Introduction
Mesenchymal stem cells (MSCs) are stem cells with
multiple differentiation ability, which have great potential for
clinical applications. Current research mainly focuses on human
bone marrow mesenchymal stem cells (1). Human bone marrow mesenchymal stem
cells are usually obtained through bone marrow puncture; however,
the quantity markedly decreases along with increasing age, which
limits investigations and applications of human bone marrow
mesenchymal stem cells (2).
Therefore, current research explores alternative sources of
mesenchymal stem cells, including the peripheral blood (3), umbilical cord blood (4,5),
baby teeth (6), umbilical cord
(7) and placenta (8). The placenta consists of amnion,
chorion and matrix decidua, and among them, the chorion is composed
of nourishing cells and tissue that originates from extra-embryonic
mesoderm, gathering large amounts of mesenchymal stem cells. As the
placenta is a waste product following pregnancy, it has a wide
source, is easy to obtain and there is no ethical debate associated
with its use. Therefore, the placenta has become the focus of stem
cell studies in the last decade (6–11).
At an international human placenta mesenchymal stem cell research
conference in 2007, the use of human placental mesenchymal stem
cells was approved for clinical application (10). The placenta has become the focus of
mesenchymal stem cell research; however, the stem cell content in
these tissues is limited. Therefore, optimizing the existing
mesenchymal stem cell culture conditions and expanding the limited
mesenchymal stem cells rapidly in vitro is crucial.
Fms-related tyrosine kinase 3 ligand (FL) is a type
of cell factor which is able to promote the generation and
differentiation of hematopoietic stem cells and their progenitor
cells (12). Previous studies have
demonstrated that the mRNA of FL was highly expressed in certain
tissues, including peripheral blood mononuclear cells, the heart,
placenta, lung, spleen and thymus tissue, and is also expressed to
a certain extent in non-hematopoietic tissues, including the
prostate, testis, ovaries, intestines, liver, kidney and skeletal
muscle. Following combination with its receptor, Flt3, FL delivers
a mitosis promotion signal to the cells to regulate cell growth and
differentiation.
Therefore, on the basis of stable cultivation of
human AMSCs and CMSCs, the present study investigated the
importance of cell factors (FL) on the proliferation of AMSCs and
CMSCs in vitro.
Materials and methods
Antibodies and reagents
The materials used included: A placenta from
cesarean delivery (agreed to by the puerpera), low-glucose
Dulbecco’s modified Eagle’s medium (L-DMEM; HyClone, Loughborough,
UK), fetal calf serum (HyClone), type IV collagen enzyme (Sigma,
St. Louis, MO, USA), pancreatic enzyme (Sigma), EDTA (Sigma),
recombinant human FL (R&D systems, Minneapolis, MN, USA),
recombinant human alkaline fibroblast growth factor (bFGF; R&D
systems), mouse anti-human monoclonal antibodies for flow
cytometry, including CD90 (BD Biosciences, Franklin Lakes, NJ,
USA), CD105 (Biolegend, San Diego, CA, USA), CD73 (BD Biosciences),
CD14 (Biolegend), CD34 (BD Biosciences), CD45 (Biolegend) and
HLA-DR (eBioscience, San Diego, CA, USA), mouse anti-human
monoclonal antibodies for immunofluorescence staining, including
CD90 (eBiosience) and CD166 (eBiosience), an avidin biotin complex
(ABC) kit (Boster Biological Tech Ltd., Fremont, CA, USA),
perilipin (PLIN), aggrecan (ACAN), runt related transcription
factor 2 (RUNX2), Flt3 and β-actin primer sequences (Genscript,
Nanjing, Jiangsu, China), an Olympus BX51 light microscope
(Olympus, Tokyo, Japan), a CO2 incubator (Jouan,
Winchester, VA, USA) and an FC500 flow cytometer (Beckman Coulter,
Miami, FL, USA). The present study was approved by the ethics
committee of Soochow University (Suzhou, Jiangsu, China) and
written informed consent was obtained from the patient.
Immunofluorescence stain of placental
tissue
After the frozen placental tissue sections were
slightly air dried, a drop of the appropriate primary antibodies
(CD90; concentration of 1:400; CD166 concentration of 1:200 and
phosphate-buffered saline (PBS) as the negative control) was added.
Next, they were placed into a 37°C water bath box for 1 h and
washed three times with PBS (3 min each time) in order to remove
unbound antibodies. Sheep anti-rat fluorescence antibody combined
with fluorescein isothiocyanate (FITC; at the concentration of
1:100) was added and then placed into a 37°C water bath box for 30
min, washed with PBS three times (3 min each time) and observed
under an Olympus BH-2 fluorescent microscope (Olympus).
Isolation and culture of human AMSCs and
CMSCs
The cesarean section placenta and the amniotic
tissue were rinsed repeatedly in PBS and the placental amniotic
membranes were cut into small pieces. They were then digested in
0.25% pancreatic enzyme at 37°C for 10 min and filtered through a
100 mesh screen. The tyrosine-digested tissues were fully cleaned
in PBS, continued to digest in 0.1% collagen IV enzyme for 15 min
at 37°C and suspended in L-DMEM containing 10% fetal calf serum by
pipetting up and down. The suspension was then filtered through a
100 mesh screen to remove any adherent cells, centrifuged at 400 g
for 10 min and then the supernatant was discarded. Next, the cells
sourced from the amniotic membrane were collected and divided into
three groups to be subjected to different cultural conditions.
Group I was cultured in complete L-DMEM containing 10% fetal calf
serum, group II was cultured in complete L-DMEM containing 5 ng/ml
bFGF and group III was cultured with complete L-DMEM complete
containing 75 ng/ml FL.
CMSCs separation and culture in
vitro
The cesarean section placenta and the cut chorion
tissue were rinsed repeatedly with PBS. Once the blood was washed
away, the chorion tissues were cut into small pieces and digested
for 30 min at 37°C with 0.1% type IV collagenase according to the
manufacturer’s instructions, and CMSCs were obtained. The two types
of MSCs (AMSCs and CMSCs) were cultured at 37°C with 5%
CO2. The medium was changed every 3 days. Following
12–14 days, the cells were passaged using conventional methods.
Flow cytometric analysis of the cellular
phenotype
The AMSCs and CMSCs of the experimental groups I, II
and III, which were cultured for more than three passages, were
collected, respectively and the cell density was adjusted to
5×105 cells/50 μl with PBS containing 1% fetal calf
serum. Then, rat anti-human monoclonal antibodies CD73-PE, CD90-PE,
CD105-PE, CD29-PE, CD44-PE, CD14-PE, CD34-FITC, CD45-PE and
HLA-DR-PE were added, respectively, and the cells were incubated at
4°C for 20 min. The cells were then washed twice with PBS and
finally, the cell phenotype was detected by flow cytometry.
