Introduction
Myocardial ischemic injury is a common pathological
process in patients suffering from cardiac conditions, including
atherosclerotic coronary artery disease, acute myocardial
infarction and cardiac transplantation (1). However, the treatment costs for these
diseases are high; therefore, it is important to investigate
low-cost therapies that impede ischemia-induced myocardial
injury.
The mechanisms underlying ischemia-induced cell
damage are complicated and remain elusive. Increasing evidence
suggested that oxidative stress is important in myocardial ischemic
injury (2). Oxidative stress is
characterized by marked increases in the production of reactive
oxygen species (ROS), including the superoxide anion
(O2•−) and the hydroxyl radical (·OH), as
well as non-radical molecules, including hydrogen peroxide
(H2O2) and singlet oxygen
(1O2) (3,4).
Environmental stress factors, including ultraviolet rays, heat
exposure, as well as ischemia and/or hypoxia, are major causes of
oxidative stress, which may damage proteins, lipids and DNA, and
eventually result in cellular death or the development of cancer
(5,6). Under normal conditions, intracellular
ROS levels are controlled by balancing ROS generation with ROS
elimination. Once the balance is disrupted, for instance, through
increased ROS generation and/or reduced elimination, ROS may
aggregate and oxidative stress arises. Eliminating excessive ROS
and enhancing endogenous antioxidation ability have been applied
clinically (7), and in myocardial
ischemic injury, oxidant scavengers, antioxidant extracts, vitamin
E and vitamin C have all demonstrated to have a potential
therapeutic value (8). However,
water-soluble vitamin C has a low transmembrane diffusion ability
and it is difficult to accumulate vitamin C up to an effective
level to eliminate ROS (9).
Conversely, the lipid-soluble vitamin E is difficult to dissolve in
the cytoplasm in order to neutralize ROS (10). These shortcomings have limited the
wide clinical use of the two vitamins. Therefore, a small
antioxidant with water- and lipid-solubility is expected to have
greater application value.
Hydrogen (H2) gas is an inexpensive
medical gas generated by electrolysis of water. Similar to other
gaseous molecules, including nitric oxide (NO), carbon monoxide
(CO) and hydrogen sulfide (H2S), H2 gas has
been demonstrated to exhibit numerous important cytoprotective
effects in the nervous, cardiovascular and digestive systems
(11–14). Unlike vitamin C and vitamin E,
H2 gas dissolves in water and lipids. In addition, the
simple molecular structure and small molecular weight render
H2 gas a good antioxidant candidate in cells. A number
of studies have suggested that H2 gas may selectively
scavenge ·OH free radicals (15,16).
Therefore, H2 gas may have broad clinical applications
in the future. However, in cardiomyocytes, it has not been reported
whether H2 gas protects against ischemia-induced injury
in vitro, which was the focus of the present study.
Abundant evidence indicated that enhancement of
endogenous antioxidation activity exerts cardioprotective effects.
For instance, upregulation of superoxide dismutase (SOD) or heme
oxygenase-1 (HO-1) protects cardiomyocytes against ischemia and/or
reperfusion-induced damage (17–19).
Notably, in addition to scavenging ·OH free radicals, H2
gas protection has been associated with induction of HO-1
controlled by NF-E2-related factor 2 (Nrf2) in rat lung
transplant-induced injury and paraquat-induced oxidative damage in
plants (20,21). H2 gas protection of
cardiomyocytes may therefore be associated with scavenging ·OH free
radicals and upregulation of the Nrf2/HO-1 signaling pathway.
H9c2 cardiomyoblasts (H9c2 cells), originating from
rat heart ventricular tissue, have widely served as an in
vitro model for cardiac muscle in virtue of their morphological
features and biochemical properties (22). In the present study, H9c2 cells
were subjected to serum and glucose deprivation (SGD) or exposed to
hypoxia provoked by a chemical hypoxia-mimicking agent, cobalt
chloride (CoCl2), to establish an
ischemia/hypoxia-induced myocardial injury model. H2
gas-rich medium was applied to investigate the cytoprotection,
antioxidation and activation of the Nrf2/HO-1 signaling pathway as
well as the involvement of scavenging ·OH free radicals and
upregulation of the Nrf2/HO-1 signaling pathway in the
cardioprotection by H2 gas.
