Introduction
Interstitial cystitis (IC) is a chronic,
noninfectious inflammatory disease of the bladder that is
characterized by pelvic or perineal pain associated with the
bladder, irritative voiding symptoms and cystoscopic or
histological features (1). In
spite of numerous studies, the etiology and pathogenesis of IC
remain to be fully elucidated. A few pieces of crucial evidence
from pathological biopsies of the human bladder suggested that mast
cells are important in the pathogenesis and pathophysiology of IC
(2,3). However, there currently is a lack of
appropriate rat models of mastocytosis.
Although IC is regarded as a heterogeneous clinical
condition that is diagnosed predominantly in females, the current
accepted theory is that toxic substances from urine infiltrate the
bladder wall owing to disruption of the glycosaminoglycan (GAG)
layer, an important permeability barrier to protect the bladder
from urine (4). This defective
protection followed by increased urothelial permeability may
activate mast cells. Mast cells, in turn, release toxic chemical
mediators preformed in granules into the surrounding tissue during
a process known as degranulation, which results in characteristic
degranulated mast cells (2–4). On
the basis of this hypothesis, Stein et al (5) developed a novel bladder injury model
that mimics IC in which protamine sulfate (PS) and the endotoxin
lipopolysaccharide (LPS) are administered intravesically to
Sprague-Dawley rats. However, this study mainly focused on the
early changes to the injured bladder and did not assess chronic
functional changes in awake voiding and degranulation of mast cells
in the bladder. Furthermore, Soler et al (6) demonstrated that urine has an
important role in the development of bladder inflammation in this
condition of increased urothelial permeability, which suggests that
a chronic bladder injury animal model including urine exposure time
to the injured mucosa is required.
Certain patients with an overactive bladder (OAB)
and urodynamic detrusor overactivity (DO) demonstrate a positive
result on the potassium sensitivity test, which is a characteristic
of IC (7). This overlap between
OAB and IC remains to be fully elucidated; however, previous
studies examining the pathophysiologies of these diseases have
provided evidence that the symptoms of patients similarly originate
from increased afferent innervations to the lower urinary tract
(8,9). Therefore, a good response to
anticholinergic medication is expected in patients with IC as
compared with patients with OAB. However, previous clinical
evidence suggests that numerous patients with IC and DO do not
respond to anticholinergic medications (10). The detailed mechanisms associated
with this ineffectiveness require investigation in a reliable
animal model of IC (11).
Urinary urgency is an essential symptom in the
diagnosis of OAB and is unanimously regarded to correspond with DO
during the filling phase according to a human urodynamic study
(12,13). A previous study by our group
demonstrated that nonvoiding contractions interpreted solely on the
basis of intravesical pressure (IVP) need to be corrected by the
simultaneous changes in intraabdominal pressures (IAP) in animal
models of various diseases in order to use those as a substitute
parameter for DO in humans (14,15).
In the present study, simultaneous measurements of IVP and IAP were
used to assess DO.
The aim of the present study was to use an animal
model of chronic IC to assess the effect of inflammation on the
histology and function of the urinary bladder in rats, with a
particular focus on the degranulation of mast cells and DO during
the filling phase of awake voiding. The measurements were made four
weeks after exposure to LPS (Sigma, St. Louis, MO, USA) following
destruction of the GAG layer by PS (Sigma). In addition, the
effects of tolterodine (Sigma) on DO were assessed in this chronic
IC model.
Materials and methods
Animals and study design
A total of 18 female Sprague-Dawley rats (250–300 g;
Orient Bio Inc., Gyeonggi-do, South Korea) were used in the present
study. In 12 rats, LPS was intravesically instilled following the
induction of IC by the administration of PS. In six rats, the
intravesical instillation of saline was used as a sham treatment.
Cystometrograms were obtained in all unanesthetized, unrestrained
rats in metabolic cages, 1 month following intravesical
instillation of LPS or saline. Following the completion of the
experiments, 15 rats survived, including five out of the six
sham-operated controls and 10 out of 12 animals in the IC group.
From the IC group, six rats were sacrificed immediately following
cystometry and the bladders were removed and examined
histologically for mast cell and inflammatory changes. In the
remaining rats from the IC group, cystometrograms were obtained
following treatment with tolterodine.
