Introduction
Diabetes mellitus (DM) is an endocrine metabolic
disease characterised by hyperglycaemia. Of all diabetic patients,
95% are classified as having type 2 DM (T2DM). In developed
countries, T2MD has become a major chronic disease that seriously
threatens human health, affecting an increasing number of people
every year. Chronic complications, particularly macroangiopathy,
are considered an important cause of death and disability in
patients with T2DM (1). Efficient
control of blood glucose is the basic strategy utilized to delay
the initiation and development of DM complications (2). Insulin effectively reduces the risk
of macroangiopathy in patients with T2DM (3). Thus, optimizing the efficacy of
insulin therapy for patients with T2DM is crucial.
Currently, ~80% of patients with T2DM receive
premixed insulin and insulin analogues in the form of two
subcutaneous injections, one in the morning and the other in the
evening, and a number of clinical studies have proven the
effectiveness of such regimen on glycaemic control (4–9).
This is a simplified approach in DM treatment, which minimizes the
number of injections patients must receive daily. However, it is
limited by its fixed ratio, which does not fulfil normal
physiological needs or provide enough flexibility, and it is
associated with increasing the risk of hypoglycaemia (4,8). The
key application of premixed insulin enabling regular monitoring of
blood glucose, which allegedly establishes good glycaemic control.
However, the majority of patients with diabetes in China still have
poor control of blood glucose or suffer from frequent
hypoglycaemia. Therefore, simplified treatment strategies that
decrease the risk of hypoglycaemia and improve blood glucose
control are essential to improve the efficacy of diabetes
therapeutics.
Several studies have investigated such novel
treatment strategies (10–14) by adopting a two-back-one strategy,
where premixed insulin/insulin analogues that were ineffective in
patients with T2DM were replaced with insulin glargine plus OADs.
Improved glycaemic control was observed in the patients following
the switch in regimen. Insulin glargine is a long-term human
insulin analogue with slow and stable absorption and is thus
capable of functioning for 24 h with stable bioavailability. When
injected once daily, it lowers the incidence of hypoglycaemia and
possibly stimulates physiological insulin secretion. When combined
with OAHs, it has the capacity to control blood glucose to a safe
range by promoting endogenous insulin secretion and glucose
advantage, as well as by inhibiting glucose absorption. However,
the two-back-one strategy currently focuses on the
standard-reaching rate of glycosylated-haemoglobin (HbA1c),
incidence of hypoglycaemia and satisfaction of patients. The
suitability of this strategy for different patients has not been
reported. Furthermore, previous studies reported that patients who
received two-back-one treatment continued to have poor glycaemic
control, which is presumably correlated with inter-patient
differences in islet function. However, to the best of our
knowledge, no studies have yet confirmed this hypothesis. In the
present study, two-back-one treatment was administered to patients
with T2DM by an injection of premixed insulin or an insulin
analogue. Patients were grouped according to islet function and the
efficacy of the new treatment in these groups was observed. The aim
was to examine the feasibility of the two-back-one strategy and its
suitability to different patients, with the purpose of providing
data that will optimise insulin therapy for T2DM patients.
Materials and methods
Subjects
Between 2010 and 2011, T2DM patients who were
injected with stable doses of premixed insulin or analogue twice a
day for at least eight weeks were selected from the First
Subsidiary Hospital of the Medical College of Dalian (Liaoning,
China). The subjects were aged 18–75 years and had been diabetic
for <15 years. These patients received an insulin dose of 50
IU/day, and had laboratory values of fasting blood glucose
(FBG)<11 mmol/l, 5.5% <HbA1c≤10% and fasting C peptide
(FCP)≥0.8 ng/ml. This study also included patients with frequent
glycopenia (frequent mild, severe nighttime glycopenia), those with
irregular life styles and those willing to reduce the number of
injections. Patients with the following conditions were excluded:
Liver dysfunction with alanine transaminase exceeding twice the
normal upper limit, obvious renal disease or serum creatinine ≥133
mmol/l, various diseases affecting blood glucose (such as
hyperthyroidism and hypercortisolism), prior systemic
corticosteroid therapy or hormone replacement therapy, diabetes
with acute complications, concomitant disease or stressful
situation, severe heart failure at level III or IV and/or left
ventricular ejection fraction <40%, pregnancy, gestation or
lactation. Patients who were allergic or intolerant to the test
drugs, had poor compliance, did not cooperate, changed food or
drugs or were lost in the follow-up period, as well as those whose
blood glucose levels were not controlled (FBG>7.0 mmol/l, 2 h
postprandial blood glucose (2hPBG)>10 mmol/l) three weeks
following the switch, were also not included. The present study was
conducted in accordance with the declaration of Helsinki and with
approval from the Ethics Committee of Henan province people’s
hospital. Written informed consent was obtained from all
participants.
