Introduction
Due to low scattering properties in exposed tissue
and deposition of the ionizing energy at an exact depth, proton
beam irradiation is a useful tool in tumor radiotherapy, with no
radiation penetrating the normal tissue neighboring the tumor. Once
a proton beam enters the body, the increased Bragg peak, the
specific energy per unit at the end of the beam’s range, induces
excellent localization to the target (1,2).
The mortality rates and prognoses in cancer patients
are determined by the metastatic potential of the tumor. The
five-year survival rate in localized breast cancer patients is
~98%; by contrast, only 27% of patients diagnosed with metastatic
breast cancer survive for five years or longer (3). Therefore, prevention of metastasis is
a required strategy to enhance the five-year survival rate for
patients.
Metastasis is a multistep series of events that
involves cancer cell dissociation from the primary tumor and
invasion and seeding at a distant site (4,5). The
metastatic potential of tumor cells is closely associated with the
expression levels of numerous proteins, including matrix
metalloproteinases (MMPs), plasminogen activator (PA), nitric oxide
synthase and cyclooxygenase (COX). COX catalyzes the synthesis of
prostaglandins from arachidonic acid and exists in two predominant
isoforms: COX-1, a constitutive enzyme, and COX-2, an inducible
protein. COX-2 accelerates cancer progression and metastasis, and
is overexpressed in various cancer types, including breast, colon,
lung and gastric cancer (6,7).
Several studies have reported that cancer cell proliferation and
metastasis are enhanced in the COX-2-overexpression system and are
reduced by downregulation of COX-2 expression by inhibitors
(8–11). Other studies have shown that MMP
and vesicular endothelial growth factor expression levels are
regulated by COX-2 (10,12). The results of these studies
indicate that COX-2 inhibition is important in the prevention of
cancer development, proliferation and metastasis.
The invasiveness of breast cancer cells is reduced
by COX-2 and MMP inhibition via the prevention of mitogen activated
protein kinase (MAPK) or phosphoinositide 3-kinase (PI3K)/Akt
signaling (10). By contrast, the
metastatic potential of breast cancer cells is increased via the
upregulation of COX-2 and MMPs by
12-O-tetradecanoylphorbol-13-acetate (TPA), activating the protein
kinase C (PKC)/MAPK and PI3K/Akt signaling pathways (13–15).
The enhancement of COX-2 and MMP-9 expression levels by TPA
requires nuclear factor-κB (NF-κB) and activator protein-1 (AP-1),
which bind to the COX-2 and MMP-9 promoters (16–18).
Numerous genes involved in cell proliferation, apoptosis,
metastasis, cancer development and inflammation are governed by
NF-κB and AP-1, which are activated by internal and external
stimuli (19–21). The metastatic potential of numerous
types of cancer cells has been shown to be determined by MMP-9 and
COX-2 activities underlying the change in NF-κB and/or AP-1
transcriptional activity (15,20–22).
The transcriptional activities of NF-κB and AP-1 regulating COX-2
and MMP-9 are closely associated with the PKC/MAPK and PI3K/Akt
signaling pathways (19–23).
Previous studies have revealed that the metastatic
potential in MDA-MB-231 and MCF-7 human breast cancer cells was
reduced by proton beam irradiation (14). In the present study, the molecular
biological mechanism of the antimetastatic activity of proton beam
irradiation in MDA-MB-231 human breast cancer cells was
investigated.
Materials and methods
Cell culture
MDA-MB-231 human breast cancer cells were purchased
from the Korean Cell Line Bank (Seoul, Korea). The cells were grown
as a monolayer in Dulbecco’s modified Eagle’s medium (DMEM)
supplemented with 10% fetal bovine serum and 1%
antibiotic-antimycotic solution at 5% CO2 and 37°C. The
cells were serum-starved for 24 h in serum-free DMEM medium prior
to proton beam irradiation.
Proton beam irradiation
Proton beam irradiation was performed with 35-MeV
proton beams at the MC-50-cyclotron of the Korean Institute of
Radiological Sciences (Seoul, Korea) (14). The cells were irradiated at the
center of the Bragg peaks, modulated to 6 cm widths.