Surgical procedures
All experimental animal procedures were performed in
accordance with the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Bethesda, MD, USA)
and were approved by the the INHA Institutional Animal Care and Use
Committee at the Inha University Medical School (Incheon, South
Korea; approval ID: INHA 100507-57). The rats were maintained under
a standard 12-h light/dark cycle and with free access to food
pellets and tap water except during the experiments. The rats were
anesthetized with ketamine (Ketamine; Yuhan Corp., Seoul, Korea; 75
mg kg−1 intraperitoneally) and xylazine (Rompun; Bayer
Korea Corp, Seoul, Korea; 15 mg kg−1 intraperitoneally)
for specific procedures.
Induction of cystitis
Cystitis was induced by intravesical instillation of
LPS following PS, as described by Stein et al (5) with certain modifications. Through an
abdominal incision under anesthesia, a 24-gauge needle with a
syringe was inserted into the dome of the bladder. After all the
urine was aspirated, 0.5 ml of PS (10 mg ml−1) was
instilled into the bladder lumen. Following 20 min, the bladder was
emptied, washed with phosphate-buffered saline (PBS) and then given
a second treatment with 0.5 ml of LPS (750 μg/ml) for 20 min. For
the sham group, normal saline of the same volume was instilled.
Procedures for intravesical,
intraabdominal and intravenous catheter implantation
Simultaneous catheterizations for IVP and IAP
recordings were performed three days prior to cystometry, as
described previously (11,13–15).
Briefly, following the induction of anesthesia, a polyethylene
catheter (PE-50; Becton-Dickinson, Parsippany, NJ, USA) with a cuff
was implanted into the dome of the bladder through an abdominal
incision. To record IAP, an abdominal balloon (Latex; Daewoo
Medical, Incheon, Korea) around the cuff of a catheter tip was
placed proximal to the bladder and was tied to another catheter
with a silk tie. A polyethylene catheter (PE-50) was heated in warm
water, elongated by ~1.5 times its original length at the tip of
the inserting side and filled with heparinized saline (100 IU
ml−1). As the bladder catheter was implanted, the
elongated catheter was inserted into the femoral vein in six out of
the 12 rats in the IC group in the same session. These catheters
were then tunneled through the subcutaneous space, exited through
the back of the animal and were anchored to the skin of the back.
Following surgery, each rat was caged individually and maintained
in the same manner.
Functional evaluation
Cystometrograms were performed on unanesthetized,
unrestrained rats in metabolic cages. The indwelling catheter to
the bladder was connected to a two-way valve that was connected via
a T-tube to a pressure transducer (Research Grade Blood Pressure
Transducer; Harvard Apparatus, Holliston, MA, USA) and a
microinjection pump (PHD22/2000 pump; Harvard Apparatus). Another
indwelling catheter connected to a fluid-filled abdominal balloon
was connected to another pressure transducer to record the IAP. The
micturition volumes were recorded continuously by means of a fluid
collector connected to a force displacement transducer (Research
Grade Isometric Transducer; Harvard Apparatus). Room-temperature
saline was infused into the bladder at a rate of 10 ml
h−1. IVP, IAP and micturition volumes were recorded
continuously using Acq Knowledge 3.8.1 software and an MP150 data
acquisition system (Biopac Systems, Goleta, CA, USA) at a sampling
rate of 50 Hz. The mean values from three reproducible micturition
cycles were used for evaluation. IAP was defined as the recorded
balloon pressure subtracted by the lowest balloon pressure in each
voiding cycle (zeroing). The detrusor pressure was defined as the
IVP minus IAP. The increase in intravesical pressure during the
filling phase was defined as increments of IVP that exceeded 2 cm
H2O from baseline, which was interpreted as DO if
occurring without simultaneous similar changes in IAP or abdominal
straining if occurring with simultaneous similar changes in
IAP.
Investigation of cystometric
parameters
Pressure- and volume-associated parameters consisted
of the lowest bladder pressure during filling (BP), bladder
pressure immediately prior to micturition (TP), maximum bladder
pressure during the micturition cycle (MP), volume of expelled
urine (MV), remaining urine following voiding (RV), MV+RV (BC) and
intervals between micturition contractions (MI).
DO-associated parameters during the
filling phase
These consisted of the time of the filling phase
(interval from the initiation of infusion through the tube and the
point immediately prior to the initiation of micturition),
frequency of abdominal straining per minute, frequency of DO per
minute and increased amplitude from base to peak of the DO spike as
IVP. These frequencies were calculated on the basis of the time of
the filling phase.
Administration of the drug
During cystometry, room temperature saline was
infused into the bladder at a rate of 10 ml h−1. The
micturitions during intravesical saline infusion served as baseline
values. After 0.2 ml tolterodine solution (0.3 mg kg−1)
was injected intravenously, there was a 30 min observation period.