Detection of the role of cell factors in
the proliferation of AMSCs and CMSCs by cell counting
Passage three AMSCs and CMSCs from the experimental
groups I, II and III were collected and seeded in 24-well plates at
a density of 1×104 cells per well, respectively. The
cells were treated with 75 ng/ml human FL, 5 ng/ml human bFGF or no
factor for the control group. On the second day, three wells were
digested for cell counting. Cells were counted using the Merck
Millipore Scepter 2.0 cell counter (Merck Millipore, Darmstadt,
Germany). An eight-day cell growth curve was generated with, the
cell count as the y-coordinate and the culture time as the
x-coordinate.
Analysis of the differentiation potential
of AMSCs and CMSCs by specific staining
The passage three AMSCs and CMSCs from the FL group
were collected and seeded in a six-well plate. After the cells were
attached completely, the culture medium was removed by aspiration.
Fat cell inducing medium (containing 10% FBS, 1 μmol/ml of
dexamethasone, 0.5 mmol/l of 3-isobutyl-1-methyl xanthine, 10 μg/ml
of insulin and 0.2 mmol/l of indomethacin DMEM), bone cell inducing
medium (100 nM of dexamethasone, 10 nM of β-glycerophosphate and
0.25 nM of phospho-l-ascorbic acid trisodium salt) and cartilage
cell inducing medium (0.5 μg/ml of insulin, 50 μM of ascorbic
acid-2 phosphate and 2 μg/ml of TGF-β) was added. Negative control
cells were conventionally cultured at 37°C with 5% CO2.
The culture solution was changed every three days. Following 2–3
weeks for the corresponding detection, cells were observed under an
Olympus BX51 light microscope (Olympus) and images were
captured.
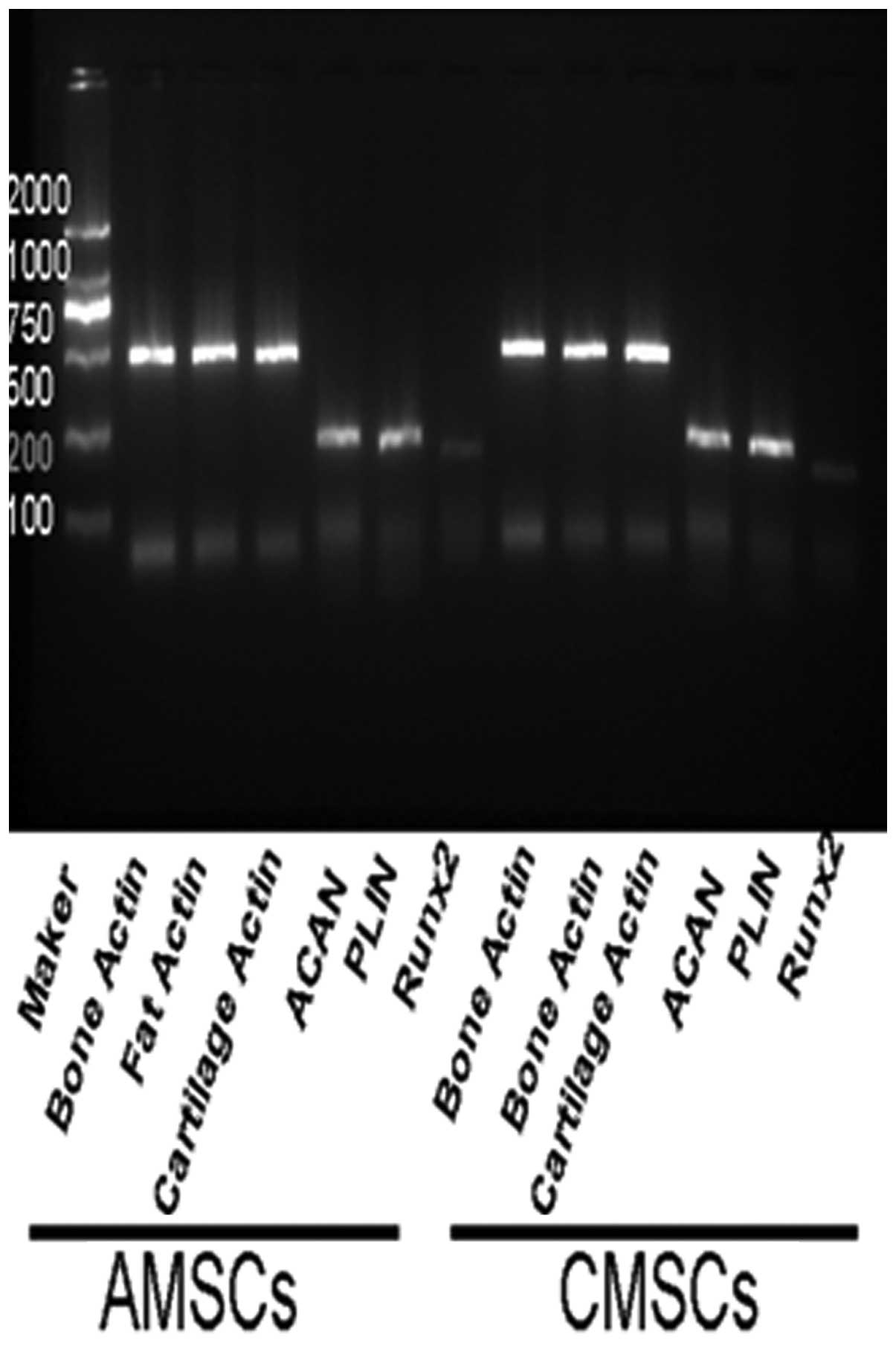
qPCR analysis of the mRNA levels of PLIN
(fat cells), ACAN (cartilage cells) and RUNX2 (bone cells) in
induced AMSCs and CMSCs
The third passage AMSCs and CMSCs of FL groups were
collected and fat, cartilage, bone cell inducing medium and 1 ml of
TRIzol (Thermo Fisher Scientific, Carlsbad, CA, USA) was added,
respectively, according to the manufacturer’s instructions. The
total RNA was extracted and then the random primers were
transcribed from RNA into cDNA. The following primers were used in
the present study: PLIN forward, 5′-AAACAGCATCAGCGTTCCCATC-3′ and
reverse, 5′-AGTGTTGGCAGCAAATTCCG-3′, fragment length, 173 bp; RUNX2
forward, 5′-CGCAAAACCACAGAACCACAAGTGCG-3′, and reverse,
5′-GTTGGTCTCGGTGGCTGGTAG-3′, fragment length, 164 bp; ACAN forward,
5′-CGGGTCTCACTGCCCAACTACCCG-3′ and reverse,
5′-GCCTTTCACCACGACTTCCAG-3′, fragment length, 200 bp; β-actin
forward, 5′-ATCCTCACCCTGAAGTACC-3′ and reverse,
5′-CTCCTTAATGTCACGCACG-3′, fragment length, 480 bp. The PCR cycle
parameters were as follows: 94°C for 30 sec, 56°C for 30 sec and
68°C for 60 sec (32 cycles). β-actin expression was used as a
control for expression quantity analysis. The PCR cycle parameters
for β-actin were as follows: 94°C for 3 min, 94°C for 30 sec, 58°C
for 30 sec and 72°C for 45 sec (30 cycles). The PCR products were
detected by agarose gel electrophoresis.