Semi-quantitative reverse transcription
polymerase chain reaction (RT-PCR)
A total of 5×105 cells/well of MDA-MB-231
human breast cancer cells were seeded on 6-well plates and grown
for 24 h at 37°C in a 5% (v/v) CO2 atmosphere. Following
serum-starvation for 24 h, the cells were irradiated with a proton
beam and then cultured for an additional 24 h, with or without 100
nM TPA. The cells were collected by centrifugation following
trypsinization. Total RNA was extracted by using the easy-BLUE™
Total RNA Extraction kit (iNtRON Biotechnology, Sungnam, Korea)
according to the manufacturer’s instructions. RT-PCR was conducted
with the Avian Myeloblastosis Virus RNA PCR kit version 3.0 (Takara
Bio, Inc., Shiga, Japan) and 1 μg total RNA. The primer sequences
were as follows: MMP-9: Forward 5′-TTCATCTTCCAAGGC CAATC-3′ and
reverse 5′-CTTGTCGCTGTCAAAGTTCG-3′ (annealing temperature, 55°C)
(24); COX-2: Forward
5′-TTCACGCATCAGTTTTTCAA-3′ and reverse 5′-ACAGCAAACCGTAGATGCTC-3′
(annealing temperature, 55°C) (25); GAPDH: Forward
5′-ATCCCATCACCATCTTCCAG-3′ and reverse 5′-TTCTAGACGGCAGGTCAGGT-3′
(annealing temperature, 58°C) (26). The PCR products were subjected to
1.2% agarose gel electrophoresis containing 0.5 μg/ml ethidium
bromide and were visualized on a UV transluminometer (CoreBio
System, Seoul, Korea). Bands were densitometrically analyzed using
Scion Image (Scion Corporation, Frederick, MD, USA).
Nuclear fractionation
The cells were washed twice with phosphate-buffered
saline (PBS), and hypotonic buffer [containing 20 mM Tris-HCl (pH
7.4), 10 mM NaCl and 3 mM MgCl2, plus protease inhibitor
cocktail and phosphatase inhibitor cocktail] was added to each
sample. The cells were scraped with a rubber policeman and held on
ice for 15 min; then 1/8 volume 10% NP-40 was added. The cells were
vortexed for 10 sec at the maximum setting and centrifuged for 10
min at 2,500 × g at 4°C after 10 min incubation on ice. The
supernatants were removed and designated as the cytosol fraction
and the pellets were designated as the nuclear fraction. The
pellets were washed with hypotonic buffer and then lysed with Cell
Extraction Buffer (Invitrogen Life Technologies, Carlsbad, CA,
USA), containing protease inhibitor cocktail and phosphatase
inhibitor cocktail (GenDepot, Barker, TX, USA), for 30 min on ice,
vortexing at 10-min intervals. The lysates were separated by
centrifugation at 14,000 ×g for 30 min at 4°C; the supernatants
were subsequently removed, stored at −80°C, and labeled as the
nuclear fractions.
Preparation of total cell lysate and
western blotting
The cells were lysed in radioimmunoprecipitation
assay lysis buffer containing phosphatase and protease inhibitor
cocktails. Total cell lysates were prepared by centrifugation of
the lysed cells at 14,000 × g for 10 min at 4°C and stored at
−80°C. The protein concentrations in the total cell lysates were
determined by the bicinchoninic acid method. The protein samples
were subjected to SDS-PAGE and transferred onto polyvinylidene
fluoride membranes. The membranes were blocked with 5% skimmed milk
in Tris-buffered saline containing 0.1% Tween-20 and then incubated
with primary antibody (1:3,000) overnight at 4°C; anti-mouse
monoclonal COX-2 (1:3,000; Invitrogen Life Technologies) and
β-actin (1:3,000; Santa Cruz Biotechnology, Dallas, TX, USA).
Anti-rabbit monoclonal Akt, p-Akt, Erk1/2, p-Erk1/2, JNK1/2,
p-JNK1/2, p38, p-p38, NF-κB, p-NF-κB, c-Jun and lamin B (1:3,000;
Cell Signaling Technology, Beverly, MA, USA). Subsequently, the
membranes were incubated for 1 h at room temperature with secondary
antibodies; goat anti-mouse and -rabbit IgG HRP (1:3,000; Santa
Cruz Biotechnology). Protein bands were visualized with West-Q
Chemiluminescent Substrate Kit Plus (GenDepot) and images were
captured by the Luminescent Image Analyzer LAS-4000 (Fujifilm,
Tokyo, Japan). The band densities were densitometrically analyzed
by Scion Image.
