Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
disease, characterized by synovial cell proliferation and excessive
production of pro-inflammatory cytokines, leading to the
destruction of diarthrodial joints cartilage and bones (1,2). RA
is considered an autoimmune disease (3) that can cause severe disability and
premature mortality (4), with ~1%
of individuals afflicted worldwide (5). Although the etiology of RA has not
been fully elucidated, numerous studies have demonstrated that it
is a multifactorial disease that results from interactions between
genetic and environmental factors (6).
Over the past 15 years, rheumatologists have
developed a number of therapeutic strategies aiming to treat RA
(7). Moreover, researchers have
shown that the characterization of the cytokine signaling pathways
involved in RA provides important opportunities for identifying
pro-inflammatory cytokines that can be targeted in the context of
novel therapeutic interventions (8–10).
However, these agents are not effective in all patients (11). Furthermore, it has long been
recognized that patients with RA have an increased risk for certain
types of cancer (12,13). For example, it was demonstrated
that RA-associated interstitial lung disease accounts for mortality
of up to 20% of RA patients (14).
Therefore, there is an urgent need to investigate the molecular
mechanisms underlying the processes involved in RA.
High-throughput mRNA sequencing (RNA-seq), which
allows simultaneous identification of transcripts and estimation of
their abundance (15,16), is a recently developed
transcriptome profiling approach that uses deep-sequencing
technologies (17). It has
fostered numerous advances in the characterization and
quantification of transcripts, since it allows a nearly complete
characterization of transcriptomes (18). A recent study making use of RNA-seq
showed that the activation and proliferation of RA synovial
fibroblasts relate to the pathogenesis of RA (19). Here, we performed a comprehensive
meta-analysis of the transcriptome data from based on the data of
Heruth et al (19), which
were derived from RNA of healthy and RA patients. Our findings may
enhance the understanding of the mechanisms underlying the process
of joint destruction, and allow a more selective and specific
application of therapeutic agents that target pro-inflammatory
cytokines and thus, a more effective treatment of patients with RA
and other inflammatory disorders.
Materials and methods
RNA-seq data
The RNA-seq data were downloaded from the Sequence
Read Archive (SRA). Two samples of synovial fibroblasts were
available for meta-analysis: One was from individuals with RA, and
the control sample (CT) was from healthy individuals (Table I). The downloaded data come from
Illumina RNA-seq (19) of
paired-end (2 × 100 bp) cDNA libraries prepared from each RNA
sample, with sequencing performed as in (20,21).
 | Table IInformation on raw RNA-seq data from
rheumatoid arthritis (RA) and control (CT) samples, downloaded from
the Sequence Read Archive (SRA). |
Table I
Information on raw RNA-seq data from
rheumatoid arthritis (RA) and control (CT) samples, downloaded from
the Sequence Read Archive (SRA).
| Sample name | Type | Library | Data size (bp) | SRA no. |
|---|
| RA | Rheumatoid
arthritis | Paired-end | 9.1 G | SRR364313 |
| CT | Healthy | Paired-end | 8.2 G | SRR364315 |
Quality analysis of raw RNA-seq
reads
SolexaQA is a software that allows investigation and
trimming of sequences based on their base quality scores. To
exclude analytical error from sequencing errors and low data
quality, the SolexaQA software (22) was used to process the raw data,
based on the read-level quality of each sequence. Raw sequences
with quality scores <20 and a length <25 bp were removed from
the final dataset that was used for downstream analyses.
Calculation of expression values and
identification of differentially expressed genes (DEGs)
First, the TopHat software version 2.0.8 (23) was used to map the sequencing reads
to the reference human genome (version hg19, UCSC Genome Browser).
TopHat is an efficient read-mapping algorithm designed to align
reads from an RNA-seq experiment to a reference genome; we used the
following settings: maximum 20 multiple hits per read and maximum 2
mismatches allowed. Second, Cufflinks software package (version
2.0.2) was used to assemble transcripts and calculate their
relative expression levels, expressed as fragments per kilobase of
exon per million fragments mapped (FPKM) using the default
parameters and an average insert size of 200 bp (24). Finally, the Cuffdiff module in
Cufflinks was used to estimate differential expression, expressed
as a ratio of RA to control expression for each transcript, along
with the statistical significance of the observed differences
(25). Genes with log2
fold change (FC) ≥1 and p-value ≤0.05 were selected as DEGs. In
addition, the expression values of the differentially expressed
genes were normalized by z-score transformation prior to
visualization with the heatmap function available in the R
statistical package (26).