Analysis of the Flt3 mRNA levels in human
AMSCs and CMSCs
Passage three AMSCs and CMSCs of three different
samples were collected and 1 ml TRIzol was added, respectively,
according to the manufacturer’s instructions. Then the total RNA
was extracted and the random primers were transcribed from RNA into
cDNA. The PCR cycle parameters were as follows: 95°C for 3 min,
95°C for 30 sec, 65°C for 30 sec, 72°C for 60 sec (35 cycles) and
72°C for 10 min. The Flt3 primer sequence was used in the present
study as follows: Flt3 forward, 5′-TCAAGTGCTGTGCATACAATTCCC-3′ and
reverse, 5′-CACCTGTACCATCTGTAGCTGGCT-3′, fragment length, 176 bp.
The PCR products were detected by agarose gel electrophoresis.
Statistical analysis
All the data were analyzed with SPSS 10.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference. All values are expressed as
the mean ± standard deviation. Fisher’s t-test was performed among
all groups.
Results
Distribution of MSCs in the amniotic and
chorionic membranes of human placenta
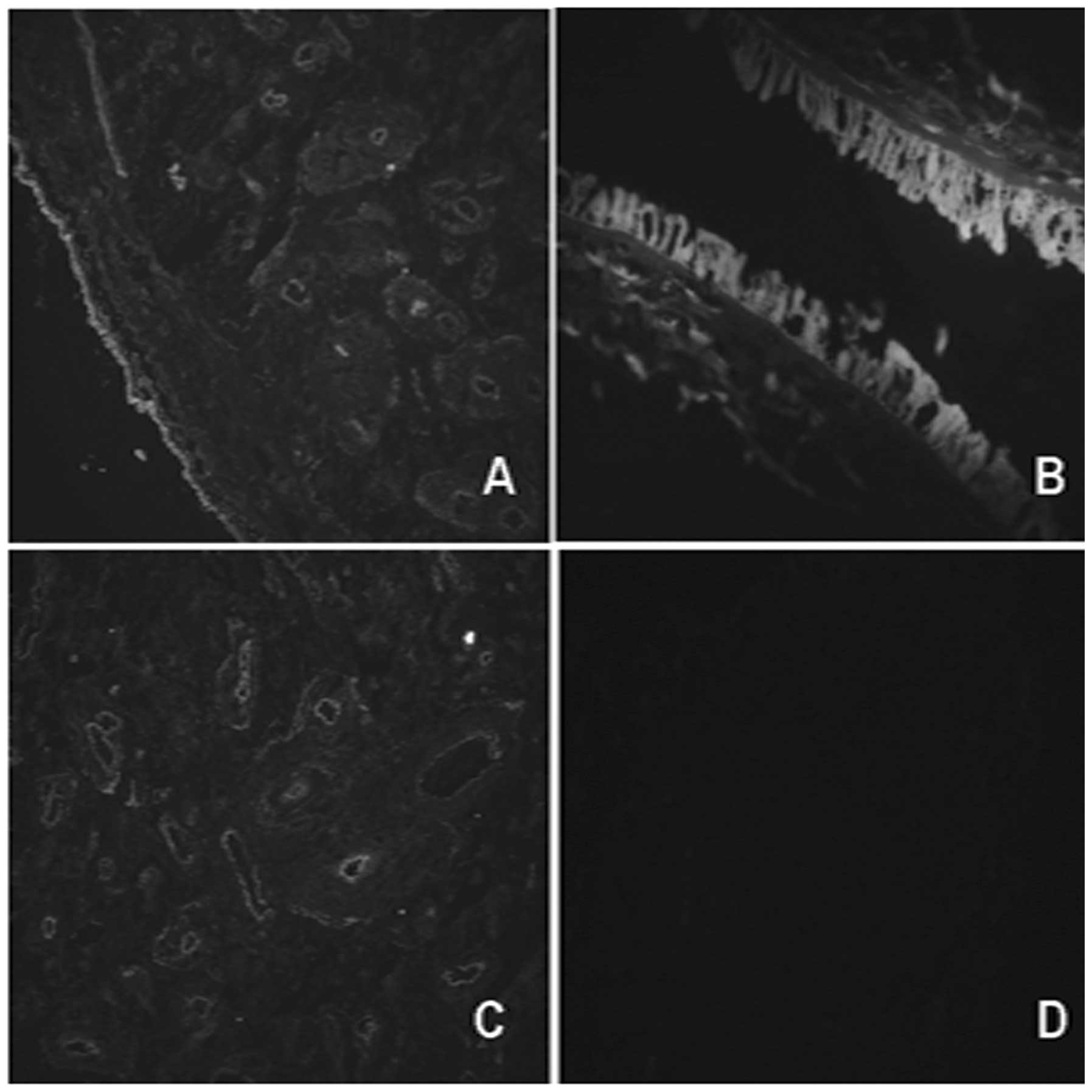
In order to identify the distribution of MSCs in
human placenta, the expression of CD90 and CD166 was separately
examined by immunofluorescence analysis. The immunofluorescence
stain demonstrated that CD90- and CD166-positive cells of amniotic
membrane tissue were distributed mainly in the amniotic epithelial
and amniotic membrane stroma, while the positive cells of the
chorionic membrane tissue were mainly in the vascular endothelium
and stroma near the blood vessels (Fig. 1). This indicated that human
placenta amniotic and chorionic membranes are tissues which are
rich in MSCs.