Methods
After the selected patients signed the informed
consent form, their treatment was switched from premixed insulin or
analogue to insulin glargine (300 U/vial; Sanofi-Aventis China Co.,
Ltd., Beijing, China) or insulin glargine with a glimepiride tablet
(2 mg/tablet; Sanofi-Aventis China Co., Ltd.). The original oral
medicine therapy was retained. The specific treatment was as
follows: Initial dose of insulin glargine was 0.15 U/kg/day, which
was subcutaneously injected prior to sleeping every night and a 2
mg glimepiride tablet was taken prior to breakfast every day. The
target value of parameters was set as 4.4 mmol/l ≤FBG≤7.0 mmol/l
and 2hPBG≤10 mmol/l. Glucose levels were monitored through
fingerstick testing to adjust drug dose. Glargine insulin (≤1 U)
was added every time FBG exceeded the target value of 1 mmol/l; in
patients with hypoglycaemia (<3.3 mmol/l), glargine insulin was
reduced by 2 U to 6 U. The doses were adjusted every 2–3 days.
Patients with 2hPBG exceeding 10 mmol/l received 50–100 mg acarbose
(50 mg/tablet; Bayer China Co., Ltd., Beijing, China) during
mealtimes, depending on their actual condition. Antihypertensive
and lipid-lowering therapy remained constant. The subjects were
monitored for eight weeks.
Evaluation indices
Efficacy indices included HbA1c, FBG, 2hPBG (with a
target range of FBG≤7.0 mmol/l, 2hPBG≤10 mmol/l and HbA1c≤7.0%) and
insulin dose.
Islet function and insulin sensitivity indices were
as follows: Homeostasis model assessment-function of β cells
(HOMA-β)=0.27xFCP/(FPG-3.5); homeostasis model assessment-insulin
resistance (HOMA-IR)=1.5+FPGxFCP/2800. Insulin therapy was
administered to all patients in the study. As the connecting
peptide or C-peptide was secreted at the same rate as insulin
(which was not affected by external insulin), FCP was used as an
alternative to fasting insulin to evaluate insulin resistance and
pancreatic β-cell function (15).
Safety indices included the following: i)
Hypoglycaemic events (hypoglycaemia and severe hypoglycaemia were
defined as blood glucose ≤4.0 mmol/l and blood glucose ≤2.8 mmol/l,
whereas symptomatic hypoglycaemia was characterised by palpitation,
sweating and hunger without a corresponding record of glycaemia or
the monitored blood glucose not reaching the above standard); ii)
body weight and body mass index (BMI) and iii) any adverse
events.
Statistical analysis
All data were analysed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). Variable values of measurement data
are presented as the mean ± standard deviation and were subjected
to normality and F-tests. The t-test was employed to compare
between the groups prior to and following the switch. The
χ2 test was used for comparing the rates. Classified
indices were described as a case number and % of each type.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
General data comparison
Among the 30 patients selected for this study, only
28 remained at the end of the investigations, due to two patients
leaving the study early. Of these two patients, one had high blood
glucose levels due to an insufficiently controlled diet and the
other switched back to premixed insulin from insulin glargine due
to high blood glucose. The subjects were divided into two groups
according to islet function: Group A, 2hCP/FCP≤3; seven males,
seven females (mean, 52.93±10.74 years old). Group B,
2hCP/FCP>3; six males, eight females (mean, 53.57±13.15 years
old).
No significant differences were observed between the
two groups in terms of age, gender ratio, disease course, body
weight, BMI, HbA1c, FBG, 2hPBG, FCP, HOMA-IR, HOMA-β and insulin
dose. The difference in 2hCP between the two groups was
statistically significant (P<0.05), as demonstrated in Table I.
 | Table IComparison of general data between two
groups. |
Table I
Comparison of general data between two
groups.