Gene Ontology (GO) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) pathway analysis
The GOstat software (27) was used to conduct GO functional
annotation and enrichment analysis of DEGs (significantly at
P<0.05). The KAAS annotation server (28) was used to identify the KEGG
metabolic pathways in which the identified DEGs are involved.
Finally, the KOBAS server 2.0 (29), integrating data from a number of
human disease databases, was used to identify DEGs related to
RA.
Results
Identification of DEGs
Quality control statistics on the RNA-seq are shown
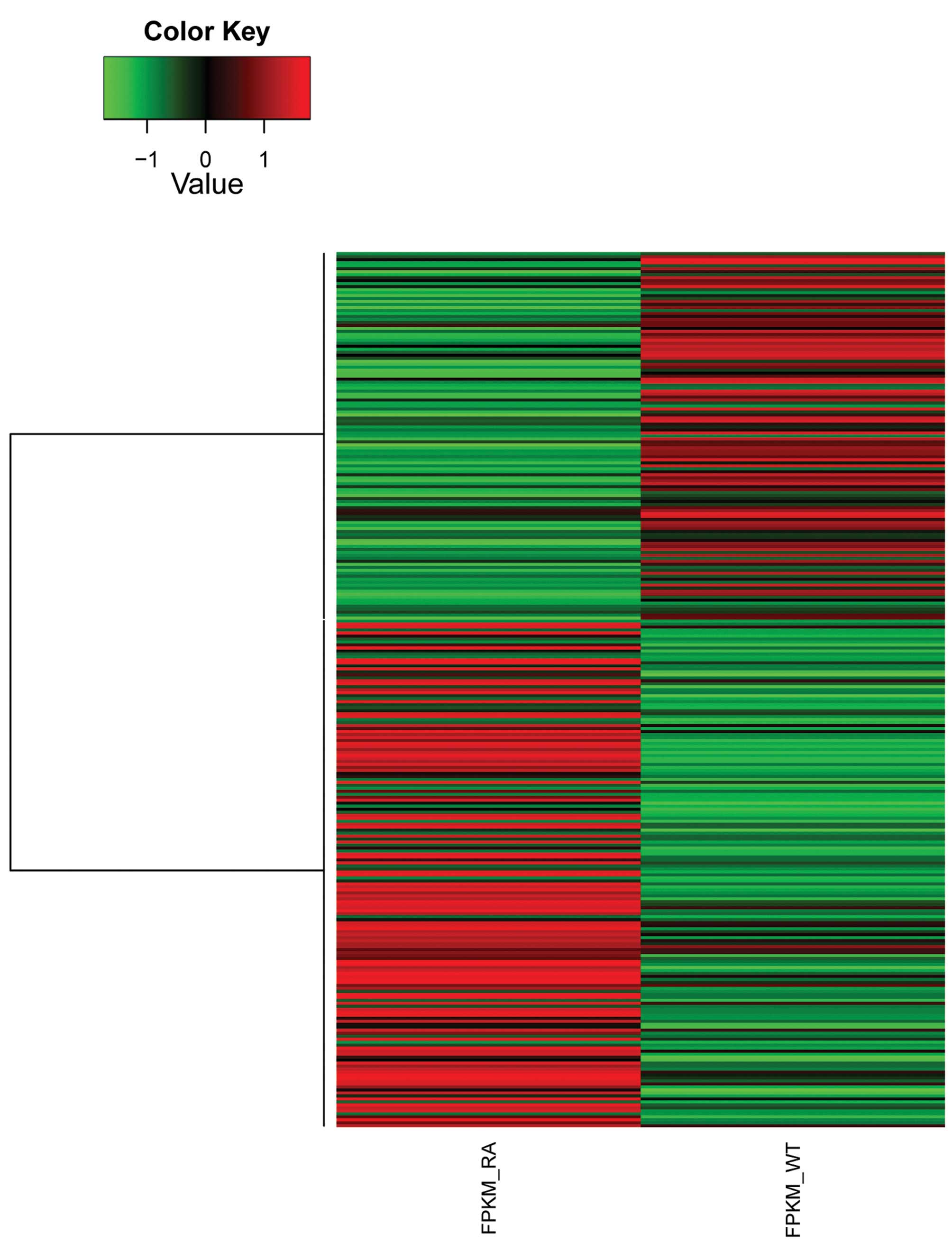
in Table II. A total of 293 genes
were identified as differentially expressed between the RA and CT
samples based on our criteria (log2 FC ≥1; p-value
≤0.05). Fig. 1 shows the
expression patterns of DEGs in the RA and CT samples. The relative
expression (FKPM), log2 FC, and associated p-values of
the top 10 DEGs (in terms of FKPM at the RA sample) are shown in
Table III.