Materials and methods
Materials
CoCl2 and protoporphyrin IX zinc (II)
(ZnPP IX) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Brusatol (BR), an inhibitor of Nrf2, was provided by BOC Sciences
(Shirley, NY, USA). A cell counting kit-8 (CCK-8) was purchased
from Dojindo Laboratories (Kyushu, Japan). Specific monoclonal
primary antibodies against rat HO-1 and Nrf2 proteins were obtained
from EPITOMICS of Abcam Company (Burlingame, CA, USA). High glucose
and glucose-free Dulbecco’s modified Eagle’s medium and fetal
bovine serum (FBS) were supplied by Gibco-BRL (Grand Island, NY,
USA). A bicinchoninic acid (BCA) protein assay kit was purchased
from Kangchen Bio-tech Inc. (Shanghai, China). An enhanced
chemiluminescence (ECL) kit was obtained from Applygen Technologies
Inc. (Beijing, China). An enzyme-linked immunosorbent assay (ELISA)
kit for detection of 8-hydroxy-2′-deoxyguanosine (8-OHdG) was
provided by Abnova Corporation (Taipei, Taiwan).
Cell culture
H9c2 cells were supplied by the Cell Bank at the
Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained in high glucose DMEM
supplemented with 15% FBS at 37°C in an atmosphere of 5%
CO2 and 95% air. The cells were passaged approximately
every two days after digestion with 0.2% trypsin.
Hypoxia or ischemia treatment
CoCl2, a chemical hypoxia inducer, was
co-incubated with H9c2 cells to induce hypoxia. An ischemia-induced
myocardial injury model generated through SGD was prepared in
vitro in the cell medium. The cell viability was used to
indicate the extent of hypoxic or ischemic injury in the H9c2
cells.
Preparation of H2 gas-rich
medium
Pure H2 gas (99.999% purity) was produced
via electrolysis of water with a M177021 H2 gas
generator, supplied by Beijing Midwest Yuanda Technology Co., Ltd.
(Beijing, China; 23). H2 gas-rich medium was prepared
freshly prior to saturating the medium with the generated
H2 gas for at least 30 min.
Determination of cell viability
Cell viability was analyzed by a CCK-8 assay
following the manufacturer’s instructions. H9c2 cells were plated
in 96-well plates at a density of 5,000 cells/well. When the cells
were grown to ~70% confluence, the indicated treatments were
administered. At the end of the treatment, the CCK-8 solution (10
μl) at 1:10 dilution with FBS-free DMEM high glucose medium (100
μl) was added to each well followed by a further 3 h incubation at
37°C. Absorbance (A) was measured at 450 nm with a microplate
reader produced by Molecular Devices, LLC (Sunnyvale, CA, USA). The
mean A was used to calculate the percentage of cell viability
according to the following equation: Percentage of viable cells =
(A treatment group − A Blank group)/(A Control group-A Blank group)
× 100%. Experiments were performed six times (24).
Western blot analysis of protein
expression
H9c2 cells were plated in 60-mm diameter petri
dishes. Following the indicated treatments, the cells were
harvested and total proteins or nuclear proteins were extracted and
quantified with the BCA kit and used to measure HO-1 or Nrf2
expression levels, respectively. Subsequent to denaturation by
heating at 100°C for 5 min, equal quantities of proteins of the
indicated groups were loaded and separated by 12% SDS-PAGE. The
proteins in the gel were then transferred to a polyvinylidene
fluoride membrane. Following blocking with 5% fat-free milk in
Tris-buffered saline with Tween 20 (TBS-T), the membranes were
incubated with rat monoclonal primary antibodies against HO-1 or
Nrf2 overnight with gentle agitation at 4°C. β-actin and Lamin B
served as loading controls. Subsequent to three washes with TBS-T,
the membranes were incubated with anti-rabbit horseradish
peroxidase-conjugated secondary antibodies at room temperature for
1 h. The membranes were washed again and developed with an ECL
system. The membranes were then exposed to X-ray films (Kodac
Company, Beijing, China). The integrated optical density of the
protein bands was calculated by Image J 1.47 Software (National
Institutes of Health, Bethesda, MD, USA).