Following cystometry, the animals were sacrificed by cervical
dislocation. The bladder and the urethra were removed en bloc and
separated at the level of the bladder neck, and the bladder was
weighed.
Histological evaluation
As previously described, the bladder was excised,
split longitudinally and fixed in 10% buffered formaldehyde and
embedded in paraffin. Thin sections (4 μm) of the bladder were cut
and stained with 1% toluidine blue (Merck, Darmstadt, Germany) to
assess inflammatory changes as well as the number of total and
degranulated mast cells. Slides were examined with an Olympus BX51
light microscope (Olympus, Tokyo, Japan) and images were captured
with an Olympus PM10SP photographic system. For quantification,
mast cells in the mucosa and muscularis were counted and averaged
from five randomly selected microscopic visual fields at ×200
magnification under an optical microscope (Olympus).
Statistical analysis
All results were analyzed using SPSS 20.0 (SPSS
Inc., Chicago, IL, USA). The results are presented as the mean
values ± standard error of the mean. Normal distributions were
confirmed by the Shapiro-Wilk W-test. Statistical significance was
determined by unpaired t-tests to detect differences in urodynamic
parameters and histological data between the sham and IC groups.
Paired t-tests were used to compare these parameters prior to and
following drug administration in half of the IC group. For multiple
comparisons, one-way analysis of variance with Tukey’s test was
used to detect differences. P<0.05 was considered to indicate a
statistically significant difference. All calculations were made on
the basis of n, which denoted the number of animals.
Results
Body weight, bladder weight and
ratio
No significant difference in the body weight between
the sham group (272.0±5.6 g) and the IC group (275.0±5.2 g) was
identified four weeks following intravesical instillations of PS
and LPS or saline. The bladder weight in the IC group (0.14±0.00 g)
did not differ significantly from that of the sham group (0.14±0.01
g) and no significant differences between the groups were
identified when the bladder weight was normalized to body weight
(data not shown).
Comparison of the urodynamic parameters
between the sham and IC groups
Rats with ICs did not differ significantly from the
sham rats in any pressure- or volume-asssociated parameters,
including BP, TP, MP, BC, MV, RV and MI (Table I). However, DO during the filling
phase was shown in all rats in the IC group (100%), but was not
apparent in the sham group (0%; Table
I). The average frequency and pressure of DO in the IC group
was 1.56±0.41 min−1 and 2.61±0.66 cm H2O,
respectively (data not shown).
 | Table ICystometric parameters in conscious,
unrestrained Sprague-Dawley rats in the sham and IC groups. |
Table I
Cystometric parameters in conscious,
unrestrained Sprague-Dawley rats in the sham and IC groups.
| Group | BP [DP (cm
H2O)] | TP [DP (cm
H2O)] | MP [DP (cm
H2O)] | BC (ml) | MV (ml) | RV (ml) | MI
(min−1) | DO positive, n
(%) |
|---|
| Sham (n=5) | 6.60±1.48 | 18.98±3.22 | 53.56±5.72 | 1.24±0.21 | 1.24±0.21 | 0.00±0.00 | 8.08±1.38 | 0 (0%) |
| IC (n=11) | 6.80±0.59 | 18.15±0.59 | 57.27±3.42 | 1.45±0.15 | 1.45±0.15 | 0.00±0.00 | 8.73±0.73 | 11 (100%) |
Changes in the urodynamic parameters
following the intravenous injection of an anticholinergic drugs in
rats during IC
Compared with prior to tolterodine injection, no
significant differences in any pressure-associated parameters,
including BP and TP with the exception of MP, were identified
following tolterodine treatment in the IC group (Table II). Following treatment, the MP
decreased significantly in IC rats from 51.08±6.36 to 18.13±5.90 cm
H2O (P<0.05; Fig.
1A). No significant differences in any volume-associated
parameters, including BC, MV, RV and MI, were identified following
tolterodine treatment (Table II).
Additionally, there were no significant changes in the frequency or
pressure of DO during the filling phase prior to and following the
medication (Table II; Fig. 1B–E).