 | Table IIQuality control statistics of mRNA
sequence reads. |
Table II
Quality control statistics of mRNA
sequence reads.
| Reads | Healthy
control | Percentage | Rheumatoid
arthritis | Percentage |
|---|
| Original | 80,782,262 | - | 89,757,726 | - |
| Low-quality | 9,605,518 | 11.89 | 6,707,374 | 7.47 |
| Remaining | 71,176,744 | 88.11 | 83,050,352 | 92.53 |
 | Table IIIThe top 10 significantly
differentially expressed genes. |
Table III
The top 10 significantly
differentially expressed genes.
| Gene | Chr | RA FPKM | CT FPKM | Log2
FC | p-value |
|---|
| CHI3L1 | Chr1 | 5,847.720 | 0.211 | −14.753 | 3.02E-14 |
| MMP-1 | Chr11 | 78.271 | 0.017 | −12.166 | 9.28E-11 |
| SMOC2 | Chr6 | 36.802 | 0.010 | −11.719 | 1.95E-07 |
| Ror2 | Chr9 | 8.913 | 0.009 | −9.995 | 1.23E-05 |
| VIT | Chr2 | 9.313 | 0.013 | −9.522 | 1.34E-04 |
| HEYL | Chr1 | 0.043 | 28.459 | 9.355 | 1.29E-14 |
| MCAM | Chr11 | 0.155 | 153.674 | 9.955 | 0 |
| EFHD1 | Chr2 | 0.054 | 103.317 | 10.893 | 5.70E-12 |
| FOXE1 | Chr9 | 0.009 | 18.429 | 11.069 | 2.92E-12 |
| WFDC1 | Chr16 | 0.020 | 76.095 | 11.882 | 5.09E-13 |
GO analysis
The GOstat tool retrieves GO annotations and
investigates over-representation of these in a given gene list
(27). We used GOstat to
functionally annotate the identified DEGs based on the cut-off
point of P<0.05. The 293 DEGs were enriched for 309 GO terms.
The top 10 GO terms in terms of p-value are shown in Table IV. This analysis indicated that
the identified DEGs are mainly involved in anatomical structure
development, cell membrane formation and stability, and biological
adhesion.
 | Table IVThe top 10 significantly enriched
Gene Ontology (GO) terms among differentially expressed genes. |
Table IV
The top 10 significantly enriched
Gene Ontology (GO) terms among differentially expressed genes.
| GO ID | Name | Description | p-value |
|---|
| 0048856 | Anatomical
structure development | The biological
process whose specific outcome is the progression of an anatomical
structure from an initial condition to its mature state. This
process begins with the formation of the structure and ends with
the mature structure, whatever form that may be including its
natural destruction. An anatomical structure is any biological
entity that occupies space and is distinguished from its
surroundings. Anatomical structures can be macroscopic such as a
carpel, or microscopic such as an acrosome. | 8.76e-44 |
| 0032502 | Developmental
process | A biological
process whose specific outcome is the progression of an integrated
living unit: An anatomical structure (which may be a subcellular
structure, cell, tissue, or organ), or organism over time from an
initial condition to a later condition. | 1.19e-36 |
| 0007275 | Multicellular
organismal development | The biological
process whose specific outcome is the progression of a
multicellular organism over time from an initial condition (e.g. a
zygote or a young adult) to a later condition (e.g. a multicellular
animal or an aged adult) | 1.36e-35 |
| 0048731 | System
development | The process whose
specific outcome is the progression of an organismal system over
time, from its formation to the mature structure. A system is a
regularly interacting or interdependent group of organs or tissues
that work together to carry out a given biological process. | 2.12e-33 |
| 0032501 | Multicellular
organismal process | Any biological
process, occurring at the level of a multicellular organism,
pertinent to its function. | 3.89e-32 |
| 0009653 | Anatomical
structure morphogenesis | The process in
which anatomical structures are generated and organized.