Biological characteristics of AMSCs and
CMSCs from human placenta
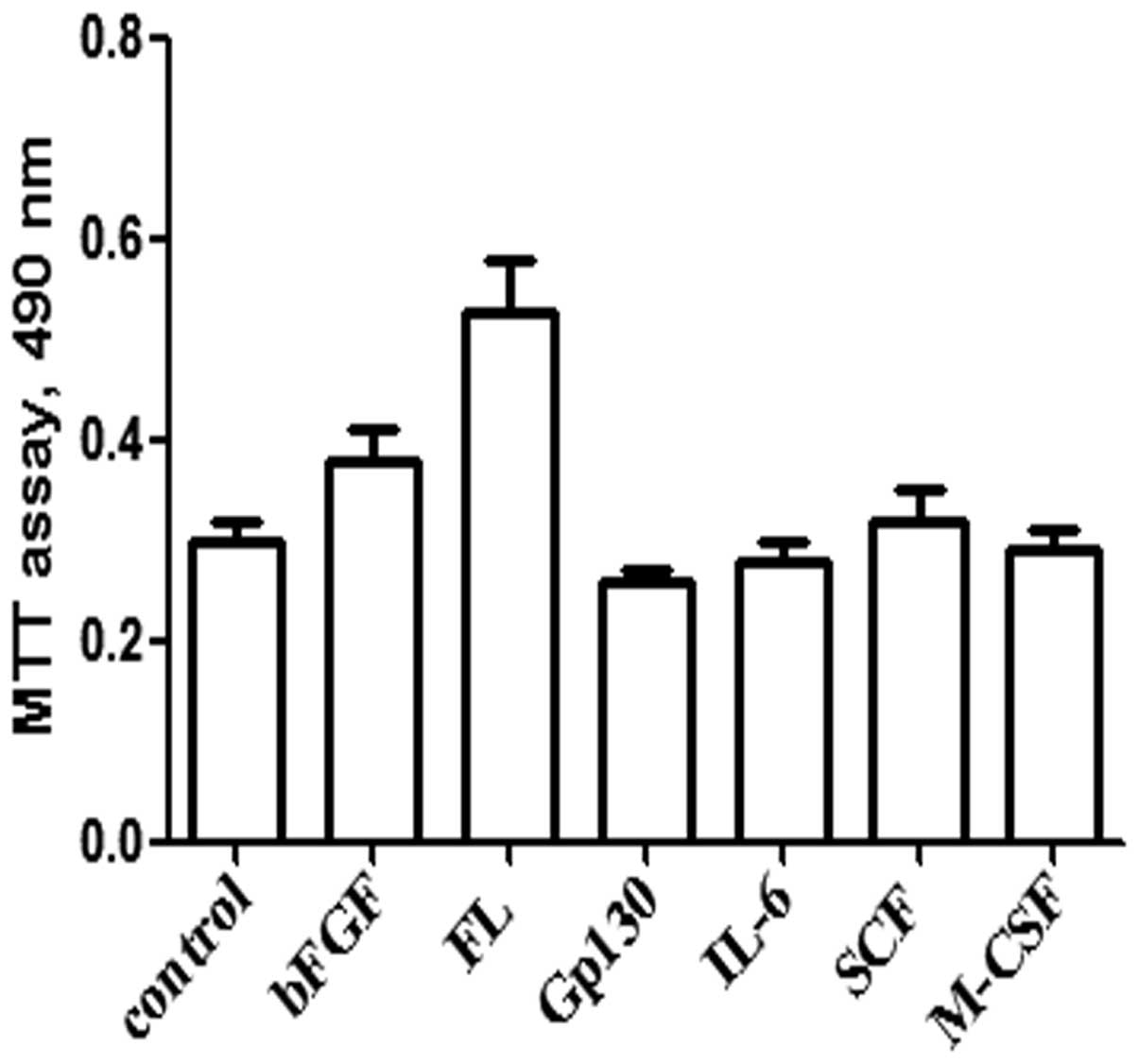
In order to optimize the protocol for growing MSCs,
the following cytokines were used: bFGF, FL, Gp130, interleukin
(IL)-6, stem cell factor (SCF) and macrophage colony stimulating
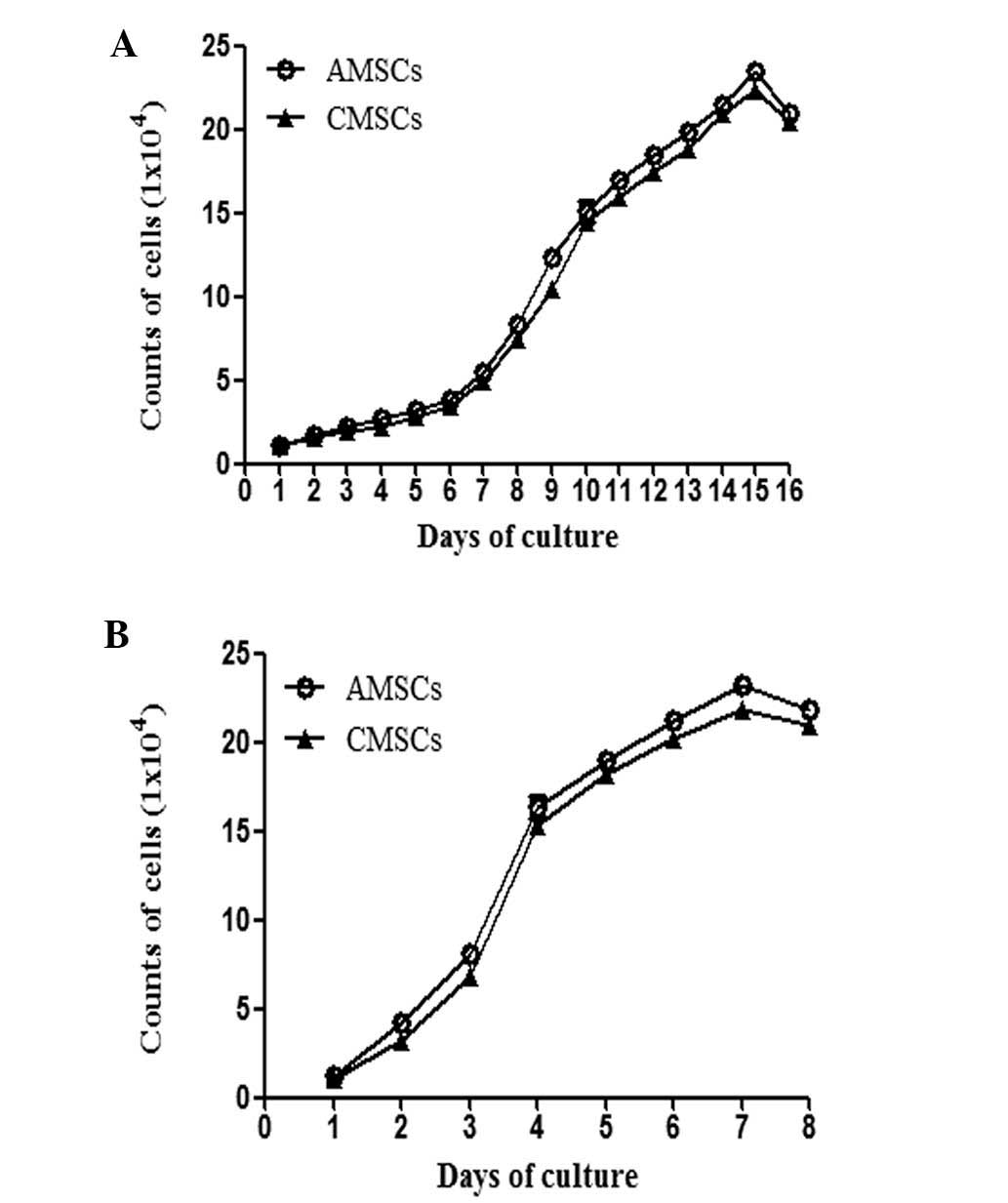
factor (M-CSF) (Fig. 2). AMSCs and
CMSCs were mainly cultured from human placenta by enzymatic
digestion and using an adherent culture method, and then the
primary generations of AMSCs and CMSCs were selected to obtain
values for the 16-day growth curve. It was confirmed that AMSCs and
CMSCs exhibited an increasing proliferation capacity with the
extension of culture time. (Fig.