| Group A | Group B |
|---|
| Gender (M/F) | 7/7 | 6/8 |
| Age (years) | 52.93±10.74 | 53.57±13.15 |
| Disease course
(year) | 8.00±3.96 | 6.25±5.26 |
| BMI
(kg/m2) | 27.76±5.53 | 26.28±2.17 |
| Bodyweight (kg) | 77.00±14.00 | 70.43±5.84 |
| FBG (mmol/l) | 8.05±1.22 | 7.64±0.87 |
| 2hPBG (mmol/l) | 11.08±1.51 | 11.32±1.66 |
| HbA1c (%) | 8.04±1.16 | 7.94±1.32 |
| FCP (ng/ml) | 2.29±1.32 | 1.64±0.65 |
| 2hCP (ng/ml) | 4.86±2.59 | 7.78±2.99* |
| HOMA-IR | 0.81±0.45 | 0.54±0.18 |
| HOMA-β | 10.87±6.95 | 8.70±4.96 |
| Insulin dose (U) | 30.79±8.15 | 26.07±6.13 |
Efficacy after switch
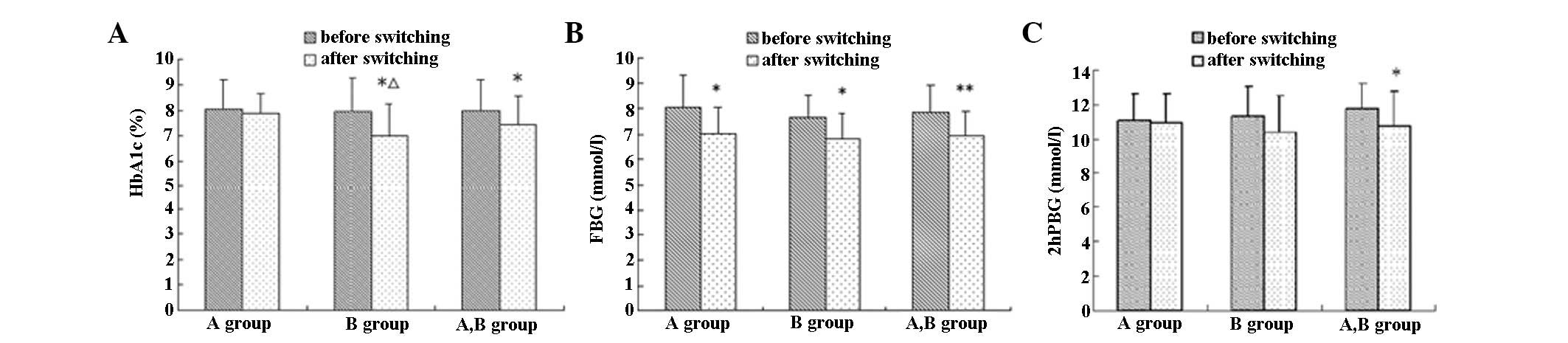
Eight weeks following the treatment switch, the
average HbA1c, FBG and 2hPBG of the 28 patients decreased by 9.18,
11.61 and 8.04%, respectively, compared with the values prior to
switching, which was a statistically significant difference
(P<0.05), particularly for FBG reduction (P<0.01). HbA1c, FBG
and 2hPBG decreased by 4.65, 12.55 and 1.17%, respectively, in
group A and by 13.81, 10.73 and 8.04%, respectively, in group B,
compared with values prior to switching. A significant decrease in
FBG was observed in group A and in the HbA1c and FBG in group B
(P<0.05). Statistical significance in HbA1c reduction was higher
in group B than in group A (P<0.05), as demonstrated in Table II and Fig. 1.
 | Table IIComparison of main efficacy indices
prior to and after switch in the selected patients (± standard
deviation). |
Table II
Comparison of main efficacy indices
prior to and after switch in the selected patients (± standard
deviation).
| Before switch | After switch |
|---|
|
|
|
|---|
| Group | A | B | A+B | A | B | A+B |
|---|
| HbA1c | 8.04±1.16 | 7.94±1.32 | 7.99±1.22 | 7.84±0.79 | 6.99±1.26*Δ | 7.42±1.12* |
| FBG (mmol/l) | 8.05±1.22 | 7.64±0.87 | 7.84±1.05 | 7.04±0.99* | 6.82±0.97* | 6.93±0.96** |