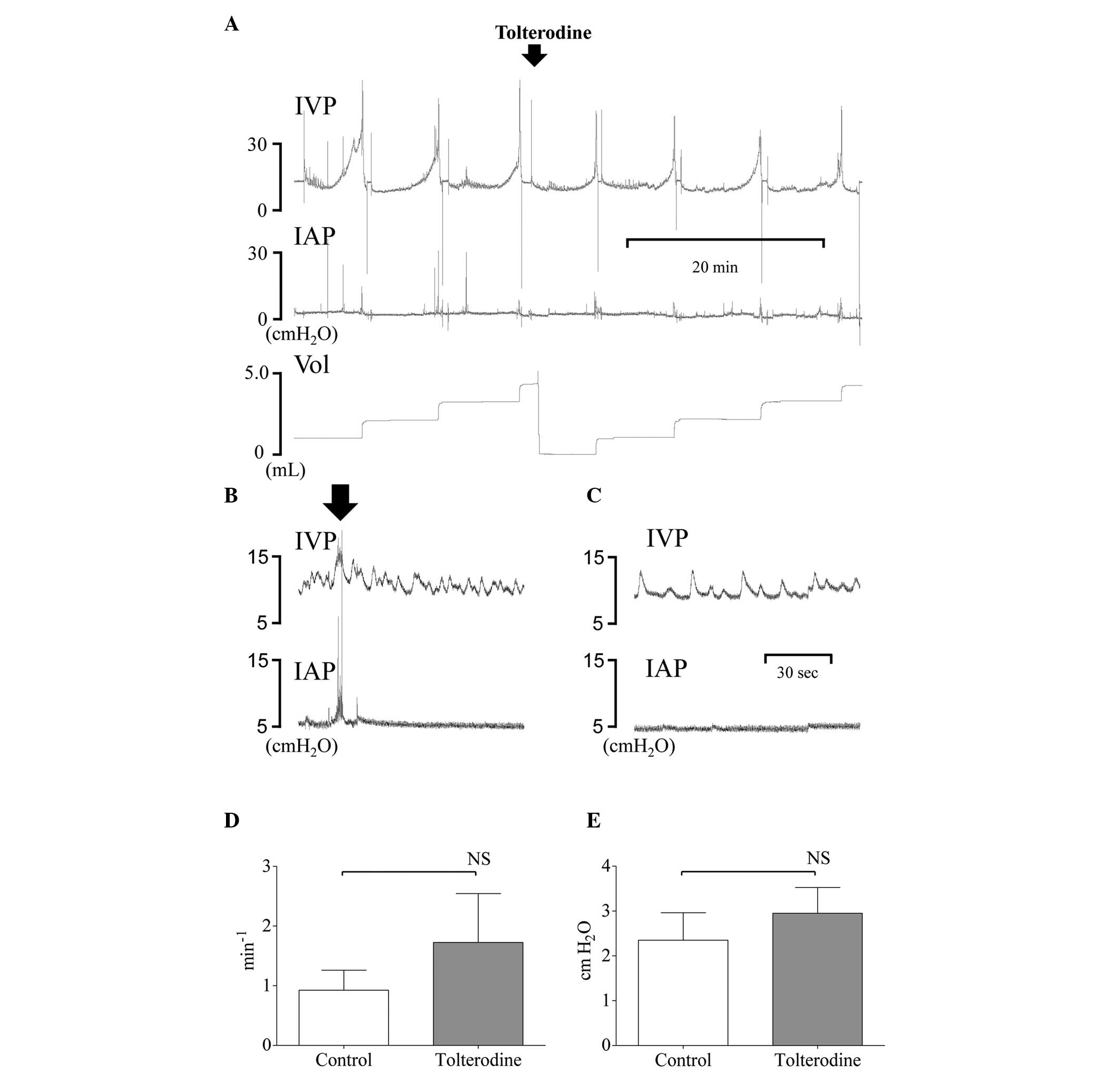 | Figure 1(A) Representative cystometrogram from
one rat demonstrating IVP, IAP and Vol prior to and following
intravenous injection of 0.3 mg kg−1 of tolterodine 1
month following the induction of IC by intravesical administration
of LPS following PS. (B) During the filling phase, the fluctuations
in IVP recordings were not interpretated as DO, if occurring with
simultaneous similar changes in IAP. The black arrow indicates the
abdominal straining. (C) IVP recordings were interpretated as DO if
occurring without simultaneous changes in IAP. Prior to the
injection, all IC rats showed DO. Following the injection, no
significant changes in (D) frequency or (E) pressure of DO were
present. NS, not significant. IVP, intravesical pressure; IAP,
intraabdominal pressure; Vol, micturition volume; IC, interstitial
cystitis; LPS, lipopolysaccharide; PS, protamine sulfate; DO,
detrusor overactivity. |
 | Table IIChanges in cystometric parameters in
conscious, unrestrained Sprague-Dawley rats in the IC group
following intravenous injection of tolterodine. |
Table II
Changes in cystometric parameters in
conscious, unrestrained Sprague-Dawley rats in the IC group
following intravenous injection of tolterodine.