3A) Based on this, the growth curve of the third generation
AMSCs and CMSCs was generated, which revealed that the
proliferation of AMSCs and CMSCs was not suppressed following
passage (Fig. 3B). Therefore,
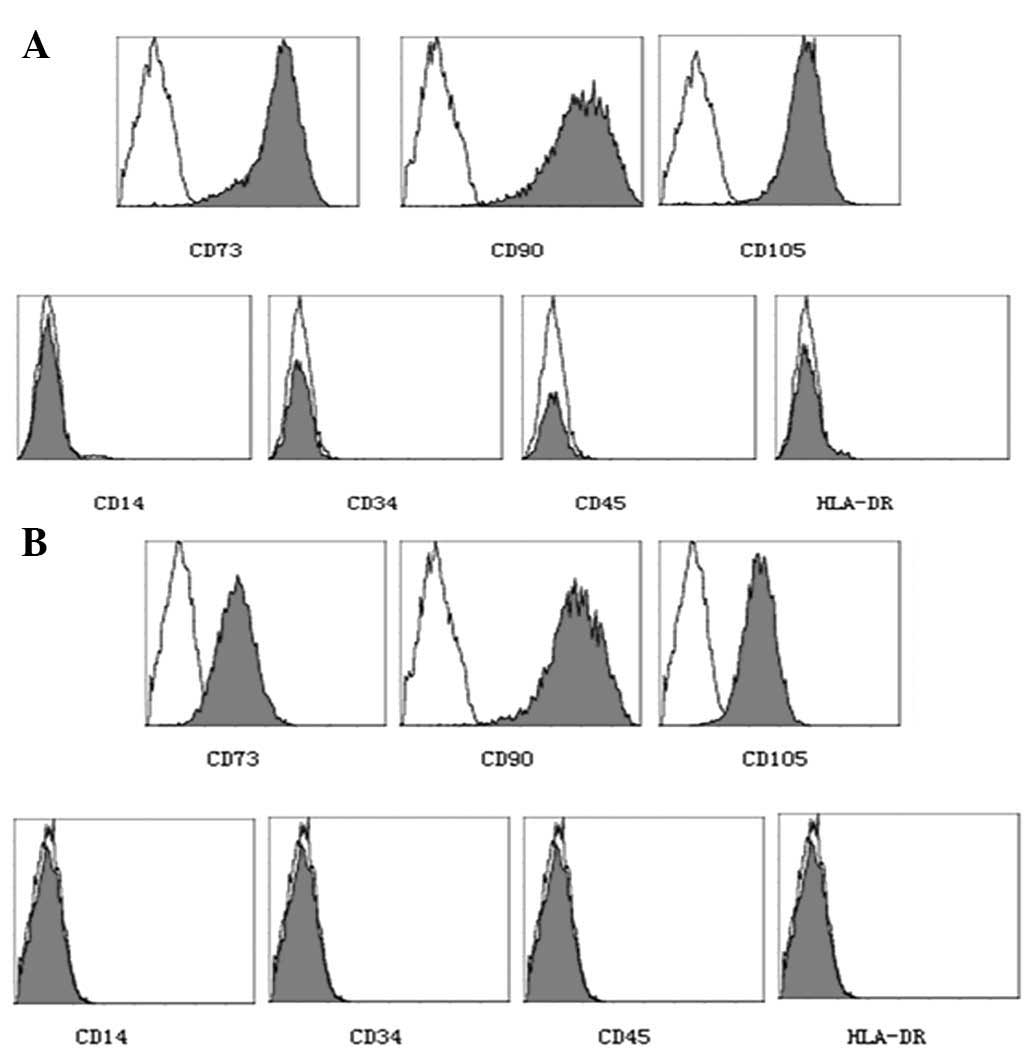
passage three cells (AMSCs and CMSCs) were selected to examine
their immune phenotypes. The results of the flow cytometric
analysis indicated that the immune phenotypes of AMSCs and CMSCs
were consistent with human placental MSCs, which were approved for
clinical use at an international meeting (10) (Fig.
4).
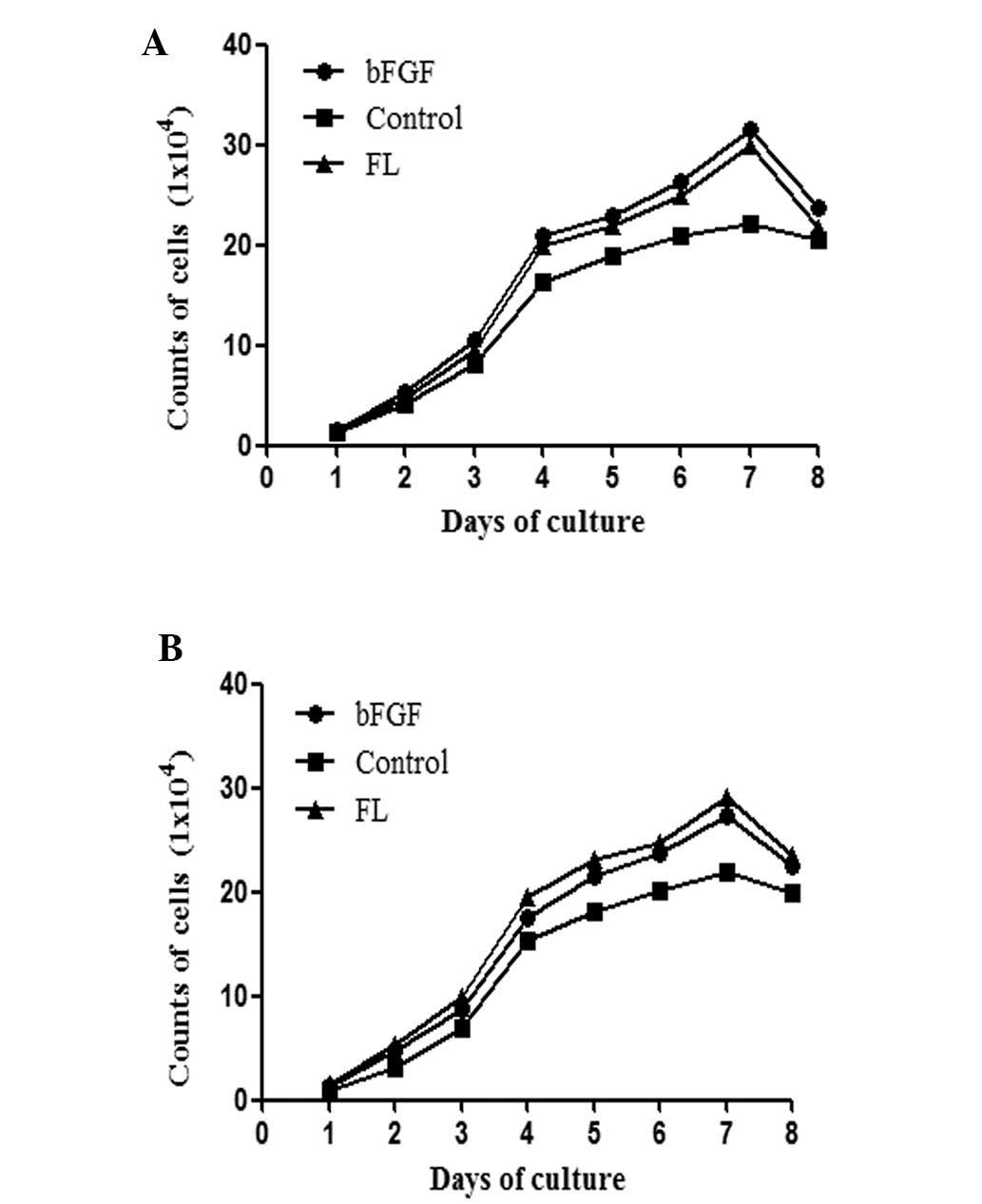
FL promotes the proliferation of AMSCs
and CMSCs
In order to identify the optimal working
concentration for each cell factor, different cell factors were
tested using an MTT assay. The results demonstrated that all
detected cell factors were able to promote the proliferation of
CMSCs; however, bFGF and FL demonstrated a higher growth
stimulation than others. Thus, the eight-day growth curve of AMSCs
and CMSCs was produced (Fig. 5).
