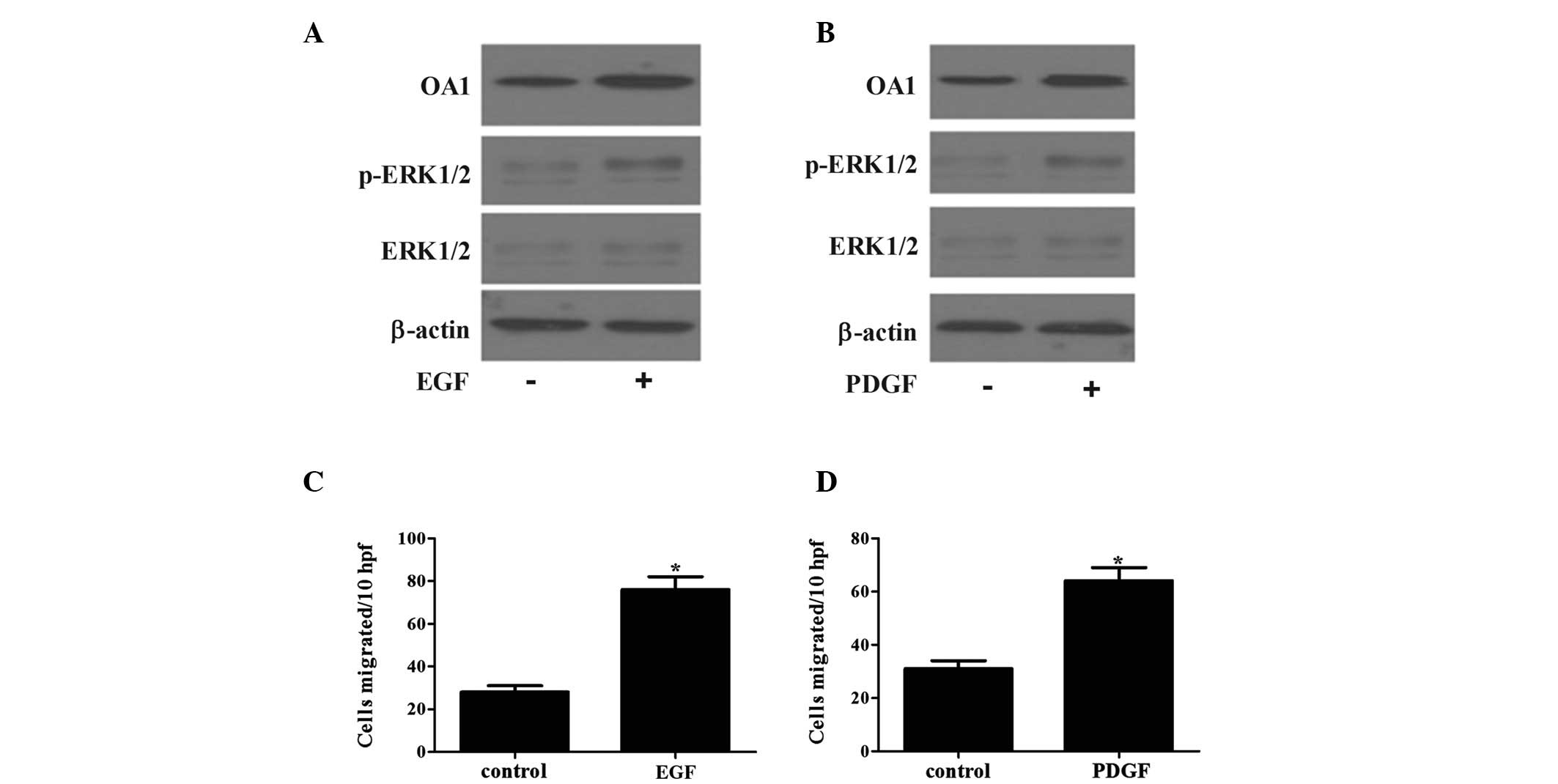
associated with the RAS/RAF/MEK/ERK signaling pathway. Two
important stimulators of the RAS/RAF/MEK/ERK cascade were used:
Epidermal growth factor (EGF) and platelet-derived growth factor
(PDGF). As shown in Fig. 4, the
stimulation of ERK activity by EGF or PDGF was associated with an
increase in OA1 protein expression and cell migration. To further
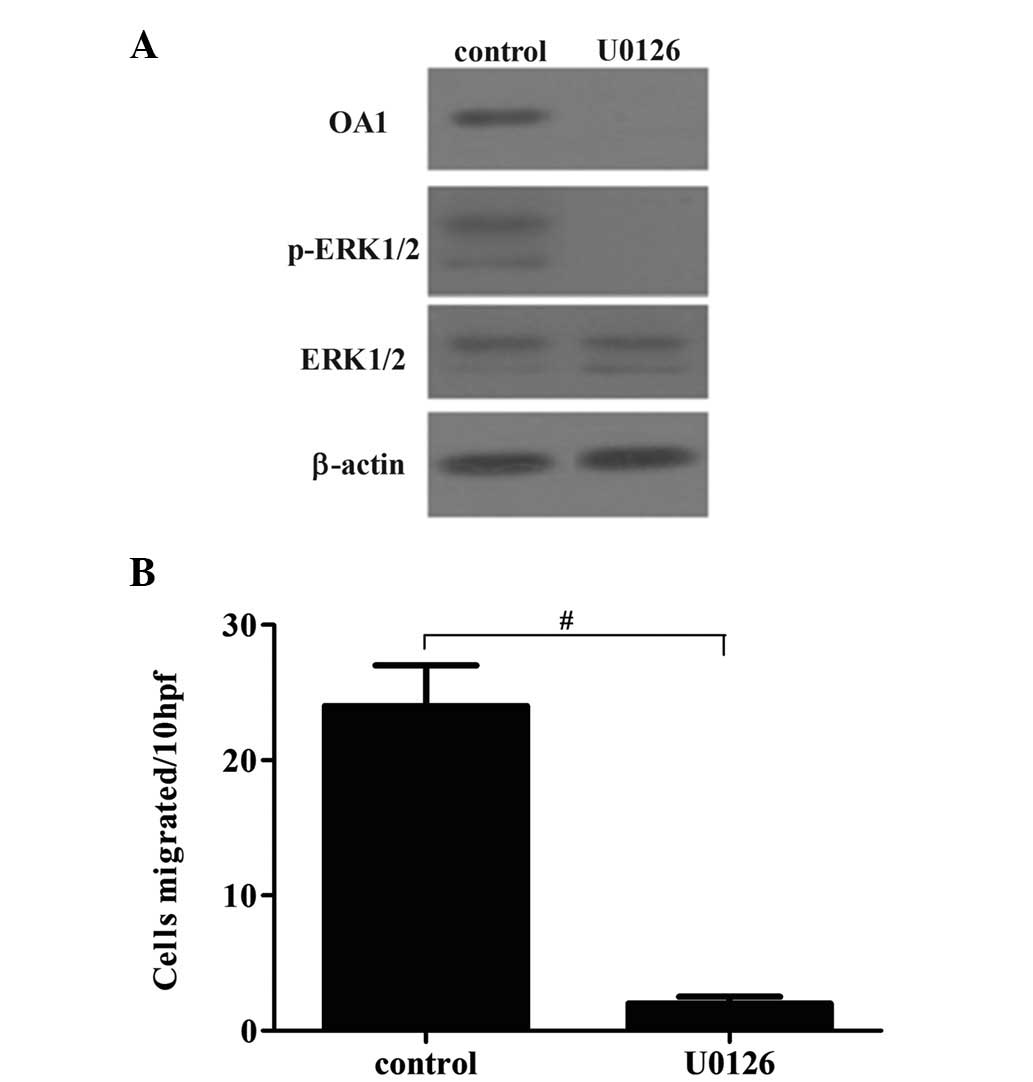
investigate the role of RAS/RAF/MEK/ERK in OA1-induced melanoma
cell migration, the effect of the RAS/RAF/MEK/ERK pathway
inhibitor, U0126, on melanoma cell migration and OA1 expression was
assessed. As shown in Fig. 5,
U0126 markedly attenuated p-ERK1/2 and OA1 levels in melanoma
cells. The cell migration rate was also significantly decreased by
U0126. These findings indicate that OA1-induced melanoma cell
migration is mediated by the RAS/RAF/MEK/ERK signaling pathway.