Results
Changes in COX-2 and MMP-9 expression
levels following proton beam irradiation
In a previous study, cell migration and MMP-9
activity in MDA-MB-231 human breast cancer cells induced by TPA
were shown to be dose-dependently reduced by proton bream
irradiation (14). MMP-9 activity
is determined by its expression levels and is important in
metastasis. Furthermore, MMP-9 expression levels are closely
associated with COX-2 expression levels. Therefore, COX-2
expression levels may be regulated and MMP-9 transcription reduced
by proton beam irradiation. In the present study, the effects of
proton beam irradiation on COX-2 expression levels and MMP-9
transcription were investigated in MDA-MB-231 human breast cancer
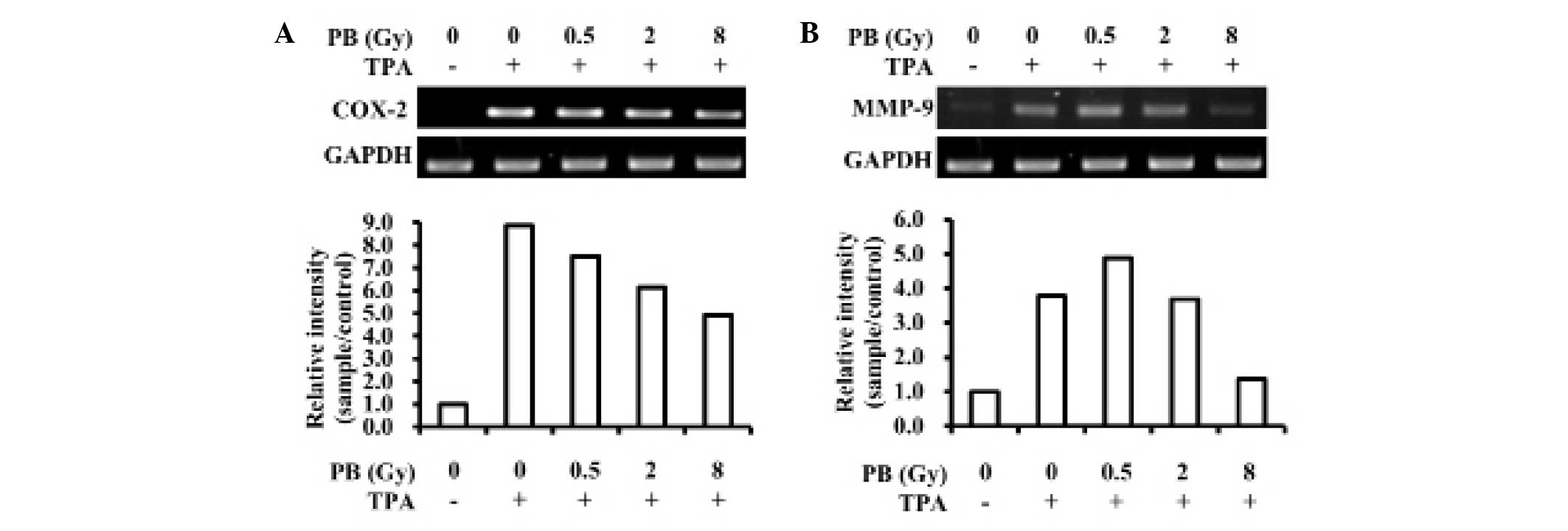
cells. As shown in Fig. 1A and B,
proton beam irradiation inhibited TPA-induced COX-2 and MMP-9
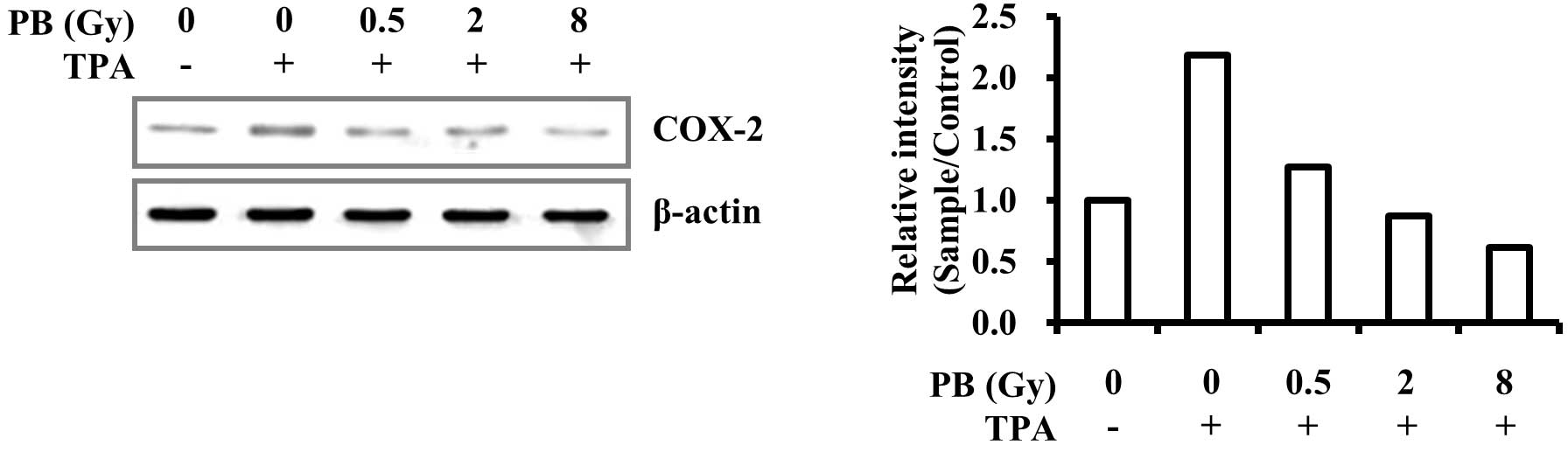
transcription. Furthermore, COX-2 protein expression levels were
dose-dependently suppressed by proton beam irradiation (Fig. 2). These results demonstrate that
proton beam irradiation may prevent increases in metastatic
potential in MDA-MB-231 triple-negative human breast cancer cells
through the downregulation of COX-2 and MMP-9 expression.