The results confirmed that FL was able to promote the proliferation
of AMSCs and CMSCs; however, the effects were more apparent in
CMSCs.
FL affects the immune phenotype of AMSCs
and CMSCs
The present study demonstrated that FL was able to
promote the proliferation of AMSCs and CMSCs. Furthermore, the
present study aimed to identify whether FL was able to alter the
immune phenotypes of AMSCs and CMSCs. Therefore, the phenotypes of
AMSCs and CMSCs collected from three groups (the control group,
bFGF group and FL group) were collected. The results demonstrated
that AMSCs and CMSCs highly expressed CD73, CD105 and CD90;
however, they did not express CD14, CD34, CD45 and HLA-DR (Tables I and II). This was consistent with the
expression profile of human placental MSCs, which were approved for
clinical use at an international meeting (10).
 | Table IFlow cytometric analysis of AMSCs. |
Table I
Flow cytometric analysis of AMSCs.
| Maker group | CD73 | CD90 | CD105 | CD14 | CD34 | CD45 | HLA-DR |
|---|
| Control | 62.6±2.52 | 84±1.00 | 78±1.00 | 0.2±0.10 | 0.13±0.05 | 0.17±0.06 | 0.27±0.05 |
| 5 ng/ml bFGF | 71.3±1.53 | 93.3±1.52 | 81.4±0.53 | 0.13±0.06 | 0.23±0.07 | 0.23±0.05 | 0.5±0.10 |
| 75 ng/ml FL | 69.7±1.54 | 95±1.00 | 86±1.00 | 0.23±0.07 | 0.33±0.06 | 0.27±0.05 | 0.23±0.08 |
 | Table IIFlow cytometric analysis of CMSCs. |
Table II
Flow cytometric analysis of CMSCs.
| Maker group | CD73 | CD90 | CD105 | CD14 | CD34 | CD45 | HLA-DR |
|---|
| Control | 70±1.00 | 85±1.00 | 81.7±1.53 | 0.27±0.06 | 0.23±0.15 | 0.33±0.16 | 0.47±0.15 |
| 5 ng/ml bFGF | 82.3±0.58 | 97.3±1.53 | 95±1.00 | 0.63±0.15 | 0.47±0.25 | 0.5±0.36 | 0.47±0.21 |
| 75 ng/ml FL | 79.7±1.53 | 97±1.00 | 91±1.00 | 0.43±0.21 | 0.6±0.20 | 0.5±0.20 | 0.4±0.20 |
FL impacts the multilineage
differentiation potential of AMSCs and CMSCs
In order to gain insight into the multilineage
differentiation potential of AMSCs and CMSCs following the
administration of FL, the ability of AMSCs and CMSCs to
differentiate into fat, bone and cartilage was detected,
respectively. The results demonstrated that AMSCs and CMSCs from
the FL group were able to differentiate into fat, bone and
cartilage. It also indicated that FL not only promoted the
proliferation, but also maintained the differentiation potential of
MSCs (Fig. 6).
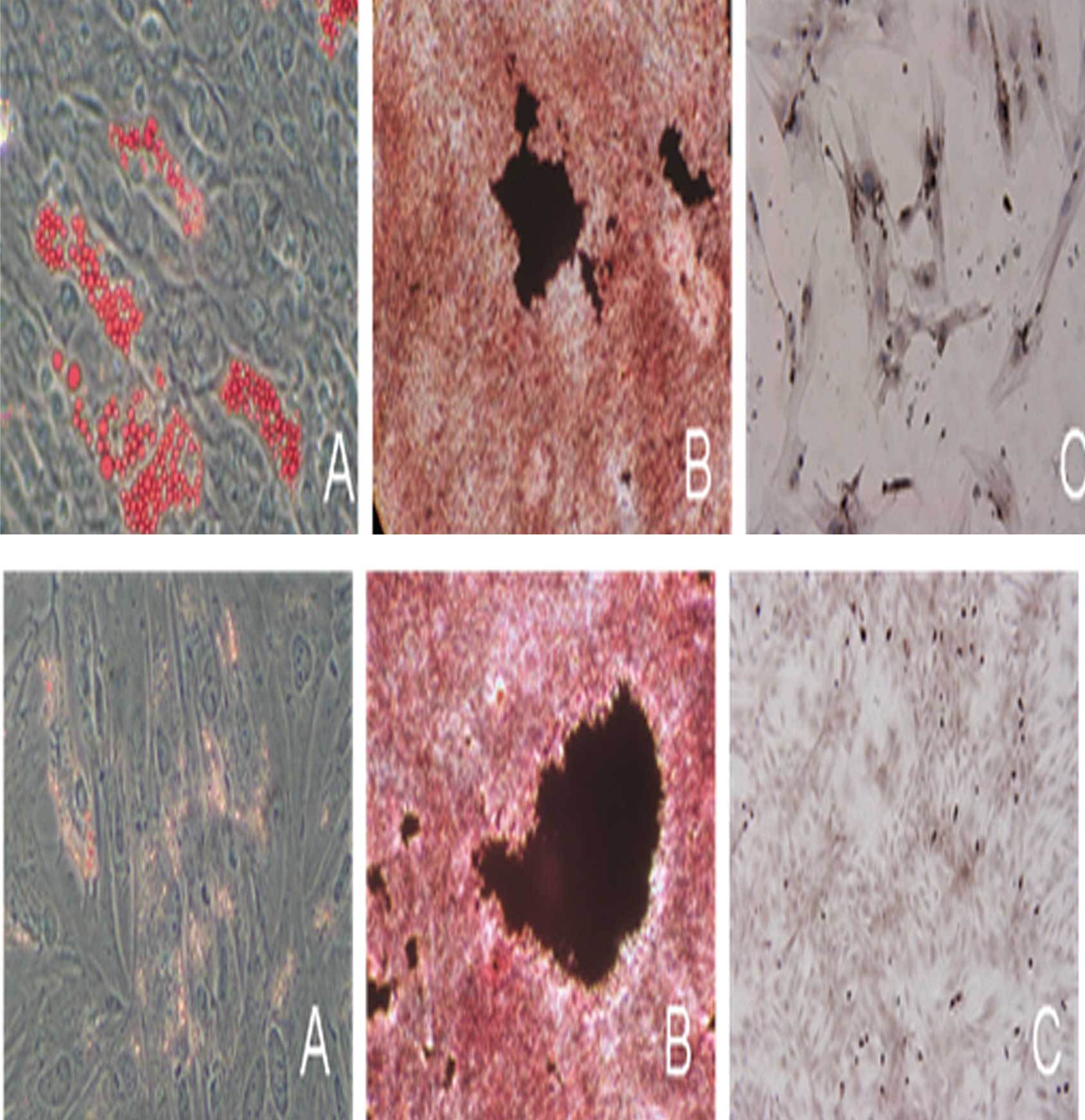 | Figure 6Identification of differentiation of
AMSCs and CMSCs into fat, bone and cartilage cells by
immunohistochemical analysis following administration of
fms-related tyrosine kinase 3 ligand. Differentiation of (top)
AMSCs and (bottom) CMSCs into (A) fat cells, oil red O staining,
(magnification, ×200), (B) bone cells, von Kossa staining
(magnification, ×100) and (C) cartilage, anti-collagen type II
immunocytochemistry stain (magnification, ×200). AMSCs, amniotic
mesenchymal stem cells; CMSCs, chorion mesenchymal stem cells. |
At the same time, qPCR results demonstrated that
specific genes, including PLIN (adipose cells), ACAN (chondrocytes)