Discussion
Malignant melanoma has the highest risk of mortality
among all types of skin cancer due to its highly metastatic
potential. The incidence and mortality rates associated with
malignant melanoma have continued to increase over recent years
(14,15). However, there is currently no
effective treatment for metastatic melanoma, partly due to the
complicated mechanism underlying its metastasis (16). In the present study, OA1 was found
to promote the migration of melanoma cells in vitro. In
addition, knockdown of OA1 using siRNA was observed to inhibit
melanoma cell migration. Furthermore, OA1-induced melanoma cell
metastasis was found to involve the RAS/RAF/MEK/ERK signaling
pathway. These findings may provide a novel target for melanoma
treatment and may improve the future treatment of the disease.
The progression and metastasis of malignant melanoma
are complex processes, which involve multiple cellular events,
including cell proliferation, survival, migration and invasion.
Metastasis is the spread of malignant tumor cells from a primary
site to distant tissue and is the most life-threatening factor
associated with cancer. A variety of metastasis-promoting and
metastasis -suppressing genes have been identified to be involved
in the metastasis of melanoma cells. Survivin is the smallest
member of the inhibitor of apoptosis protein family (17). Certain studies have indicated that
survivin enhances cell migration and the invasion of human melanoma
cells (18). A previous report
demonstrated that survivin promoted cell motility through
activation of the protein kinase B signaling pathway and
upregulation of α5 integrin (18).
Furthermore, the survivin-mediated promotion of melanoma cell
invasion was found to be dependent upon the activation of the
mitogen-activated protein kinase pathway (18). Nm23-H1 was the first gene to be
identified in a class of metastasis suppressor genes and the
overexpression of Nm23-H1 in metastatic melanoma cells has been
reported to reduce cell motility in vitro, as well as reduce
metastatic potential in a xenograft model (19). In the present study, the effect of
OA1 on human melanoma cell migration was investigated. OA1 was
found to enhance melanoma cell migration in a dose-dependent
manner. Moreover, siRNA-induced OA1 knockdown reduced melanoma cell
migration. These findings indicate that OA1 may serve as a
metastasis-promoting gene in melanoma development.
The RAS/RAF/MEK/ERK pathway has been reported to be
activated in >80% of all cutaneous melanomas, thus has been the
focus of numerous investigations on melanoma (20). RAS/RAF/MEK/ERK signaling has been
shown to promote cell proliferation, cell survival and tumor
metastasis, as well as to be frequently aberrantly activated in
cancer, in particular by the activation of upstream growth factors
(21,22). In the present study, the EGF and
PDGF growth factors, which are important in melanoma development
and progression, were found to stimulate ERK activity and increase
OA1 expression. Inhibition of RAF and MEK kinase activities are the
most investigated approaches for inhibiting ERK signaling (23,24).
RNA interference-mediated knockdown of oncogenic RAF has been
reported to markedly reduce melanoma cell migration and ERK
phosphorylation (25). In the
present study, inhibition of the RAS/RAF/MEK/ERK signaling pathway
using U0126 was found to decreases the levels of p-ERK1/2 and OA1
in melanoma cells. In addition, the number of migrated cells was
decreased by U0126. Therefore, although the mechanism underlying
the regulation of OA1 expression by the RAS/RAF/MEK/ERK signaling
pathway has yet to be elucidated, the results of the present study
indicate that OA1-induced melanoma cell migration depends on the
RAS/RAF/MEK/ERK signaling pathway.
In conclusion, the present study demonstrated that
OA1 is involved in the regulation of melanoma cell migration.
Therefore, OA1 may be significant in human melanoma and may
represent a novel therapeutic target for the prevention of
melanoma.
References
|
1
|
Chakraborty R, Wieland CN and Comfere NI:
Molecular targeted therapies in metastatic melanoma. Pharmgenomics
Pers Med. 6:49–56. 2013.PubMed/NCBI
|
|
2
|
Tas F: Metastatic behavior in melanoma:
timing, pattern, survival, and influencing factors. J Oncol.