Effect of proton beam irradiation on
TPA-induced MAPK phosphorylation
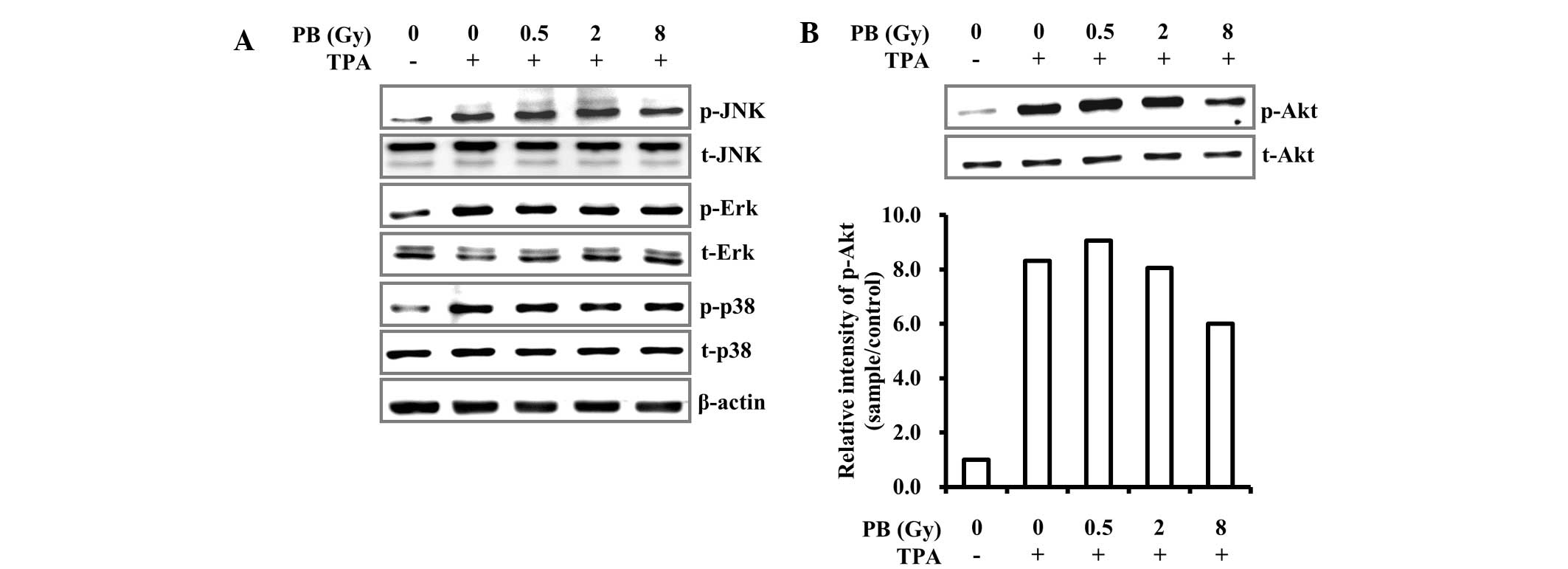
TPA-induced expression of COX-2 and MMP-9 is
primarily regulated via the PKC/MAPK signaling pathway. Therefore,
the effect of proton beam irradiation on the TPA-induced PKC/MAPK
signaling pathway was investigated. Despite proton beam
irradiation, the phosphorylation levels of the MAPKs, including
c-Jun terminal kinase, extracellular signal-regulated kinase and
p38, were not changed (Fig. 3A).
This result suggests that the reduction in COX-2 and MMP-9
expression levels induced by proton beam irradiation did not
involve MAPK signaling.
Effect of proton beam irradiation on
TPA-induced Akt phosphorylation
The metastatic activity induced by TPA is also
mediated by the PI3K/Akt signaling pathway (15). As shown in Fig. 3A, proton beam irradiation did not
prevent TPA-induced MAPK activation. Thus, the change of
TPA-induced Akt phosphorylation by proton beam irradiation was
investigated. Phosphorylation following TPA stimulation was
significantly reduced in MDA-MB-231 human breast cancer cells
irradiated by proton beams (Fig.