and RUNX2 (osteocytes) were able to be detected following induction
of differentiation of AMSCs and CMSCs from the FL group,
respectively. The evidence also suggested that FL did not affect
the ability of AMSCs and CMSCs to differentiate into fat, cartilage
and bone cells (Fig. 7).
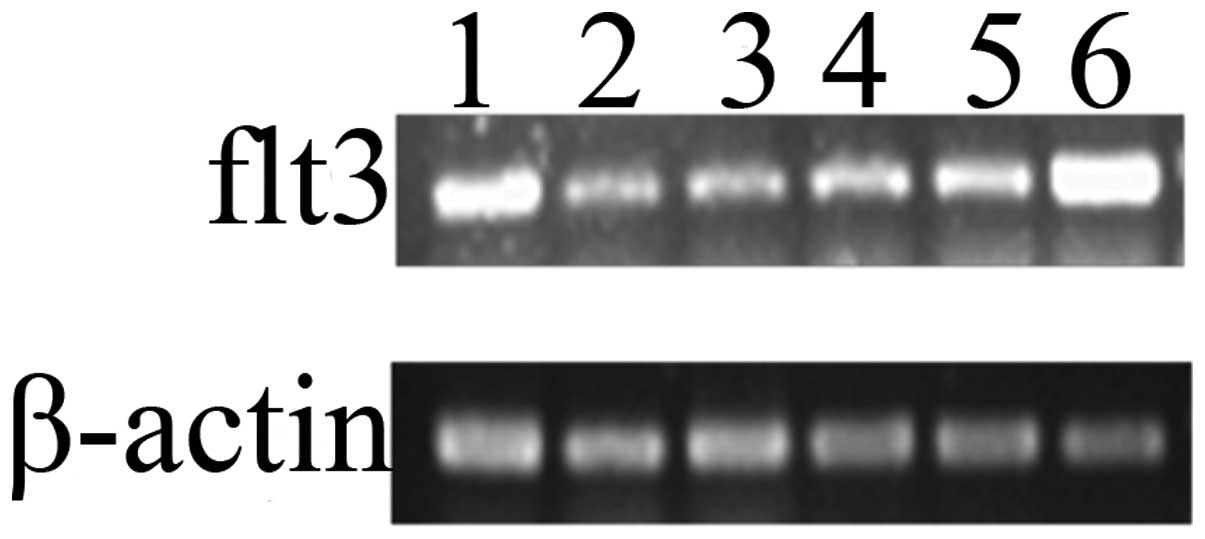
Expression levels of Flt3 mRNA in AMSCs
and CMSCs
The present study demonstrated that FL was able to
promote the proliferation of AMSCs and CMSCs; however, the possible
mechanisms remained elusive. It was possibly associated with the
expression of its receptor Flt3. Therefore, three different sources
of AMSCs and CMSCs were randomly selected from three independent
specimens as test objects. The qPCR results demonstrated that Flt3
mRNA was expressed in the AMSCs and CMSCs which were respectively
collected from three puerperas’ placenta (Fig. 8). This indicated that Flt3 may be
important in the process. These experimental data reinforce the
important role of FL in promoting the proliferation of AMSCs and
CMSCs.
Discussion
The placental tissue structure and cellular
components are relatively complex. Even if non-adherent cells were
eliminated through altering the culture solution, the cultured
cells may remain mixed with fibroblasts and endothelial cells.
Therefore, by shearing placental tissue selectively, it is possible
to obtain homogeneous mesenchymal stem cells. In the present study,
human placental amnion and chorion were selected for extracting
cells. The cells came from the two layers of placenta and were
demonstrated to be uniform in our previous experiment and were able
to proliferate well in vitro.
The placenta amnion and chorion develop from the
neural epiblast progenitors and extra-embryonic mesoderm in
embryonic development, respectively. However, due to the different
origins of amnion and chorion, and combined with amonion and
chorion structural changes in development, it was presumed that the
differentiation potentials of amnion and chorion mesenchymal stem
cells were different, in addition to their general mesenchymal stem
cell characteristics. Further study of the biological
characteristics and the molecular mechanisms of mesenchymal stem
cells gathering in placental structures, which develop from
different germ layers, is important for the use of mesenchymal stem
cells in the clinic.
As MSCs become increasingly focused upon, seeking a
source which contains MSCs of high quality and at high amounts is
clearly important. The present study optimized the culturing
conditions on the basis of existing placental mesenchymal stem cell
culture technology and selected stem cell factors associated with
proliferation, M-CSF, FL and SCF. The present study identified that
FL was able to promote the proliferation of AMSCs and CMSCs. FL is
the ligand of Flt3 and is mainly expressed in bone marrow stromal
cells and certain other cells, which mediates the synthesis of
certain growth factors to facilitate the proliferation of stem
cells, progenitor cells, dendritic cells and natural killer cells.