Morphogenesis pertains to the creation of form. | 1.62e-28 |
| 0005886 | Plasma
membrane | The membrane
surrounding a cell that separates the cell from its external
environment. It consists of a phospholipid bilayer and associated
proteins. | 2.66e-28 |
| 0031226 | Intrinsic component
of plasma membrane | The component of
the plasma membrane consisting of gene products and protein
complexes that have some covalently attached part (e.g. peptide
sequence or GPI anchor), which spans or is embedded in one or both
leaflets the membrane. | 8.71e-25 |
| 0005887 | Integral component
of plasma membrane | The component of
the plasma membrane consisting of gene products and protein
complexes that have some part that penetrates at least one leaflet
of the membrane bilayer. This component includes gene products that
are buried in the bilayer with no exposure outside the
bilayer. | 8.01e-24 |
| 0022610 | Biological
adhesion | The attachment of a
cell or organism to a substrate or other organism. | 1.01e-21 |
KEGG pathway analysis
The 293 DEGs were found to be involved in 131
pathways, including Wnt signaling, cell adhesion molecules (CAMs),
neuroactive ligand-receptor interaction, PI3K-Akt signaling,
cytokine-cytokine receptors interactions, calcium signaling,
regulation of actin cytoskeleton and focal adhesion (Table V). By combining the functional
annotation of DEGs with data from disease databases, we found that
16 genes among the DEGs have been previously associated with the
occurrence of RA (Table VI).
 | Table VKEGG pathways that DEGs are mainly
involved in. |
Table V
KEGG pathways that DEGs are mainly
involved in.
| Pathway id | Description | Count |
|---|
| 4080 | Neuroactive
ligand-receptor interaction | 10 |
| 4151 | PI3K-Akt signaling
pathway | 9 |
| 4060 | Cytokine-cytokine
receptor interaction | 9 |
| 4020 | Calcium signaling
pathway | 7 |
| 4512 | ECM-receptor
interaction | 7 |
| 4514 | Cell adhesion
molecules | 7 |
| 4810 | Regulation of actin
cytoskeleton | 7 |
| 4510 | Focal adhesion | 7 |
| 4974 | Protein digestion
and absorption | 7 |
| 5410 | Hypertrophic
cardiomyopathy | 7 |
 | Table VIGenbank information on differentially
expressed genes (n=16) that have been associated with rheumatoid
arthritis. |
Table VI
Genbank information on differentially
expressed genes (n=16) that have been associated with rheumatoid
arthritis.
| Acc. no. | Gene symbol | Name | Expression |
|---|
| NM_003881 | WISP2 | WNT1 inducible
signaling pathway protein 2 | Down |
| NM_001066 |
TNFRSF1B | Tumor necrosis
factor receptor superfamily, member 1B | Down |
| NM_000877 | IL1R1 | Interleukin 1
receptor, type 1 | Down |
| NM_002996 | CX3CL1 | Chemokine (C-X3-C
motif) ligand 1 | Up |
| NM_007365 | PADI2 | Peptidyl arginine
deiminase, type II | Up |
| NM_001276 | CHI3L1 | Chitinase 3-like
1 | Down |
| NM_004864 | GDF15 | Growth
differentiation factor 15 | Down |
| NM_000214 | JAG1 | Jagged 1 | Up |
| NM_000612 | IGF2 | Insulin-like growth
factor 2 | Up |
| NM_004878 | PTGES | Prostaglandin E
synthase | Down |
| NM_001145938 | MMP-1 | Matrix
metalloproteinase-1 | Down |
| NM_019111 | HLA-DRA | Major
histocompatibility complex, class II, DR α | Up |
| NM_000396 | CTSK | Cathepsin K | Down |
| NM_005118 | TNFSF15 | Tumor necrosis
factor superfamily, member 15 transcript variant 1 | Up |
| NM_006379 | SEMA3C | Sema domain, Ig,
short basic domain, secreted, semaphorin 3C | Down |
| NM_000692 | ALDH1B1 | Aldehyde
dehydrogenase 1 family, member B1 | Up |
Discussion
RA is a systemic inflammatory disorder that commonly
affects the diarthrodial joints (30). The pathogenesis of RA is
characterized by the influx of immune system cells (31), which induce the production of
pro-inflammatory cytokines, decreased synthesis of
anti-inflammatory cytokines and the subsequent activation and
proliferation of synovial fibroblasts (32). Remission in RA is an increasingly
attainable goal, but there is no widely used definition of
remission that is stringent yet achievable, and that could be
uniformly applied as a criterion of clinical outcome (33). Therefore, the identification and
characterization of genes related to RA is important for the
understanding of the pathogenesis of this disease and the
identification of novel anti-inflammatory therapeutic targets.