3B). Akt is a key effector in cancer survival and enhances
apoptotic resistance through NF-κB activation that induces COX-2,
MMP-9 and urokinase-type (u)PA expression (27,28).
Consequently, this suggests that proton beam irradiation may
prevent COX-2 and MMP-9 expression via downregulation of the Akt
signaling pathway.
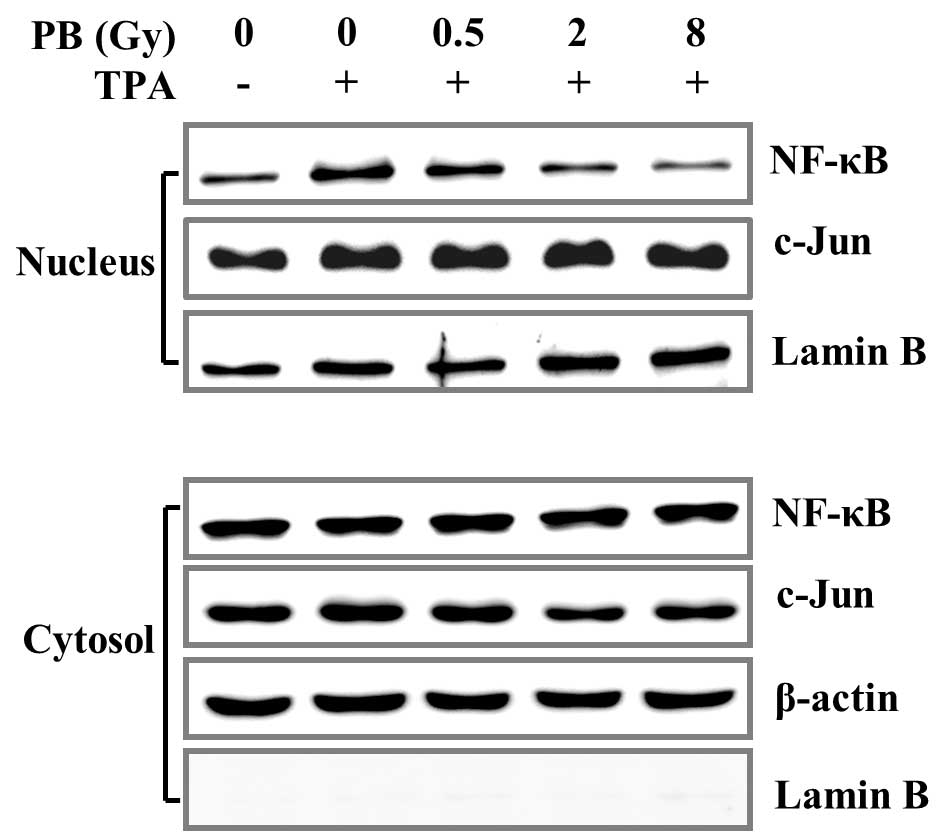
Effects of proton beam irradiation on
c-Jun expression levels, NF-κB phosphorylation and subsequent
nuclear translocation
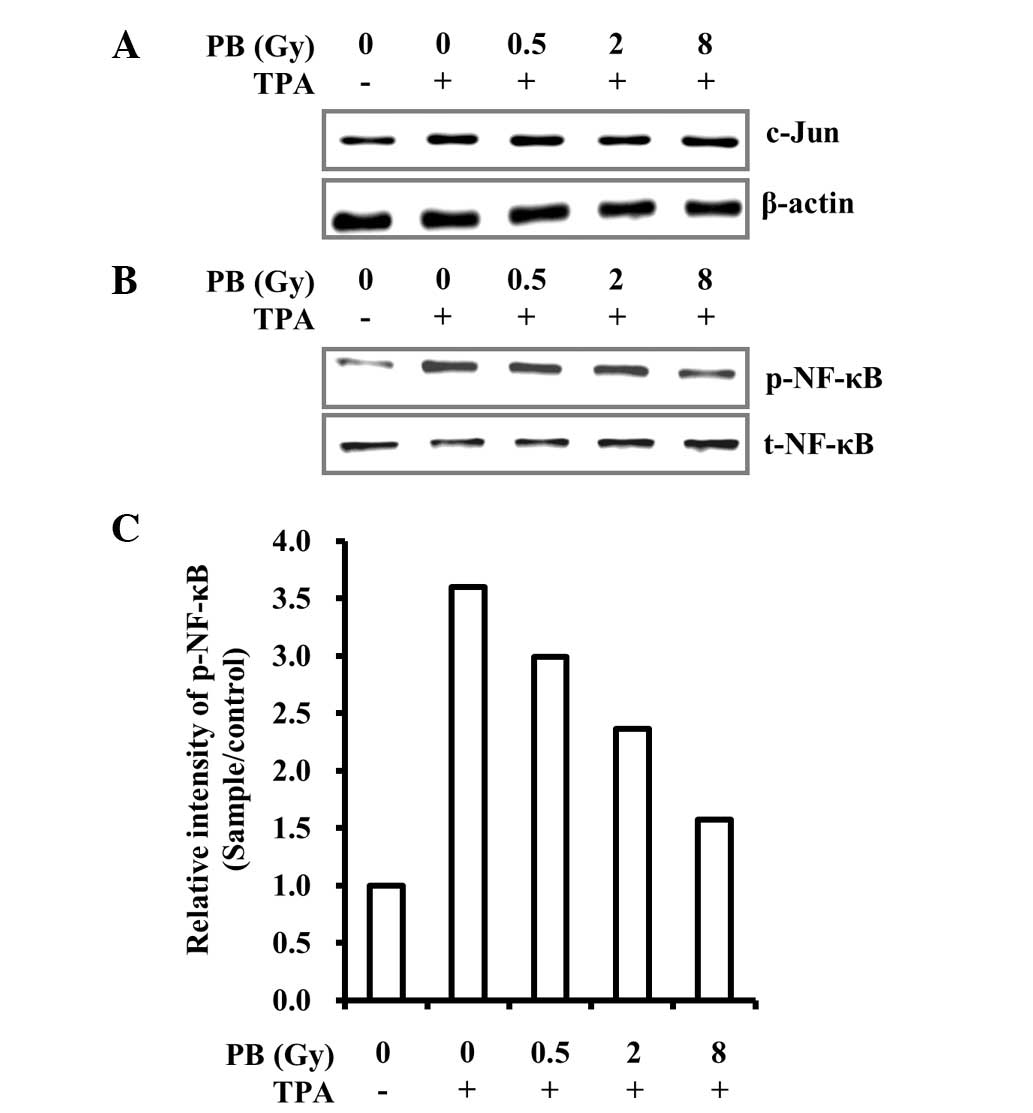
COX-2 and MMP-9 transcription levels are regulated
by c-Jun and NF-κB, responsive transcription factors that involve
various physiological responses, via binding to cis-acting elements
on promoters (16,29). c-Jun and NF-κB activities are
regulated by MAPK and Akt. Akt phosphorylation induced by TPA was
effectively reversed by proton beam irradiation in MDA-MB-231 cells
(Fig. 3B). Due to this result, the
effect of proton beam irradiation on c-Jun expression levels and
NF-κB activation was analyzed. The effects of proton beam
irradiation on nuclear translocation of c-Jun and NF-κB were also
investigated. The proton beam irradiation suppressed NF-κB
activation and subsequent nuclear translocation but not that of
c-Jun (Figs. 4 and 5). These results indicate that proton
beam irradiation downregulates TPA-induced COX-2 and MMP-9
expression through the inhibition of NF-κB activation and
subsequent nuclear translocation.
Discussion
Breast cancer is the primary cause of cancer-related
mortality worldwide in females. The five-year survival rate in