Competitive ELISA for measurement of
8-OHdG
Intracellular ·OH free radicals cause oxidative
damage to DNA to form 8-OHdG. Therefore, by measuring 8-OHdG levels
in the cells, ·OH free radical content may be analyzed indirectly.
H9c2 cells were plated in six-well plates and treated as indicated.
At the end of the treatment, the cells were harvested and lysed.
8-OHdG levels in the lysate were determined according to the
manufacturer’s instructions (Abnova Corporation). The experiment
was performed at least six times with similar outcomes.
Gene knockdown
Small interfering RNA (Si-RNA) against rat Nrf2 mRNA
(GenBank accession no. AF037350; https://www.ncbi.nlm.nih.gov/genbank/) was synthesized
by GenePharma Co., Ltd (Shanghai, China). The Si-RNA of Nrf2
(Si-Nrf2) and random non-coding RNA (Si-NC) were transfected into
H9c2 cells using Lipofectamine 2000, according to the
manufacturer’s instructions (Invitrogen Life Technologies,
Carlsbad, CA, USA). Si-Nrf2 and Si-NC (20 nmol/l) were incubated
with the cells for 6 h followed by further incubation for 24 h in
order to transfect the cells.
Statistical analysis
All data were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA) and the results are expressed as the
mean ± standard deviation. The significance of intergroup
differences was evaluated by one-way analyses of variance.
Differences were considered to be significant if the two-sided
probability (P) was <0.05.
Results
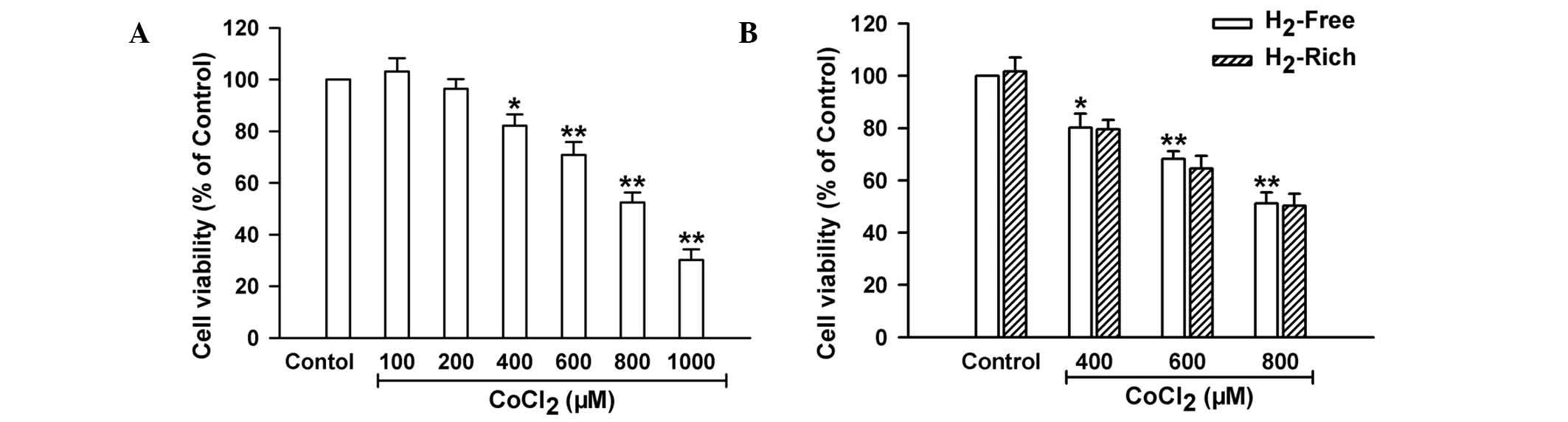
H2 gas-rich medium does not
affect chemical hypoxia-induced myocardial injury
Hypoxemia can be imitated in vitro by
chemical hypoxia or SGD treatment. H2 gas protection in
chemical hypoxia-induced myocardial injury was firstly assessed by
treatment of H9c2 cells with CoCl2 in H2
gas-rich medium. As shown in Fig.
1A, treatment of H9c2 cells with increasing concentrations of
CoCl2 significantly reduced cell viability at
concentrations of 400–1,000 μM. In order to observe H2
gas effectiveness, the cell culture medium was saturated with
H2 gas generated by electrolysis of water. No difference
in cell viability between the cells cultivated in H2
gas-rich medium and those cultivated in H2 gas-free
medium was identified under the conditions of CoCl2
exposure or rest (Fig. 1B). This
result indicated that H2 gas treatment did not influence
chemical hypoxia-induced myocardial injury.