In the present study, we identified genes
differentially expressed between RA and healthy individuals. A
total of 293 genes were identified as DEGs, including these
encoding the receptor tyrosine kinase-like orphan receptor 2
(Ror2), chitinase-3-like 1 (CHI3L1), matrix metalloproteinases
(MMPs), interleukin (IL)-26, and v-maf musculoaponeurotic
fibrosarcoma oncogene homolog B (MafB). Ror2 has been associated
with RA. A previous study indicated that the Wnt5a-Ror2 pathway is
crucial for osteoclastogenesis in physiological and pathological
environments, and may represent a therapeutic target for bone
diseases, including RA (34).
Sonomoto et al (35)
demonstrated that IL-1β can effectively and rapidly induce the
differentiation of human mesenchymal stem cells into osteoblasts,
as well as mineralization, mainly through the non-canonical
Wnt5a-Ror2 pathway.
The CHI3L1 or YKL40 gene encodes for
the human cartilage glycoprotein 39 (HC-gp39), which is secreted by
synovial fibroblasts, macrophages, neutrophil granulocytes, and
chondrocytes. Its expression is regulated by NF-κB (36). It was recently suggested that the
YKL-40 protein might be implicated in the pathogenesis of RA and
that its level may indicate the degree of joint inflammation
(37). The carbohydrate-binding
motif in YKL-40 specifically activates the Akt signaling pathway in
colonic epithelial cells (38).
The level of YKL-40 in the serum varies depending on the RA status
of patients, and is increased in 54% of patients with clinically
active disease (39). YKL-40 may
thus be suitable for assessing the disease activity and
pathophysiology of RA.
MMPs are members of an enzyme family that contain a
zinc ion on their active site, which is required for their
catalytic activity. MMPs are critical for maintaining tissue
allostasis. These enzymes are active at neutral pH, and can
therefore catalyze the physiological turnover of extracellular
matrix (ECM) macromolecules (40).
A previous study showed that the levels of MMP-8 and -9 in the
systemic circulation are representative of the levels of these
enzymes in the inflamed joint, and suggested that MMP-9 may be
involved in degradation of the joint collagen. The study further
confirmed the hypothesis of an MMP/TIMP imbalance in RA (41). In addition, inhibition of MMP-13
was shown to reduce cartilage erosions in two out of three tested
animal models of RA, strongly supporting the development of drugs
targeting MMPs in order to reduce or halt joint destruction in
patients with RA (42).
IL-26, a member of the IL-10 cytokine family,
induces the production of pro-inflammatory cytokines by epithelial
cells. It was recently demonstrated that IL-26 is constitutively
produced by fibroblast-like cells known as synoviocytes in RA, and
that it induces the secretion of pro-inflammatory cytokines by
myeloid cells and favors the formation of T helper cells producing
interleukin 17 (Th17). The authors suggested that IL-26 is a
pro-inflammatory cytokine located upstream of the pro-inflammatory
cascade, and may constitute a promising target to treat RA and
chronic inflammatory disorders (43). Although synoviocytes are present in
all joints, only synoviocytes from RA patients can produce IL-26
(44).
MafB was another gene found as differentially
expressed in our study. The MafB protein is a putative tumor
suppressor in the myeloid lineage, with a key role in
monocytopoiesis (45), as well as
in monocyte-dendritic cell differentiation (46).
The GO functional annotation analysis showed that
DEGs are enriched for a total of 309 GO terms. The top 10 GO terms
mainly referred to anatomical structure development, membrane
formation and stability, and biological adhesion. It is notable
that alterations in biological adhesion are involved in RA. For
example, COL5A1 (collagen, type V, α1) was expressed at
significantly higher levels in chondrocytes from the damaged region
of osteoarthritic cartilage than in those from the intact region
(47).