| 2hPBG (mmol/l) | 11.08±1.51 | 11.32±1.66 | 11.69±1.51 | 10.95±1.63 | 10.41±2.04 | 10.75±2.00* |
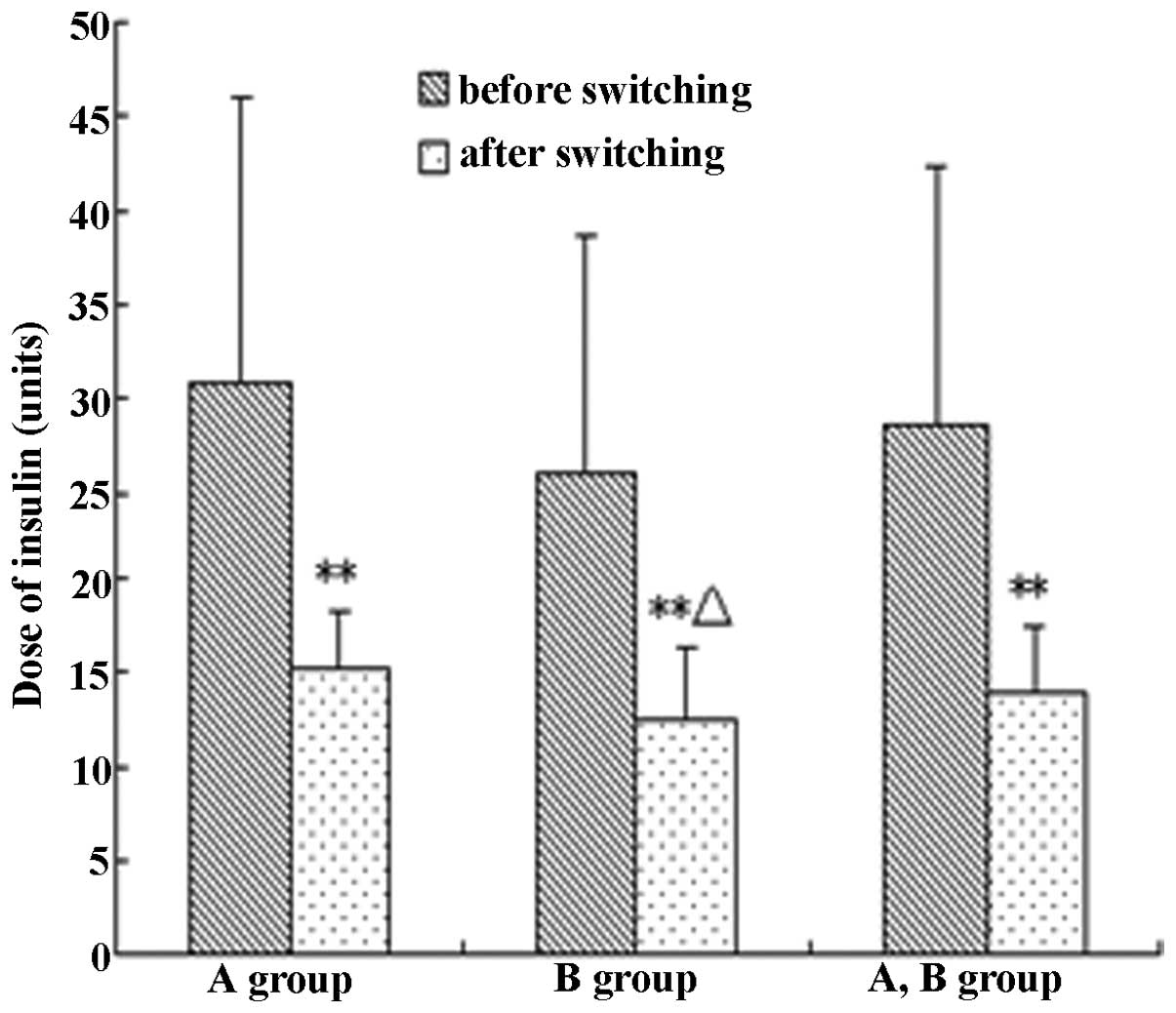
| Insulin dose (U) | 30.79±8.15 | 26.07±6.13 | 28.43±7.48 | 15.29±3.00** | 12.57±3.72**Δ | 13.93±3.59** |
| Body weight (kg) | 77.0±14.0 | 70.43±5.84 | 73.71±11.04 | 76.36±13.67 | 70.29±6.17 | 73.32±10.86 |
| BMI
(kg/m2) | 27.76±5.53 | 26.28±2.17 | 27.02±4.19 | 27.16±5.29 | 26.05±2.03 | 26.76±0.76 |
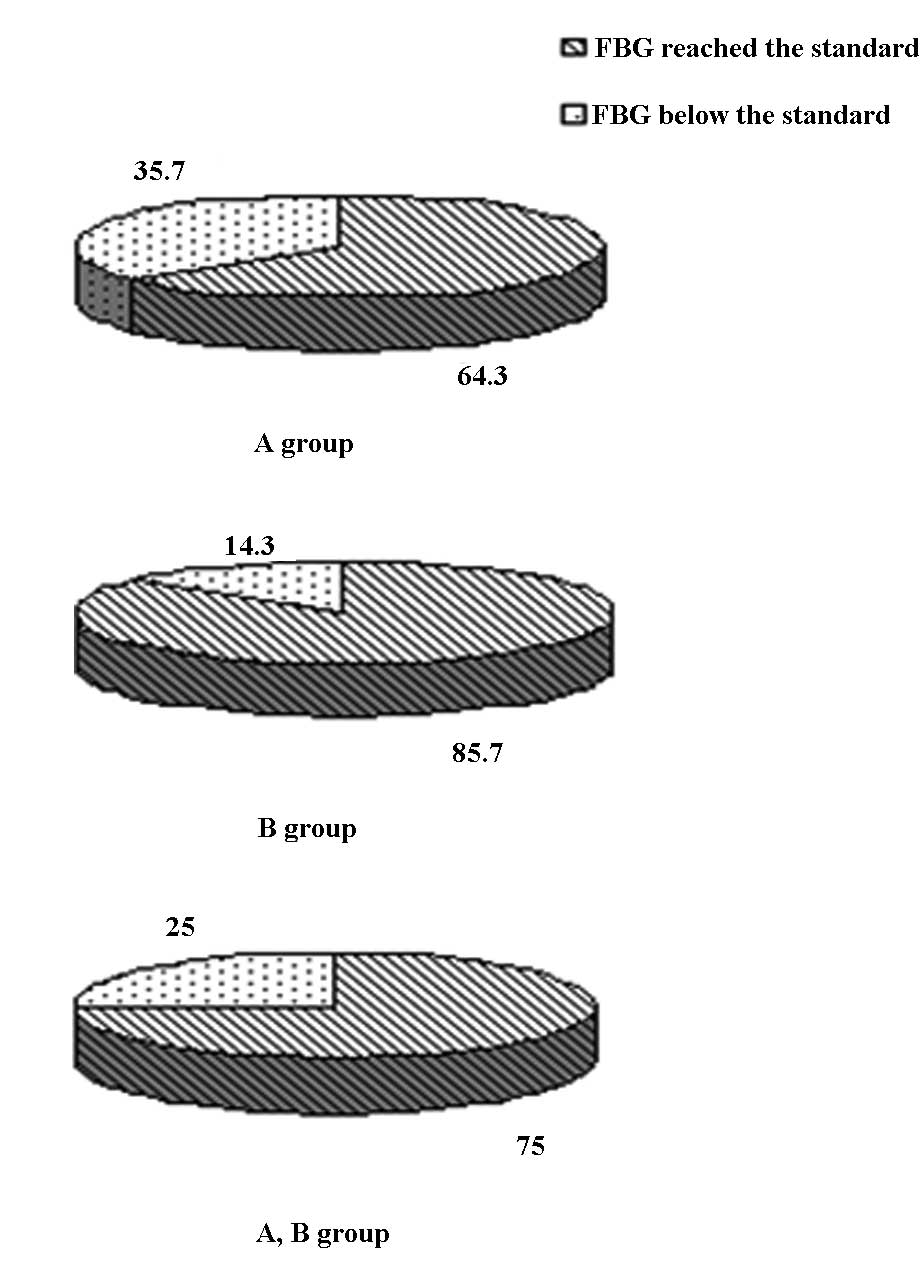
Prior to the switch, standard values of HbA1c, FBG
and 2hPBG were noted in six, five and three cases, respectively
(two, two and one of the cases were in group A and four, three and
two of the cases were in group B, respectively). No significant
difference was observed between the two groups. Eight weeks after
the switch in treatment, the standard-reaching rates of HbA1c, FBG
and 2hPBG in the 28 patients were 39.3, 75.0 and 42.9%,
respectively. This result demonstrates that the standard-reaching
rate of FBG was markedly higher compared with that of HbA1c and
2hPBG (P<0.05). The standard-reaching rates of HbA1c, FBG and
2hPBG were 21.4, 64.3 and 28.6% in group A and 57.1, 85.7 and 57.1%
in group B (Fig. 2). The
standard-reaching rates of HbA1c in group B were markedly higher
compared with those in group A (P<0.05), as demonstrated in
Table III.
 | Table IIIComparison of glycemic
standard-reaching rate. |
Table III
Comparison of glycemic
standard-reaching rate.
| Group | A | B | A+B |
|---|
|
|
|
|
|---|
|
Standard-reaching | Cases (n) | Rate (%) | Cases (n) | Rate (%) | Cases (n) | Rate (%) |
|---|
| FBG (mmol/l) | 9 | 64.3 | 12 | 85.7 | 21 | 75.0 |
| 2hPBG (mmol/l) | 4 | 28.6 | 8 | 57.1 | 12 | 42.9* |
| HbA1c (%) | 3 | 21.4* | 8 | 57.1Δ | 11 | 39.3* |
Comparison of insulin dose
The average insulin dose decreased by 51.0% in the
28 patients following the switch in treatment, and statistical