| IC rats | BP [DP (cm
H2O)] | TP [DP (cm
H2O)] | MP [DP (cm
H2O)] | BC (ml) | MV (ml) | RV (ml) | MI
(min−1) | DO positive, n
(%) |
|---|
| Control (n=4) | 6.40±0.86 | 16.08±1.37 | 51.08±6.36 | 1.62±0.28 | 1.56±0.24 | 0.06±0.06 | 9.52±1.82 | 4 (100%) |
| Tolterodine IV
(n=4) | 6.60±0.65 | 11.78±1.04 | 18.13±5.90* | 1.63±0.22 | 1.56±0.17 | 0.07±0.07 | 10.24±1.47 | 4 (100%) |
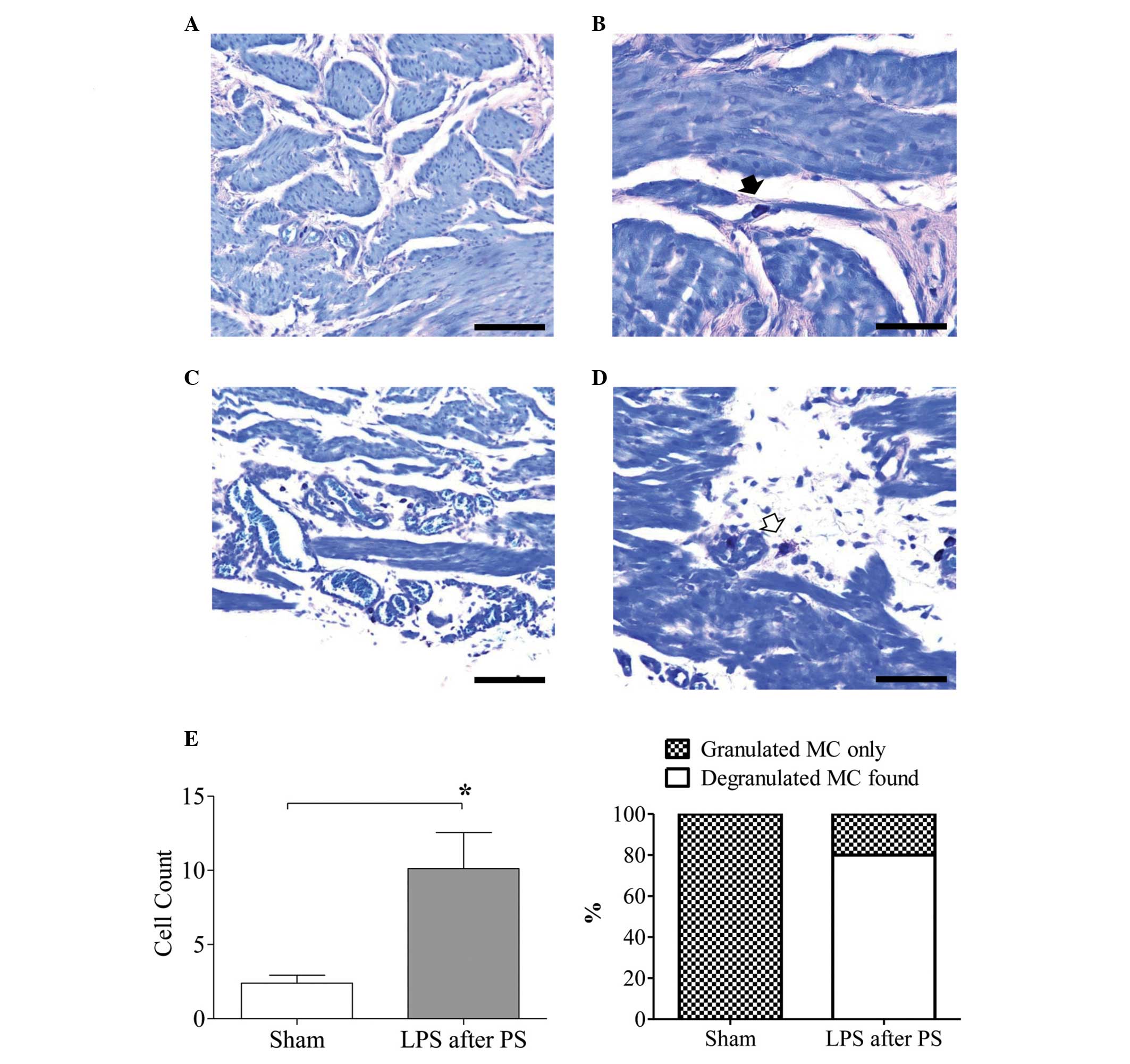
Comparison of the histological findings
between the sham and IC groups
In total, five rats formed the IC group as one rat
was excluded owing to a technical problem. In the sham rats, the
urothelium, lamina propria and muscularis propria appeared normal.