KEGG signaling pathway analysis revealed that the
DEGs are predicted to be involved in a total of 131 pathways,
including Wnt and calcium signaling, as well as CAM-related
pathways. The Wnt signaling pathway plays a key role in cell
renewal. A few studies showed that it is also involved in RA
pathogenesis (48,49). In the synovial membrane of patients
with RA, the Wnt and Fz genes are expressed at higher
levels compared to those observed in patients without RA (50). The Wnt proteins are glycoproteins
that bind to the Fz receptors on the cell surface, thereby
affecting a number of important biological processes, such as cell
differentiation, embryonic development, limb development, and joint
formation (51). Enhanced
knowledge of the role(s) of the Wnt signaling pathway in RA is
expected to improve our understanding of the different RA clinical
features and improve prognosis. Both calcium signaling and
CAM-related pathways, upregulated in RA patients showing a
traditional Chinese medicine heat pattern, have been suggested to
be important for T-lymphocyte interactions, and thus, constitute
candidate targets for RA therapy (52).
Cross-referencing with disease databases revealed
that 16 of the identified DEGs have been previously associated with
RA; these include genes encoding MMP-1, interleukin-1 receptor type
1 (IL1R1) and PADI2. The aggressive phenotype of synovial
fibroblasts in RA is characterised by the increased expression of
MMP-1 (53). Collagenases MMP-1
and -13 play an important role in collagen degradation in RA and
osteoarthritis, while gelatinases MMP-2 and -9 may be involved in
arthritis by degrading non-collagen matrix components in the joints
(54). The IL1R1 receptor, once
activated upon IL-1 binding, activates NF-κB, which is a modulator
of expression of inflammatory and immune genes (55). Nevertheless, further experiments
are needed to study the roles that these genes may play in the
occurrence of RA.
Overall, our study provided a list of candidate
molecular targets for RA treatment and further research. In recent
years, gene-targeted therapy of RA has become a popular approach,
and related ongoing research appears promising. Despite significant
therapeutic advances, RA treatment remains an unsolved medical
issue. Therefore, the molecular mechanism(s) underlying this
disease need to be further investigated.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China of the year 2011 (81171734) and the
Applied Basic Research Project of Yunnan Province (2011FZ313).
References
|
1
|
Marrelli A, Cipriani P, Liakouli V, et al:
Angiogenesis in rheumatoid arthritis: a disease specific process or
a common response to chronic inflammation? Autoimmun Rev.
10:595–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lajas C, Abasolo L, Bellajdel B, et al:
Costs and predictors of costs in rheumatoid arthritis: a
prevalence-based study. Arthritis Rheum. 49:64–70. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smolen JS, Aletaha D, Koeller M, Weisman
MH and Emery P: New therapies for treatment of rheumatoid
arthritis. Lancet. 370:1861–1874. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aletaha D, Neogi T, Silman AJ, et al: 2010
Rheumatoid arthritis classification criteria: an American College
of Rheumatology/European League Against Rheumatism collaborative
initiative. Arthritis Rheum. 62:2569–2581. 2010. View Article : Google Scholar
|
|
5
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tobon GJ, Youinou P and Saraux A: The
environment, geo-epidemiology, and autoimmune disease: Rheumatoid
arthritis. Autoimmun Rev. 9:A288–A292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smolen JS, Aletaha D, Bijlsma JW, et al:
Treating rheumatoid arthritis to target: recommendations of an
international task force. Ann Rheum Dis. 69:631–637. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guma M, Hammaker D, Topolewski K, et al:
Antiinflammatory functions of p38 in mouse models of rheumatoid
arthritis: advantages of targeting upstream kinases MKK-3 or MKK-6.
Arthritis Rheum. 64:2887–2895. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Le Goff B, Blanchard F, Berthelot JM,
Heymann D and Maugars Y: Role for interleukin-6 in structural joint
damage and systemic bone loss in rheumatoid arthritis. Joint Bone
Spine. 77:201–205. 2010.PubMed/NCBI
|
|
10
|
Damjanov N, Kauffman RS and Spencer-Green
GT: Efficacy, pharmacodynamics, and safety of VX-702, a novel p38
MAPK inhibitor, in rheumatoid arthritis: results of two randomized,
double-blind, placebo-controlled clinical studies. Arthritis Rheum.
60:1232–1241. 2009. View Article : Google Scholar
|
|
11
|
Buch MH and Emery P: New therapies in the
management of rheumatoid arthritis. Curr Opin Rheumatol.