significance was measured at P<0.01. The average insulin dose
markedly decreased by 50.34 and 53.7% in groups A and B,
respectively (P<0.01). However, the insulin doses in group B
were distinctly lower than those in group A (P<0.05), as shown
in Table II and Fig. 3.
Safety evaluation
During the study, one case of mild hypoglycaemia
(1/28, 3.6%) was identified, but no cases of severe hypoglycaemia
were observed. No significant difference was identified between
groups A and B in terms of the incidence of hypoglycaemia
(P>0.05). Table IV
demonstrated that there were hypoglycaemic cases observed in group
A (0%), but one case of mild hypoglycaemia was seen among the 14
cases in group B (7.1%).
 | Table IVComparison of incidence of
hypoglycemia between group A and B. |
Table IV
Comparison of incidence of
hypoglycemia between group A and B.
| Cases (n) | Patients with
hypoglycemia (n) | Incidence of
hypoglycemia (%) | χ2 | P-value |
|---|
| Group A | 14 | 0 | 0 | | |
| Group B | 14 | 1 | 7.1 | 1.04 | 0.05 |
Eight weeks following the treatment switch, there
was no significant change in body weight and BMI (P>0.05). The
difference in body weight and BMI between groups A and B was also
non-significant (P>0.05), as demonstrated in Table II.
Discussion
Previous studies (10–14)
have demonstrated improved glycaemic control after premixed
insulin/insulin analogue regimens, that were ineffective in
treating T2DM patients, were replaced with insulin glargine plus
OADs. In the present study, the treatment of 28 T2DM patients was
switched from premixed insulin or analogue to insulin glargine with
OADs. HbA1c, FBG and 2hPBG decreased by 9.18, 11.61 and 8.04%,
respectively, eight weeks after the switch (P<0.05). FBG
reduction was statistically significant (P<0.01), which is
consistent with that observed in previous studies (10–14).