breast cancer patients depends on whether cancer is localized or
metastasized (3). Metastasis is a
sign of cancer ingravescence and markedly interferes with cancer
therapy (30). Therefore, to treat
breast cancer successfully, metastasis requires monitoring. COX-2
activity is closely associated with metastatic potential and tumor
growth in cancer. Certain studies have demonstrated that the level
of newly synthesized prostaglandin E2 in the blood is associated
with tumor growth and metastasis (11,31).
Several other studies have observed COX-2 involvement in tumor
growth and metastasis, and evidence suggests that COX-2 inhibitors
reduced cancer cell growth and metastasis in vitro and in
vivo (10–12,31).
These studies indicate the importance of COX-2 targeting in cancer
therapy. In the present study, proton beam irradiation was found to
reduce COX-2 expression levels in MDA-MB-231 invasive human breast
cancer cells (Figs. 1A and
2). This suggests that proton beam
irradiation may prevent cancer progression and metastasis in
invasive breast cancer.
During cancer metastasis, degradation of the
extracellular matrix (ECM) and basement membrane (BM) is required
for the release of cancer cells from the primary tumor and for the
attachment of the cells to distant sites. The degradation is
catalyzed by membrane proteases, such as MMPs and uPA. MMP-9, one
of two gelatinases (MMP-2 and MMP-9), is important in the
degradation of ECM and BM in breast cancer metastasis. In addition,
poor prognosis and relapse in various cancer patients have been
closely associated with MMP-9 overexpression (32,33).