The rats treated with LPS following PS had a thickened bladder wall
and focally atrophied uroepithelium. Histological examination
further revealed a significant increase in the total numbers of
infiltrated mast cells in the IC rats compared with the sham rats
(P<0.05; Fig. 2). Four out of
the five treated rats demonstrated degranulated mast cells (80%),
whereas the sham rats did not (Fig.
2). However, among the total counted mast cells, only 14.2%
were degranulated and 85.8% were granulated mast cells (data not
shown).
Discussion
A central observation of the present study was that
chronic IC with similar characteristics to the human disease was
induced in Sprague-Dawley rats by intravesical instillations of LPS
following PS. All rats in this chronic model except one exhibited
degranulated mast cells, which was crucial evidence for the
successful establishment of a model resembling the human disease.
None of the sham rats exhibited degranulated mast cells. PS was
used to destroy the GAG layer, which protects the bladder from the
adherence of bacteria, to facilitate the action of LPS.
Furthermore, irritation by urine over four weeks appeared to have
an important role in this model. Although the urodynamic results in
these model rats did not match up to all the characteristic
features of IC in humans, all rats demonstrated DO during the
filling phase, which is a characteristic of OAB.
The pathophysiological mechanisms that have been
implicated in IC include uroepithelial dysfunction, mast cell
activation and neurogenic inflammation (2,3,8).
Among these, the most important is mast cell activation, which can
be precipitated by diverse nonimmunological stimulants, including
bacteria, chemicals, kinins, neuropeptides and acetylcholine
(2,3,16).
Although IC is not an infectious disease, LPS, an endotoxin from
E. coli, is commonly used as an acute precipitating factor
in animal models of IC (17). In
the normal urinary bladder, however, the GAG layer prevents the
adherence of bacteria by covering possible receptor sites (4,18).
Thus, intravesical instillation of PS, a highly alkaline
polycationic peptide, is used to destroy this GAG layer on
urothelial cells prior to the instillation of LPS (19). Until recently, the majority of
studies have focused on the acute effects of this dual treatment on
the bladder in animal models and few studies have investigated the
chronic effects on awake voiding and mast cell degranulation. In
the model used in the present study, the injured uroepithelium was
further exposed to urine as a chronic irritating stimulant for 1
month, which is expected to be similar to the conditions in
patients with IC.
Mast cells are essential in the initiation and
propagation of inflammation, leading to numerous symptoms of acute
and chronic inflammatory diseases (3,16,20).
They function as immune sentinels in almost all vascularized
tissues or organs and co-ordinate all the steps of the inflammatory
response, with positive and negative actions in the course of
numerous diseases, including the defense against microbial
infection (16,20). They store proinflammatory
mediators, neurotrophic factors and immunoregulatory cytokines in
their vesicles and discharge their large population of secretary
granules outside the cell into the tissue by stimulating factors.
These mediators are important in the immediate and late phase
inflammatory reactions. When chronically stimulated, mast cells
exhibit characteristic features of degranulated mast cells, also
known as phantom cells (3,21). Furthermore, they are associated
with neuropathic pain, a characteristic of IC, mediated by
neurotrophins, including nerve growth factor (22). The exact etiology and
pathophysiology of IC remain to be fully elucidated; however, mast
cells are known to be important in IC pathogenesis (2,23).
Furthermore, the importance of degranulated mast cells in IC was
demonstrated by crucial pathological evidence from numerous human
studies (2,3). The present study demonstrated that
80% of IC model rats exhibited degranulated mast cells, with a
significant increase in the total number of mast cells compared
with that in the sham rats. However, among the total mast cells,
only 14.2% were degranulated, which suggests that a longer duration
of IC is necessary.