In a study by the AT.LANTUS group (12), the treatment of 384 T2DM patients
with poor glycaemic control was switched from premixed insulin plus
OADs to insulin glargine plus OADs and the level of HbA1c and FBG
highly improved. In the study by Hammer et al (10), treatment was switched from premixed
insulin to insulin glargine plus OADs, in 6308 T2DM patients who
had an average disease course of 8.6 years; 8.3% of HbA1c, 9.9
mmol/l of FBG and 10.8 mmol/l of 2hPBG. A distinct decrease in the
patients’ HbA1c, FBG and 2hPBG was observed 12 weeks following the
switch. Yang et al (14)
performed a multicentre, prospective study where treatment was
switched to insulin glargine in 313 T2DM patients, who were not
responsive to premixed insulin. The average FBG and 2hPBG improved,
compared with the baseline values. Average HbA1c also markedly
decreased 16 weeks after the switch.
In the present study, HbA1c≤7.0%, FBG≤7.0 mmol/l and
2hPBG≤10 mmol/l were defined as the standard. Their corresponding
standard-reaching rates eight weeks after the switch were 39.3, 75
and 42.9%, respectively, which further confirmed the effectiveness
of the two-back-one strategy. Of note, the differences in
standard-reaching rates that have been reported in various studies
were mainly due to differences in the standards adopted. Hammer
et al (10) set the
standard as HbA1c<7.5%, FBG<6.7 mmol/l and 2hPBG<7.2
mmol/l. The corresponding standard-reaching rates were 73.9, 48.9
and 38.4%, respectively. In the study by Yang et al
(14), the standard was
HbA1c<7.0% and the standard-reaching rate was only 19.5%, which
is lower than the value in the present study, which is mainly due
to the effects of the course of treatment. The average treatment
course of the patients in the present study was shorter (group A,
8±3.96 years; group B, 6.25±5.26 years), whereas that of patients
in the study by Yang et al was increased by ~10 years.
Patients with a shorter treatment course may have maintained
relatively good islet function and therefore, the converted
compliance rate would have been higher as a result. In the study by
Yang et al, the standard-reaching rate of FBG is evidently
higher than that of HbA1c and PBG (P<0.05), because basic
insulin effectively reduces FBG and glycogen output, enhances
insulin sensitivity and increases insulin secretion in DM patients
(7). By contrast, in the present
study, the standard-reaching rate of FBG was higher and the
standard-reaching rate of HbA1c was lower, compared with the
results of other studies, because the target values were
different.
Following the switch, our results demonstrated a
significant reduction in average insulin dose (51%) of the patients
(P<0.01). In another study, Ligthelm et al demonstrated
that long-acting insulin analogues combined with oral drugs reduce
the amount of insulin approximately by half, but deliver similar
blood glucose control compared with premixed insulin/analogues
(16). Although the insulin dose
decreased by 20% following the switch in regimen in the study by
Yang et al (14), the
present study yielded a higher reduction in insulin dose, which is
correlated with the FBG standard setting (FBG≤6.0 mmol/l).
Furthermore, in the present study, certain patients had short
disease courses, whereas others had better insulin function and
achieved HbA1c≤7.0% prior to the switch, and thus, the insulin dose
was lower.
Hypoglycaemia was observed in only one case (3.6%),
in which the insulin dose was not reduced, according to the
decrease in blood glucose. However, hypoglycaemia was no longer
evident when the insulin dose was decreased. Thus, the incidence of
hypoglycemia was low in this study, as it was observed in only nine
of all the patients who received premixed insulin or insulin
analogues eight weeks prior to the treatment switch. No significant
change was identified in the body weight and BMI of the patients
following the switch. Domestic and foreign studies have
demonstrated a dramatic decrease in the incidence of hypoglycaemia
after the switch in regimen. Furthermore, patients were more
satisfied with the fewer injections and easy administration of
insulin glargine, proving that the glargine treatment promotes
increased safety and compliance (17,18),
which is inseparable from the biological characteristics of the
long-acting insulin analogues (19).
The treatment of diabetes should be individualised,
and each treatment program, including long-acting insulin
analogues, has its adaptation (20). Therefore, treatment conversions are
often only successful in a number of patients, while the glucose
levels of certain patients are not controlled effectively after
converting. Summarizing the findings of several recent studies
(10–14), patients with the following
characteristics are suitable to undergo a treatment switch: i) Poor
glycaemic control from premixed insulin (FPG ~10 mmol/l,
HbAlc<9%); ii) frequent hypoglycaemia, severe hypoglycaemia or
nocturnal hypoglycaemia; iii) evident increase in body weight; iv)
poor life quality, including receiving too many/inconvenient
injections, as well as extra meals or limited mealtimes; and v)
certain pancreatic β-cell function. Relevant studies were
summarised to determine the conditions of patients who were ready
for the treatment switch (Table
V).
 | Table VConditions of patients who are
suitable for switching of diabetes treatment as indicated by
associated studies. |
Table V
Conditions of patients who are
suitable for switching of diabetes treatment as indicated by
associated studies.