2012:6476842012.PubMed/NCBI
|
|
3
|
Tsao H, Chin L, Garraway LA and Fisher DE:
Melanoma: from mutations to medicine. Gene Dev. 26:1131–1155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zigler M, Kamiya T, Brantley EC, Villares
GJ and Bar-Eli M: PAR-1 and thrombin: the ties that bind the
microenvironment to melanoma metastasis. Cancer Res. 71:6561–6566.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li B, Castano AP, Hudson TE, Nowlin BT,
Lin SL, Bonventre JV, Swanson KD and Duffield JS: The
melanoma-associated transmembrane glycoprotein Gpnmb controls
trafficking of cellular debris for degradation and is essential for
tissue repair. FASEB J. 24:4767–4781. 2010. View Article : Google Scholar
|
|
6
|
Das SK, Bhutia SK, Sokhi UK, Azab B, Su
ZZ, Boukerche H, Anwar T, Moen EL, Chatterjee D, Pellecchia M,
Sarkar D and Fisher PB: Raf kinase inhibitor RKIP inhibits
MDA-9/syntenin-mediated metastasis in melanoma. Cancer Res.
72:6217–6226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez LP, Milne RL, Pita G, Floristan
U, Sendagorta E, Feito M, Avilés JA, Martin-Gonzalez M, Lázaro P,
Benítez J and Ribas G: Pigmentation-related genes and their
implication in malignant melanoma susceptibility. Exp Dermatol.
18:634–642. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schiaffino MV: Signaling pathways in
melanosome biogenesis and pathology. Int J Biochem Cell Biol.
42:1094–1104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giordano F, Simoes S and Raposo G: The
ocular albinism type 1 (OA1) GPCR is ubiquitinated and its traffic
requires endosomal sorting complex responsible for transport
(ESCRT) function. Proc Natl Acad Sci USA. 108:11906–11911. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Palmisano I, Bagnato P, Palmigiano A,
Innamorati G, Rotondo G, Altimare D, Venturi C, Sviderskaya EV,
Piccirillo R, Coppola M, et al: The ocular albinism type 1 protein,
an intracellular G protein-coupled receptor, regulates melanosome
transport in pigment cells. Hum Mol Genet. 17:3487–3501. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hilger RA, Scheulen ME and Strumberg D:
The Ras-Raf-MEK-ERK pathway in the treatment of cancer. Onkologie.
25:511–518. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marshall C: Specificity of receptor
tyrosine kinase signaling: transient versus sustained extracellular
signal-regulated kinase activation. Cell. 80:179–185. 1995.
View Article : Google Scholar
|
|
14
|
Bogenrieder T and Herlyn M: The molecular
pathology of cutaneous melanoma. Cancer Biomark. 9:267–286.
2011.
|
|
15
|
Forsea A, Del Marmol V, de Vries E, Bailey
EE and Geller AC: Melanoma incidence and mortality in Europe: new
estimates, persistent disparities. Br J Dermatol. 167:1124–1130.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bhatia S, Tykodi SS and Thompson JA:
Treatment of metastatic melanoma: an overview. Oncology (Williston
Park). 23:488–496. 2009.
|
|
17
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKenzie JA, Liu T, Goodson AG and
Grossman D: Survivin enhances motility of melanoma cells by
supporting Akt activation and alpha5 integrin upregulation. Cancer
Res. 70:7927–7937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Marino N, Marshall JC and Steeg PS:
Protein-protein interactions: a mechanism regulating the
anti-metastatic properties of Nm23-H1. Naunyn Schmiedebergs Arch
Pharmacol. 384:351–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agell N, Bachs O, Rocamora N and
Villalonga P: Modulation of the Ras/Raf/MEK/ERK pathway by
Ca2+, and Calmodulin. Cell Signal. 14:649–654. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang F, Steelman LS, Lee JT, Shelton JG,
Navolanic PM, Blalock WL, Franklin RA and McCubrey JA: Signal
transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine
receptors to transcription factors: potential targeting for
therapeutic intervention. Leukemia. 17:1263–1293. 2003. View Article : Google Scholar
|
|
23
|
Panka DJ, Wang W, Atkins MB and Mier JW:
The Raf inhibitor BAY 43–9006 (Sorafenib) induces
caspase-independent apoptosis in melanoma cells. Cancer Res.
66:1611–1619. 2006.
|
|
24
|
Sebolt-Leopold JS: MEK inhibitors: a
therapeutic approach to targeting the Ras-MAP kinase pathway in
tumors. Curr Pharm Des. 10:1907–1914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hingorani SR, Jacobetz MA, Robertson GP,
Herlyn M and Tuveson DA: Suppression of BRAF(V599E) in human
melanoma abrogates transformation. Cancer Res. 63:5198–5202.
2003.PubMed/NCBI
|