23:245–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Isomaki HA, Hakulinen T and Joutsenlahti
U: Excess risk of lymphomas, leukemia and myeloma in patients with
rheumatoid arthritis. J Chronic Dis. 31:691–696. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Smitten AL, Simon TA, Hochberg MC and
Suissa S: A meta-analysis of the incidence of malignancy in adult
patients with rheumatoid arthritis. Arthritis Res Ther. 10:R452008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olson AL, Swigris JJ, Sprunger DB, et al:
Rheumatoid arthritis-interstitial lung disease-associated
mortality. Am J Respir Crit Care Med. 183:372–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cloonan N, Forrest AR, Kolle G, et al:
Stem cell transcriptome profiling via massive-scale mRNA
sequencing. Nat Methods. 5:613–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mortazavi A, Williams BA, McCue K,
Schaeffer L and Wold B: Mapping and quantifying mammalian
transcriptomes by RNA-Seq. Nat Methods. 5:621–628. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Gerstein M and Snyder M: RNA-Seq:
a revolutionary tool for transcriptomics. Nat Rev Genet. 10:57–63.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ozsolak F and Milos PM: RNA sequencing:
advances, challenges and opportunities. Nat Rev Genet. 12:87–98.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heruth DP, Gibson M, Grigoryev DN, Zhang
LQ and Ye SQ: RNA-seq analysis of synovial fibroblasts brings new
insights into rheumatoid arthritis. Cell Biosci. 2:432012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang LQ, Cheranova D, Gibson M, et al:
RNA-seq reveals novel transcriptome of genes and their isoforms in
human pulmonary microvascular endothelial cells treated with
thrombin. PLoS One. 7:e312292012. View Article : Google Scholar
|
|
21
|
Cheranova D, Gibson M, Chaudhary S, et al:
RNA-seq analysis of transcriptomes in thrombin-treated and control
human pulmonary microvascular endothelial cells. J Vis Exp.
e43932013. View
Article : Google Scholar
|
|
22
|
Cox MP, Peterson DA and Biggs PJ:
SolexaQA: At-a-glance quality assessment of Illumina
second-generation sequencing data. BMC Bioinformatics. 11:4852010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trapnell C, Pachter L and Salzberg SL:
TopHat: discovering splice junctions with RNA-Seq. Bioinformatics.
25:1105–1111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Trapnell C, Williams BA, Pertea G, et al:
Transcript assembly and quantification by RNA-Seq reveals
unannotated transcripts and isoform switching during cell
differentiation. Nat Biotechnol. 28:511–515. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Trapnell C, Roberts A, Goff L, et al:
Differential gene and transcript expression analysis of RNA-seq
experiments with TopHat and Cufflinks. Nat Protoc. 7:562–578. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
R Core Team. R: A language and environment
for statistical computing. R Foundation for Statistical Computing;
Vienna, Austria: 2013
|
|
27
|
Beissbarth T and Speed TP: GOstat: find
statistically overrepresented Gene Ontologies within a group of
genes. Bioinformatics. 20:1464–1465. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moriya Y, Itoh M, Okuda S, Yoshizawa A and
Kanehisa M: KAAS: an automatic genome annotation and pathway
reconstruction server. Nucleic Acids Res. 35:W182–W185. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xie C, Mao X, Huang J, et al: KOBAS 2.0: a
web server for annotation and identification of enriched pathways
and diseases. Nucleic Acids Res. 39:W316–W322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Brooker DS: Rheumatoid arthritis:
otorhinolaryngological manifestations. Clin Otolaryngol Allied Sci.
13:239–246. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gierut A, Perlman H and Pope RM: Innate
immunity and rheumatoid arthritis. Rheum Dis Clin North Am.
36:271–296. 2010. View Article : Google Scholar
|
|
32
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Felson DT, Smolen JS, Wells G, et al:
American College of Rheumatology/European League against Rheumatism
provisional definition of remission in rheumatoid arthritis for
clinical trials. Ann Rheum Dis. 70:404–413. 2011. View Article : Google Scholar
|
|
34
|
Maeda K, Kobayashi Y, Udagawa N, et al:
Wnt5a-Ror2 signaling between osteoblast-lineage cells and
osteoclast precursors enhances osteoclastogenesis. Nat Med.