The symptoms of IC and OAB overlap considerably,
with numerous patients with IC demonstrating urgency (7). By the definition of the International
Continence Society (24), IC can
be included, in part, in the category of OAB. However, according to
the National Institute of Diabetes and Digestive and Kidney
Diseases database study, which was the first major prospective
trial on IC, none of the urodynamic parameters add enough
diagnostic evidence to characterize the voiding behavior of
patients with IC as OAB (25),
possibly due to the disease heterogeneity. Other population-based
studies on IC reported a prevalence of urodynamically verified DO
ranging from 14–30% (26,27). The animal model used in the present
study similarly demonstrated no significantly different urodynamic
parameters compared with those in the controls; however, all rats
demonstrated DO. These findings are similar to those of a previous
rat model of IC induced by intravesical administration of HCl
(17). In the present study, rats
with IC and DO demonstrated no response in DO parameters to an
anticholinergic drug, which differs from the findings of other OAB
animal models, including the models of bladder outlet obstruction
or intravesical instillation of prostaglandin E2 (11,28).
It is suggested that the dissimilar effects of anticholinergic
drugs may be associated with the complex cellular and molecular
mechanisms in the bladder, which remain to be elucidated.
In conclusion, an improved understanding of the
disease heterogeneity through the use of an animal model of chronic
IC, which is similar to the human disease condition in view of
degranulated mast cells and bladder function, may provide major
novel insights into unidentified pathophysiological mechanisms,
including those underlying the dissimilar effects of
anticholinergic drugs on the different diseases. In addition it may
also lead to further progress in the detection and treatment of
this complex disease.
Acknowledgements
This study was supported by Inha University Research
grant and Astellas Research grant (ARK-VC-2013-02). The funders had
no role in the design, data collection and analysis, decision to
publish or preparation of the manuscript.
Abbreviations:
|
BC
|
MV+RV
|
|
BP
|
basal pressure
|
|
DO
|
detrusor overactivity
|
|
GAG
|
glycosaminoglycan
|
|
IAP
|
intraabdominal pressures
|
|
IC
|
interstitial cystitis
|
|
IVP
|
intravesical pressure
|
|
LPS
|
lipopolysaccharide
|
|
MI
|
intervals between micturition
contractions
|
|
MP
|
maximum bladder pressure during the
micturition cycle
|
|
MV
|
micturition volume
|
|
OAB
|
overactive bladder
|
|
PS
|
protamine sulfate
|
|
RV
|
remaining urine after voiding
|
|
SEM
|
standard error of the mean
|
|
TP
|
threshold pressure
|
References
|
1
|
Gillenwater JY and Wein AJ: Summary of the
National Institute of Arthritis, Diabetes, Digestive and Kidney
Diseases Workshop on Interstitial Cystitis, National Institutes of
Health, Bethesda, Maryland, August 28–29, 1987. J Urol.
140:203–206. 1988.PubMed/NCBI
|
|
2
|
Theoharides TC, Kempuraj D and Sant GR:
Mast cell involvement in interstitial cystitis: a review of human
and experimental evidence. Urology. 57:47–55. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sant GR and Theoharides TC: The role of
the mast cell in interstitial cystitis. Urol Clin North Am.
21:41–53. 1994.PubMed/NCBI
|
|
4
|
Parsons CL: The role of a leaky epithelium
and potassium in the generation of bladder symptoms in interstitial
cystitis/overactive bladder, urethral syndrome, prostatitis and
gynaecological chronic pelvic pain. BJU Int. 107:370–375. 2011.
View Article : Google Scholar
|
|
5
|
Stein PC, Pham H, Ito T and Parsons CL:
Bladder injury model induced in rats by exposure to protamine
sulfate followed by bacterial endotoxin. J Urol. 155:1133–1138.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soler R, Bruschini H, Freire MP, Alves MT,
Srougi M and Ortiz V: Urine is necessary to provoke bladder
inflammation in protamine sulfate induced urothelial injury. J
Urol. 180:1527–1531. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chung MK, Butrick CW and Chung CW: The
overlap of interstitial cystitis/painful bladder syndrome and
overactive bladder. JSLS. 14:83–90. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimura N and de Groat WC: Increased
excitability of afferent neurons innervating rat urinary bladder
after chronic bladder inflammation. J Neurosci. 19:4644–4653.
1999.PubMed/NCBI
|
|
9
|
Fowler CJ: Bladder afferents and their
role in the overactive bladder. Urology. 59:37–42. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minaglia S, Ozel B, Bizhang R and Mishell
DR Jr: Increased prevalence of interstitial cystitis in women with
detrusor overactivity refractory to anticholinergic therapy.