MMP-9 not only enhances metastasis but also promotes cancer
development and progression. Chang and Werb (34) reported that MMP-9 contributes to
cancer proliferation and growth of primary tumors in prostate
carcinoma, lymphoma, neuroblastoma and glioblastoma. This suggests
that inhibiting MMP-9 expression is important in preventing cancer
growth and metastasis. In the present study, proton beam
irradiation significantly suppressed the increases in MMP-9
expression levels induced by TPA (Fig.
1B). The result demonstrates that breast cancer growth and
metastasis may be inhibited by proton beam irradiation through the
inhibition of MMP-9.
COX-2 and MMP-9 expression levels in MDA-MB-231
human breast cancer cells have been shown to be predominantly
enhanced by TPA through AP-1 and NF-κB activation regulated by the
PI3K/Akt and/or PKC/MAPK signaling pathways (32,33).
Various agents that suppress metastasis and tumor growth
downregulate COX-2 and MMP-9 expression via inhibition of the
PI3K/Akt and/or PKC/MAPK signaling pathways (17,19,22).
The present study demonstrated that proton beam irradiation not
only reduced Akt and NF-κB phosphorylation but also inhibited NF-κB
nuclear translocation (Figs. 3B,
4 and 5). However, proton beam irradiation did
not affect MAPK phosphorylation or c-Jun transcriptional activity
(Figs. 3A, 4 and 5).
Therefore, the results suggest that the reduction in TPA-induced
COX-2 and MMP-9 expression levels induced by proton beam
irradiation is regulated through the inhibition of NF-κB
phosphorylation, thus inhibiting subsequent NF-κB nuclear
translocation governed by the Akt signaling pathway. In conclusion,
the present study indicated that a proton beam may prevent cancer
growth and metastasis in triple-negative breast cancer via the
suppression of COX-2 and MMP-9 expression through the inhibition of
Akt signaling pathway.
Acknowledgements
This study was supported by the National Research
Foundation of Korea grant funded by the Ministry of Science, ICT
and Future Planning (grant no. 2012M2B2A4029604).
References
|
1
|
Lim YK, Park BS, Lee SK, Kim KR and Yang
TK: A proton beam irradiation method for a uniform dose
distribution over a sample volume. J Korean Phys Soc. 48:777–780.
2006.
|
|
2
|
Suit H and Urie M: Proton beams in
radiation therapy. J Natl Cancer Inst. 84:155–164. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nieves-Alicea R, Colburn NH, Simeone AM
and Tari AM: Programmed cell death 4 inhibits breast cancer cell
invasion by increasing tissue inhibitor of metalloproteinases-2
expression. Breast Cancer Res Treat. 114:203–209. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effect of selenite on invasion of HT1080 tumor cells. J Biol Chem.
276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chan G, Boyle JO, Yang EK, et al:
Cyclooxygenase-2 expression is up-regulated in squamous cell
carcinoma of the head and neck. Cancer Res. 59:991–994.
1999.PubMed/NCBI
|
|
7
|
Tucker ON, Dannenberg AJ, Yang EK, et al:
Cyclooxygenase-2 expression is up-regulated in human pancreatic
cancer. Cancer Res. 59:987–990. 1999.PubMed/NCBI
|
|
8
|
Adhim Z, Matsuoka T, Bito T, et al: In
vitro and in vivo inhibitory effect of three Cox-2 inhibitors and
epithelial-to-mesenchymal transition in human bladder cancer cell
lines. Br J Cancer. 105:393–402. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bocca C, Bozzo F, Bassignana A and
Miglietta A: Antiproliferative effects of COX-2 inhibitor celecoxib
on human breast cancer cell lines. Mol Cell Biochem. 350:59–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kang JH, Song KH, Jeong KC, et al:
Involvement of Cox-2 in the metastatic potential of
chemotherapy-resistant breast cancer cells. BMC Cancer. 11:3342011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kundu N and Fulton AM: Selective
cyclooxygenase (COX)-1 or COX-2 inhibitors control metastatic
disease in a murine model of breast cancer. Cancer Res.
62:2343–2346. 2002.PubMed/NCBI
|
|
12
|
Morita Y, Hata K, Nakanishi M, Nishisho T,
Yura Y and Yoneda T: Cyclooxygenase-2 promotes tumor
lymphangiogenesis and lymph node metastasis in oral squamous cell
carcinoma. Int J Oncol. 41:885–892. 2012.PubMed/NCBI
|
|
13
|
Kim S, Kim SH, Hur SM, et al: Silibinin
prevents TPA-induced MMP-9 expression by down-regulation of COX-2
in human breast cancer cells. J Ethnopharmacol. 126:252–257. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KS, Mo JY, Shon YH and Nam KS:
Inhibition of metastatic activities in human breast cancer cells
irradiated by a proton beam. J Korean Phys Soc. 59:653–656. 2011.