18:405–412. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sonomoto K, Yamaoka K, Oshita K, et al:
Interleukin-1β induces differentiation of human mesenchymal stem
cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like
orphan receptor 2 pathway. Arthritis Rheum. 64:3355–3363. 2012.
|
|
36
|
Srivastava SK, Antal P, Gal J, et al: Lack
of evidence for association of two functional SNPs of CHI3L1 gene
(HC-gp39) with rheumatoid arthritis. Rheumatol Int. 31:1003–1007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kazakova M, Batalov A, Deneva T, Mateva N,
Kolarov Z and Sarafian V: Relationship between sonographic
parameters and YKL-40 levels in rheumatoid arthritis. Rheumatol
Int. 33:341–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen CC, Llado V, Eurich K, Tran HT and
Mizoguchi E: Carbohydrate-binding motif in chitinase 3-like 1
(CHI3L1/YKL-40) specifically activates Akt signaling pathway in
colonic epithelial cells. Clin Immunol. 140:268–275. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johansen JS, Stoltenberg M, Hansen M, et
al: Serum YKL-40 concentrations in patients with rheumatoid
arthritis: relation to disease activity. Rheumatology (Oxford).
38:618–626. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Malemud CJ: Matrix metalloproteinases
(MMPs) in health and disease: an overview. Front Biosci.
11:1696–1701. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tchetverikov I, Ronday HK, Van El B, et
al: MMP profile in paired serum and synovial fluid samples of
patients with rheumatoid arthritis. Ann Rheum Dis. 63:881–883.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jungel A, Ospelt C, Lesch M, et al: Effect
of the oral application of a highly selective MMP-13 inhibitor in
three different animal models of rheumatoid arthritis. Ann Rheum
Dis. 69:898–902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Corvaisier M, Delneste Y, Jeanvoine H, et
al: IL-26 is overexpressed in rheumatoid arthritis and induces
proinflammatory cytokine production and Th17 cell generation. PLoS
Biol. 10:e10013952012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sedwick C: IL-26 kick-starts rheumatoid
arthritis. PLoS Biol. 10:e10013982012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gemelli C, Montanari M, Tenedini E, et al:
Virally mediated MafB transduction induces the monocyte commitment
of human CD34+ hematopoietic stem/progenitor cells. Cell Death
Differ. 13:1686–1696. 2006.PubMed/NCBI
|
|
46
|
Bakri Y, Sarrazin S, Mayer UP, et al:
Balance of MafB and PU.1 specifies alternative macrophage or
dendritic cell fate. Blood. 105:2707–2716. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sato T, Konomi K, Yamasaki S, et al:
Comparative analysis of gene expression profiles in intact and
damaged regions of human osteoarthritic cartilage. Arthritis Rheum.
54:808–817. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Trenkmann M, Brock M, Gay RE, et al:
Expression and function of EZH2 in synovial fibroblasts: epigenetic
repression of the Wnt inhibitor SFRP1 in rheumatoid arthritis. Ann
Rheum Dis. 70:1482–1488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
de Rooy DP, Yeremenko NG, Wilson AG, et
al: Genetic studies on components of the Wnt signalling pathway and
the severity of joint destruction in rheumatoid arthritis. Ann
Rheum Dis. 72:769–775. 2013.PubMed/NCBI
|
|
50
|
Sen M, Reifert J, Lauterbach K, et al:
Regulation of fibronectin and metalloproteinase expression by Wnt
signaling in rheumatoid arthritis synoviocytes. Arthritis Rheum.
46:2867–2877. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sen M: Wnt signalling in rheumatoid
arthritis. Rheumatology (Oxford). 44:708–713. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu C, Xiao C, Chen G, et al: Cold and heat
pattern of rheumatoid arthritis in traditional Chinese medicine:
distinct molecular signatures indentified by microarray expression
profiles in CD4-positive T cell. Rheumatol Int. 32:61–68. 2012.
View Article : Google Scholar
|
|
53
|
Maciejewska-Rodrigues H, Karouzakis E,
Strietholt S, et al: Epigenetics and rheumatoid arthritis: the role
of SENP1 in the regulation of MMP-1 expression. J Autoimmun.
35:15–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kim KS, Choi HM, Lee YA, et al: Expression
levels and association of gelatinases MMP-2 and MMP-9 and
collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of
patients with arthritis. Rheumatol Int. 31:543–547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nakki A, Kouhia ST, Saarela J, et al:
Allelic variants of IL1R1 gene associate with severe hand
osteoarthritis. BMC Med Genet. 11:502010. View Article : Google Scholar : PubMed/NCBI
|