Urology. 66:702–706. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin LH, Park CS, Shin HY, Yoon SM and Lee
T: Dissimilar effects of tolterodine on detrusor overactivity in
awake rats with chemical cystitis and partial bladder outlet
obstruction. Int Neurourol J. 15:120–126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garnett S and Abrams P: The natural
history of the overactive bladder and detrusor overactivity. A
review of the evidence regarding the long-term outcome of the
overactive bladder. J Urol. 169:843–848. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee T and Yoon SM: The role of
intra-abdominal pressure measurement in awake rat cystometry. Int
Neurourol J. 17:44–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee T, Andersson KE, Streng T and Hedlund
P: Simultaneous registration of intraabdominal and intravesical
pressures during cystometry in conscious rats--effects of bladder
outlet obstruction and intravesical PGE2. Neurourol Urodyn.
27:88–95. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin LH, Shin HY, Kwon YH, Park CS, Yoon SM
and Lee T: Urodynamic findings in an awake chemical cystitis rat
model observed by simultaneous registrations of intravesical and
intraabdominal pressures. Int Neurourol J. 14:54–60. 2010.
View Article : Google Scholar
|
|
16
|
Galli SJ: New concepts about the mast
cell. N Engl J Med. 328:257–265. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saban MR, Saban R, Hammond TG,
Haak-Frendscho M, Steinberg H, Tengowski MW and Bjorling DE:
LPS-sensory peptide communication in experimental cystitis. Am J
Physiol Renal Physiol. 282:F202–F210. 2002.PubMed/NCBI
|
|
18
|
Parsons CL: The role of the urinary
epithelium in the pathogenesis of interstitial
cystitis/prostatitis/urethritis. Urology. 69:9–16. 2007. View Article : Google Scholar
|
|
19
|
Parsons CL, Stauffer CW and Schmidt JD:
Reversible inactivation of bladder surface glycosaminoglycan
antibacterial activity by protamine sulfate. Infect Immun.
56:1341–1343. 1988.PubMed/NCBI
|
|
20
|
Yong LC: The mast cell: origin,
morphology, distribution, and function. Exp Toxicol Pathol.
49:409–424. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Claman HN, Choi KL, Sujansky W and Vatter
AE: Mast cell ‘disappearance’ in chronic murine graft-vs-host
disease (GVHD)-ultrastructural demonstration of ‘phantom mast
cells’. J Immunol. 137:2009–2013. 1986.
|
|
22
|
Lowe EM, Anand P, Terenghi G,
Williams-Chestnut RE, Sinicropi DV and Osborne JL: Increased nerve
growth factor levels in the urinary bladder of women with
idiopathic sensory urgency and interstitial cystitis. Br J Urol.
79:572–577. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park CS and Bochner BS: Potential
targeting of siglecs, mast cell inhibitory receptors, in
interstitial cystitis. Int Neurourol J. 15:61–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abrams P, Cardozo L, Fall M, Griffiths D,
Rosier P, Ulmsten U, van Kerrebroeck P, Victor A and Wein A: The
standardisation of terminology of lower urinary tract function:
report from the Standardisation Sub-committee of the International
Continence Society. Neurourol Urodyn. 21:167–178. 2002. View Article : Google Scholar
|
|
25
|
Hanno PM, Landis JR, Matthews-Cook Y,
Kusek J and Nyberg L Jr: The diagnosis of interstitial cystitis
revisited: lessons learned from the National Institutes of Health
Interstitial Cystitis Database study. J Urol. 161:553–557. 1999.
View Article : Google Scholar
|
|
26
|
Nigro DA, Wein AJ, Foy M, Parsons CL,
Williams M, Nyberg LM Jr, Landis JR, Cook YL and Simon LJ:
Associations among cystoscopic and urodynamic findings for women
enrolled in the Interstitial Cystitis Data Base (ICDB) Study.
Urology. 49:86–92. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanno P, Lin A, Nordling J, Nyberg L, van
Ophoven A, Ueda T and Wein A: Bladder Pain Syndrome Committee of
the International Consultation on Incontinence. Neurourol Urodyn.
29:191–198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh JH, Lee YS, Jin LH, Kwon YH, Park WH
and Lee T: Urodynamic effects of propiverine on detrusor
overactivity and abdominal straining during voiding in awake rats
with intravesical prostaglandin E(2) instillation. Korean J Urol.
51:64–69. 2010. View Article : Google Scholar : PubMed/NCBI
|