| Conditions of
patients |
|---|
|
|
|---|
| FBG (mmol/l) | HbA1c (%) | Course of disease
(years) | Dose of premixed
insulin (U/day) |
|---|
| Chinese study
(14) Group A | 10.2±1.8 | 8.8 | 10 (6–13) | 35±19 |
| Group B | 10.9±3.0 | 8.9 | 11 (8–20) | 32±10 |
| Hammer’s study
(10) | | 9.9 | 8.3 | 8.6±6.1
35.5±15.0 |
| Optimization study
(14) | | 8.94±2 | 8.36 | 7.42±2.49 34.4 |
| Treatment switch
recommended | ~10 | <9% | <10 | <40 |
However, the majority of studies investigating the
two-back-one strategy have focused only on the standard-reaching
rate of HbA1c, incidence of hypoglycaemia and satisfaction of
patients. The suitability of different patients for this strategy
has not been reported. Therefore, the T2DM patients in this study
were divided into two groups according to the 2hCP/FCP ratio and
administered two-back-one treatment by injection with premixed
insulin or an insulin analogue to examine the feasibility of the
strategy and identify suitable patients based on islet function.
The higher standard-reaching rate of HbA1c in group B
(2hCP/FCP>3) compared with group A (2hCP/FCP≤3) was significant
(P<0.05), whereas the difference in the standard-reaching rates
of FBG and 2hPBG was not significant. The evident reduction in
HbA1c in group B was higher compared with group A after the switch
(P<0.05). The decrease in the amount of insulin injection in the
patients (group A, 50.34% and group B, 53.70%) was significant
(P<0.01). The patients in group B received less insulin compared
with those in group A, indicating a higher standard-reaching rate,
more significant decrease in blood glucose and less insulin dosage
in patients with better islet function. In addition, insulin
glargine combined with OADs was suitable as a treatment for
diabetes patients who received premixed insulin or insulin
analogues in the case of FCP≥0.8 ng/ml, particularly when
2hCP/FCP>3.
Two patients left the study because in one case, the
blood glucose of the patient was too high during the follow-up,
which was caused by an insufficiently controlled diet. In the other
case, the patient switched back to premixed insulin from insulin
glargine due to high blood glucose. These two cases indicated that
diet control remains an important basis of T2DM treatment. The
blood glucose of patients did not reach the standard owing to the
following reasons: i) Uncontrolled diet (unscheduled timing or
quantity) or insufficient exercise; ii) inadequate dosage of
hypoglycaemic drugs or non-adherence to the drug combination and
iii) unsystematic monitoring of blood glucose. Therefore, it is
concluded that drug therapy is only one component of diabetes
therapeutics, and efficacious treatment relies also on diet control
and exercise adjustment, good compliance and regular blood glucose
monitoring, to ensure qualified blood glucose levels (21,22).
The present study adopted only a small number of patients and no
specific subgroup was subjected to different oral hypoglycaemic
drugs. In future investigations, the sample size should be
increased to study the efficacy of the two-back-one strategy more
reliably.
In conclusion, switching diabetic treatment regimens
from premixed insulin or insulin analogues to insulin glargine plus
OADs, i.e., the two-back-one strategy, allows better glycaemic
control, shorter injection times, more convenient application and
lower incidence of hypoglycaemia without body weight increase in
T2DM patients who have certain islet functions. Insulin glargine
plus OADs is particularly suitable for T2DM patients with high FBG,
minimal amounts of insulin injections and relatively good islet
function. The 2hCP and FCP ratios, as demonstrated in this study,
indicated the success rate of the two-back-one strategy in the
treatment of T2DM.
References
|
1
|
Yang Y, Yao JJ, Du JL, et al: Primary
prevention of macroangiopathy in patients with short-duration type
2 diabetes by intensified multifactorial intervention: seven-year
follow-up of diabetes complications in Chinese. Diabetes Care.
36:978–984. 2013.
|
|
2
|
Gaede P, Lund-Andersen H, Parving HH and
Pedersen O: Effect of a multifactorial intervention on mortality in
type 2 diabetes. N Engl J Med. 358:580–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Holman RR, Paul SK, Bethel MA, Matthews DR
and Neil HA: 10-year follow-up of intensive glucose control in type
2 diabetes. N Engl J Med. 359:1577–1589. 2008.PubMed/NCBI
|
|
4
|
Rizvi AA and Ligthelm RJ: The use of
premixed insulin analogues in the treatment of patients with type 2
diabetes mellitus: advantages and limitations. Insulin. 2:68–79.
2007. View Article : Google Scholar
|
|
5
|
Miser WF, Arakaki R, Jiang H, Scism-Bacon
J, Anderson PW and Fahrbach JL: Randomized, open-label,
parallel-group evaluations of basal-bolus therapy versus insulin
lispro premixed therapy in patients with type 2 diabetes mellitus
failing to achieve control with starter insulin treatment and
continuing oral antihyperglycemic drugs: a noninferiority
intensification substudy of the DURABLE trial. Clin The.