View Article : Google Scholar
|
|
15
|
Park SY, Kim YH, Kim Y and Lee SJ:
Aromatic-turmerone attenuates invasion and expression of MMP-9 and
COX-2 through inhibition of NF-kappaB activation in TPA-induced
breast cancer cells. J Cell Biochem. 113:3653–3662. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takahra T, Smart DE, Oakley F and Mann DA:
Induction of myofibroblast MMP-9 transcription in three-dimensional
collagen I gel cultures: regulation by NF-kappaB, AP-1 and Sp1. Int
J Biochem Cell Biol. 36:353–363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin Y, Yoon SH, Choe EY, et al:
PMA-induced up-regulation of MMP-9 is regulated by a
PKCalpha-NF-kappaB cascade in human lung epithelial cells. Exp Mol
Med. 39:97–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KS, Shin JS and Nam KS: Starfish
polysaccharides downregulate metastatic activity through the MAPK
signaling pathway in MCF-7 human breast cancer cells. Mol Biol Rep.
40:5959–5966. 2013. View Article : Google Scholar
|
|
19
|
Cho HJ, Kang JH, Kwak JY, et al:
Ascofuranone suppresses PMA-mediated matrix metalloproteinase-9
gene activation through the Ras/Raf/MEK/ERK- and Ap1-dependent
mechanisms. Carcinogenesis. 28:1104–1110. 2007. View Article : Google Scholar
|
|
20
|
Garg A and Aggarwal BB: Nuclear
transcription factor-kappaB as a target for cancer drug
development. Leukemia. 16:1053–1068. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pastore S, Mascia F, Mariotti F, Dattilo
C, Mariani V and Girolomoni G: ERK1/2 regulates epidermal chemokine
expression and skin inflammation. J Immunol. 174:5047–5056. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park SK, Hwang YS, Park KK, Park HJ, Seo
JY and Chung WY: Kalopanaxsaponin A inhibits PMA-induced invasion
by reducing matrix metalloproteinase-9 via PI3K/Akt− and
PKCdelta-mediated signaling in MCF-7 human breast cancer cells.
Carcinogenesis. 30:1225–1233. 2009.
|
|
23
|
Cragg GM, Newman DJ and Weiss RB: Coral
reefs, forests, and thermal vents: the worldwide exploration of
nature for novel antitumor agents. Semin Oncol. 24:156–163.
1997.PubMed/NCBI
|
|
24
|
Jang JY, Jeon YK and Kim CW: Degradation
of HER2/neu by ANT2 shRNA suppresses migration and invasiveness of
breast cancer cells. BMC Cancer. 10:3912010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Degner SC, Kemp MQ, Bowden GT and
Romagnolo DF: Conjugated linoleic acid attenuates cyclooxygenase-2
transcriptional activity via an anti-AP-1 mechanism in MCF-7 breast
cancer cells. J Nutr. 136:421–427. 2006.PubMed/NCBI
|
|
26
|
Guttilla IK, Phoenix KN, Hong X, Tirnauer
JS, Claffey KP and White BA: Prolonged mammosphere culture of MCF-7
cells induces an EMT and repression of the estrogen receptor by
microRNAs. Breast Cancer Res Treat. 132:75–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng S, Qiao M and Pardee AB: Metastasis
and AKT activation. J Cell Physiol. 218:451–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agarwal A, Das K, Lerner N, et al: The
AKT/I kappa B kinase pathway promotes angiogenic/metastatic gene
expression in colorectal cancer by activating nuclear factor-kappa
B and beta-catenin. Oncogene. 24:1021–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kumar R, Alam S, Chaudhari BP, et al:
Ochratoxin A-induced cell proliferation and tumor promotion in
mouse skin by activating the expression of cyclin-D1 and
cyclooxygenase-2 through nuclear factor-kappa B and activator
protein-1. Carcinogenesis. 34:647–657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shao W, Wang D and He J: The role of gene
expression profiling in early-stage non-small cell lung cancer. J
Thorac Dis. 2:89–99. 2010.PubMed/NCBI
|
|
31
|
Altundag K and Ibrahim NK: Aromatase
inhibitors in breast cancer: an overview. Oncologist. 11:553–562.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kupferman ME, Fini ME, Muller WJ, Weber R,
Cheng Y and Muschel RJ: Matrix metalloproteinase 9 promoter
activity is induced coincident with invasion during tumor
progression. Am J Pathol. 157:1777–1783. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stamenkovic I: Matrix metalloproteinases
in tumor invasion and metastasis. Semin Cancer Biol. 10:415–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang C and Werb Z: The many faces of
metalloproteases: cell growth, invasion, angiogenesis and
metastasis. Trends Cell Biol. 11:S37–S43. 2001. View Article : Google Scholar : PubMed/NCBI
|