Flt3 has high sequence homology with the tyrosine kinase receptor
family III (RTK III) (13). Flt3,
FMS, platelet-derived growth factor receptor (PDGFR) and KIT are
all members of the RTK III family and all consist of the five
immunoglobulin constituted membrane crossing areas and cytoplasm
area, which contains a tyrosine-based motif (14). Flt3 is mainly expressed in infant
hemopoietic stem cells, the placenta, gonads and the brain
(15,16).
Studies concerning the mechanisms of Flt3 signal
transmission preceded FL cloning. Rottapel et al (17) constructed a chimeric molecule of
FMS and Flt3 (FF3). When affected by colony stimulating factor 1
receptor (CSF-1), certain Flt3-specific hapten-cell conjugates were
detected, whose C terminal phosphoinositide 3-kinase has the
ability to combine with the SH2 domain of growth factor
receptor-bound protein 2 (17). By
combining with CSF-1, FF3 regulates the automatic phosphorylation
of tyrosine, leading to a cell mitosis signal. By the Flt3
tissue-specific expression and its signal transmission mechanism,
the present study hypothesized that the addition of FL was able to
promote the proliferation of placental AMSCs and CMSCs cultured
in vitro, and our experimental results supported this
hypothesis. In addition, a previous study by our group demonstrated
that AMSCs had suitable neurobiological characteristics for being
neural precursors and also demonstrated that the transplantation of
AMSCs could repair focal cerebral ischemic injury in rats (18). Previous studies by our group
concentrated on AMSC-derived microvesicles (MVs), as it had been
suggested that tissue regeneration triggered by exogenous stem
cells may depend on the release of MVs rather than on stem cell
transdifferentiation (19–23).
Through scientific tissue source selection and
optimizing MSC culture conditions by the addition of cell factors
in order to obtain MSCs at high quality and in large quantities,
and examining the biological characteristics of mesenchymal stem
cells and their associated molecular regulation mechanisms, the
present study may have laid a foundation for further research and
clinical application.
Acknowledgements
This study was supported by the Suzhou Science and
Technology Project (nos. SYS201042 and SYS201311).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rao MS and Mattson MP: Stem cells and
aging: expanding the possibilities. Mech Ageing Dev. 122:713–734.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernández M, Simon V, Herrera G, Cao C,
Del Favero H and Minguell JJ: Detection of stromal cells in
peripheral blood progenitor cell collections from breast cancer
patients. Bone Marrow Transplant. 20:265–271. 1997.PubMed/NCBI
|
|
4
|
Erices A, Conget P and Minguell JJ:
Mesenchymal progenitor cells in human umbilical cord blood. Br J
Haematol. 109:235–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multi-potent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miura M, Gronthos S, Zhao M, Lu B, Fisher
LW, Robey PG and Shi S: SHED: stem cells from human exfoliated
deciduous teeth. Proc Natl Acad Sci USA. 100:5807–5812. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Romanov YA, Svintsitskaya VA and Smirnov
VN: Searching for alternative sources of postnatal human
mesenchymal stem cells: candidate MSC-like cells from umbilical
cord. Stem Cells. 21:105–110. 2003.PubMed/NCBI
|
|
8
|
Yen BL, Huang HI, Chien CC, Jui HY, Ko BS,
Yao M, Shun CT, Yen ML, Lee MC and Chen YC: Isolation of
multipotent cells from human term placenta. Stem Cells. 23:3–9.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goodwin HS, Bicknese AR, Chien SN, Bogucki
BD, Quinn CO and Wall DA: Multilineage differentiation activity by
cells isolated from umbilical cord blood: expression of bone, fat,
and neural markers. Biol Blood Marrow Transplant. 7:581–588. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parolini O, Alviano F, Bagnara GP, et al:
Concise review: isolation and characterization of cells from human
term placenta: outcome of the first international Workshop on
Placenta Derived Stem Cells. Stem Cells. 26:300–311. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fukuchi Y, Nakajima H, Sugiyama D, Hirose
I, Kitamura T and Tsuji K: Human placenta-derived cells have
mesenchymal stem/progenitor cell potential. Stem cells. 22:649–658.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gilliland DG and Griffin JD: The roles of
FLT3 in hematopoiesis and leukemia. Blood. 100:1532–1542. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rosnet O and Birnbaum D: Hematopoietic
receptors of class III receptor-type tyrosine kinases. Crit Rev
Oncog. 4:595–613. 1993.PubMed/NCBI
|
|
14
|
Agnès F, Shamoon B, Dina C, Rosnet O,
Birnbaum D and Galibert F: Genomic structure of the downstream part
of the human FLT3 gene:exon/intron structure conservation among
genes encoding receptor tyrosine kinases (RTK) of subclass III.
Gene. 145:283–288. 1994.PubMed/NCBI
|
|
15
|
deLapeyrière O, Naquet P, Planche J,
Marchetto S, Rottapel R, Gambarelli D, Rosnet O and Birnbaum D:
Expression of Flt3 tyrosine kinase receptor gene in mouse
hematopoietic and nervous tissues. Differentiation. 58:351–359.
1995.PubMed/NCBI
|
|
16
|
Maroc N, Rottapel R, Rosnet O, Marchetto
S, Lavezzi C, Mannoni P, Birnbaum D and Dubreuil P: Biochemical
characterization and analysis of the transform ing potential of the
FLT3/FLK2 receptor tyrosine kinase. Oncogene. 8:909–918.
1993.PubMed/NCBI
|
|
17
|
Rottapel R, Turck CW, Casteran N, Liu X,
Birnbaum D, Pawson T and Dubreuil P: Substrate specificities and
identification of a putative binding site for PI3K in the carboxy
tail of the murine Flt3 receptor tyrosine kinase. Oncogene.
9:1755–1765. 1994.PubMed/NCBI
|
|
18
|
Li F, Miao ZN, Xu YY, Zheng SY, Qin MD, Gu
YZ and Zhang XG: Transplantation of human amniotic mesenchymal stem
cells in the treatment of focal cerebral ischemia. Mol Med Report.
6:625–630. 2012.PubMed/NCBI
|
|
19
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar
|
|
20
|
Lai RC, Arslan F, Lee MM, et al: Exosome
secreted by MSC reduces myocardial ischemia/reperfusion injury.
Stem Cell Res. 4:214–222. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruno S, Grange C, Deregibus MC, et al:
Mesenchymal stem cell-derived microvesicles protect against acute
tubular injury. J Am Soc Nephrol. 20:1053–1067. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schweitzer KS, Johnstone BH, Garrison J,
et al: Adipose stem cell treatment in mice attenuates lung and
systemic injury induced by cigarette smoking. Am J Respir Crit Care
Med. 183:215–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrera MB, Fonsato V, Gatti S, Deregibus
MC, Sordi A, Cantarella D, Calogero R, Bussolati B, Tetta C and
Camussi G: Human liver stem cell-derived microvesicles accelerate
hepatic regeneration in hepatectomized rats. J Cell Mol Med.
14:1605–1618. 2010. View Article : Google Scholar : PubMed/NCBI
|