32:896–908. 2010.
|
|
6
|
Levit S, Toledano Y and Wainstein J:
Improved glycaemic control with reduced hypoglycaemic episodes and
without weight gain using long-term modern premixed insulins in
type 2 diabetes. Int J Clin Pract. 65:165–171. 2011. View Article : Google Scholar
|
|
7
|
Vaag A and Lund SS: Insulin initiation in
patients with type 2 diabetes mellitus: treatment guidelines,
clinical evidence and patterns of use of basal vs premixed insulin
analogues. Eur J Endocrinol. 66:159–170. 2012. View Article : Google Scholar
|
|
8
|
Fritsche A, Larbig M, Owens D and Häring
HU; the GINGER study group. Comparison between a basal-bolus and a
premixed insulin regimen in individuals with type 2
diabetes-results of the GINGER study. Diabetes Obes Metab.
12:115–123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Giugliano D, Maiorino MI, Bellastella G,
Chiodini P, Ceriello A and Esposito K: Efficacy of Insulin Analogs
in Achieving the Hemoglobin A1c Target of <7% in Type 2
Diabetes: Meta-analysis of randomized controlled trials. Diabetes
Care. 34:510–517. 2011.
|
|
10
|
Hammer H and Klinge A: Patients with type
2 diabetes inadequately controlled on premixed insulin: effect of
initiating insulin glargine plus oral antidiabetic agents on
glycaemic control in daily practice. Int J Clin Pract.
61:2009–2018. 2007. View Article : Google Scholar
|
|
11
|
Bu S, Guo XH, Yang WY, et al: Post-hoc
analyses of type 2 diabetes patients switch from premixed insulin
regimen to basal insulin plus oral hypoglycemic agents regimen.
Zhong Hua Yi Xue Za Zhi. 87:3115–3118. 2007.(In Chinese).
|
|
12
|
Davies M, Lavalle-González F, Storms F and
Gomis R; AT, LANTUS Study Group. Initiation of insulin glargine
therapy in type 2 diabetes subjects suboptimally controlled on oral
antidiabetic agents: results from the AT.LANTUS trial. Diabetes
Obes Metab. 10:387–399. 2008. View Article : Google Scholar
|
|
13
|
Gómez-Peralta F, Carramiñana-Barrera F,
Félix-Redondo FJ and Fraile-Gómez J; Extreme Rescue Study Group.
Glycaemic control in patients with type 2 diabetes switching from
premixed insulin to long-acting basal insulin analogue plus oral
antidiabetic drugs: an observational study. Int J Clin Pract.
66:959–968. 2012.PubMed/NCBI
|
|
14
|
Yang W, Lv X, Li Q, Jia W and Tian H: A
prospective study to optimize insulin treatment by switching to
insulin glargine in type 2 diabetic patients previously
uncontrolled on premixed insulin: the optimization study. Curr Med
Res Opin. 28:533–541. 2012. View Article : Google Scholar
|
|
15
|
Li X, Zhou ZG, Qi HY, Chen XY and Huang G:
Replacement of insulin by fasting C-peptide in modified homeostasis
model assessment to evaluate insulin resistance and islet beta cell
function. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 29:419–423. 2004.(In
Chinese).
|
|
16
|
Ligthelm RJ, Gylvin T, DeLuzio T and
Raskin P: A comparison of twice-daily biphasic insulin aspart 70/30
and once-daily insulin glargine in persons with type 2 diabetes
mellitus inadequately controlled on basal insulin and oral therapy:
a randomized, open-label study. Endocr Pract. 17:41–50. 2011.
View Article : Google Scholar
|
|
17
|
Standl E, Maxeiner S, Raptis S,
Karimi-Anderesi Z and Schweitzer MA; HOE901/4009 Study Group. Good
glycemic control with flexibility in timing of basal insulin
supply: a 24-week com-parison of insulin glargine given once daily
in the morning or at bedtime in combination with morning
glimepiride. Diabetes Care. 28:419–420. 2005. View Article : Google Scholar
|
|
18
|
Janka HU, Plewe G, Riddle MC,
Kliebe-Frisch C, Schweitzer MA and Yki-Järvinen H: Comparison of
basal insulin added to oral agents versus twice-daily premixed
insulin as initial insulin therapy for type 2 diabetes. Diabetes
Care. 28:254–259. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Porcellati F, Lucidi P, Rossetti P, et al:
Differential effects of adiposity on pharmacodynamics of basal
insulins NPH, glargine, and detemir in type 2 diabetes mellitus.
Diabetes Care. 34:2521–2523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nathan DM, Buse JB, Davidson MB, et al:
Medical management of hyperglycemia in type 2 diabetes: a consensus
algorithm for the initiation and adjustment of therapy: a consensus
statement of the American Diabetes Association and the European
Association for the Study of Diabetes. Diabetes Care. 32:193–203.
2009. View Article : Google Scholar
|
|
21
|
Romero-Márquez RS, Díaz-Veja G and
Romero-Zepeda H: Style and quality of life in patients with type 2
diabetes. Rev Med Inst Mex Seguro Soc. 49:125–136. 2011.(In
Spanish).
|
|
22
|
Shrestha P and Ghimire L: A review about
the effect of life style modification on diabetes and quality of
life. Glob J Health Sci. 4:185–190. 2012.PubMed/NCBI
|