H2 gas-rich medium alleviates
SGD-induced cell injury
SGD treatment acts as another in vitro
hypoxemia model by withdrawing FBS and glucose to mimic acute
infarction-induced myocardial injury in vivo. When the cells
were exposed to SGD for different time periods followed by further
culture for 30 h, the cell viability of H9c2 cells was
significantly reduced (P<0.01; Fig.
2A). Following exposure to SGD for 6–18 h, the H9c2 cells were
cultured in H2 gas-rich medium for 30 h. The findings
demonstrated that, compared with H2 gas-free culture,
H2 gas-rich culture significantly increased the
viability of the cells subjected to SGD for 6 or 12 h (P<0.05),
but not 18 h. The results suggested that when the cell injury
induced by SGD was not severe, H2 gas was able to
promote cell survival.
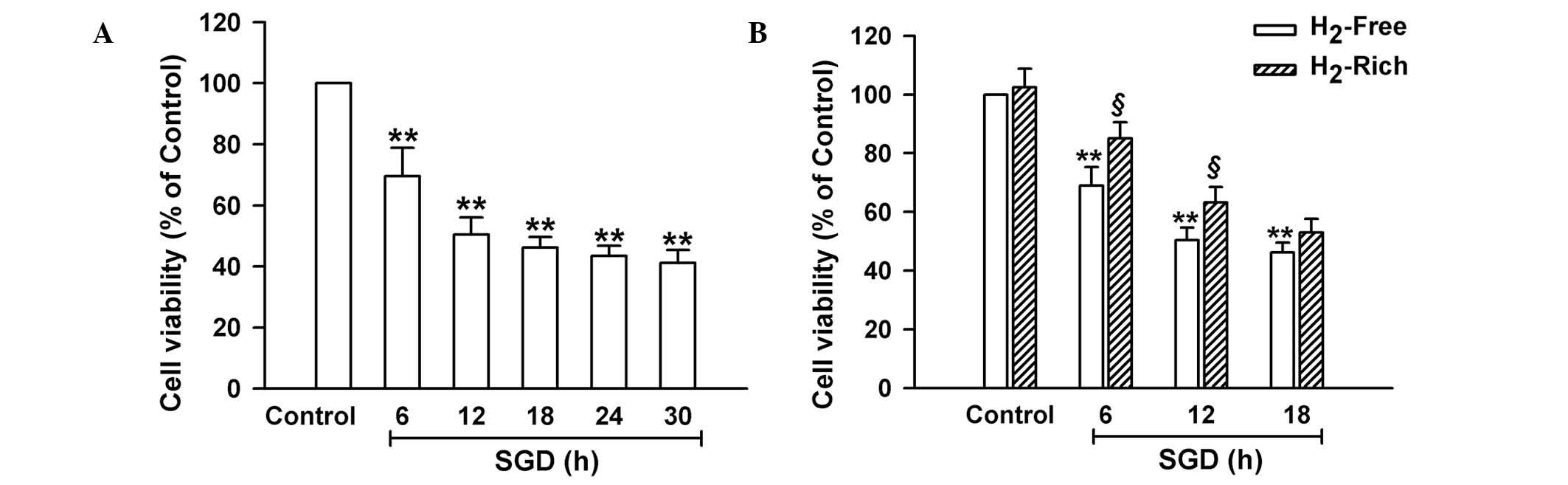 | Figure 2Effects of H2 gas on
SGD-induced injury in H9c2 cells. Following the indicated
treatments, cell viability was measured with a cell counting kit-8
assay. (A) H9c2 cells were exposed to SGD between 6 and 30 h,
followed by a further 30 h in normal culture. (B) H9c2 cells were
exposed to SGD for 6, 12 or 18 h, respectively, in the absence
(H2-Free) or presence (H2-Rich) of
H2 gas treatment. Data are presented as the mean ±
standard deviation, n=6. *P<0.05,
**P<0.01, compared with the control group.
§P<0.05, compared with the SGD group. SGD, serum and
glucose deprivation. |
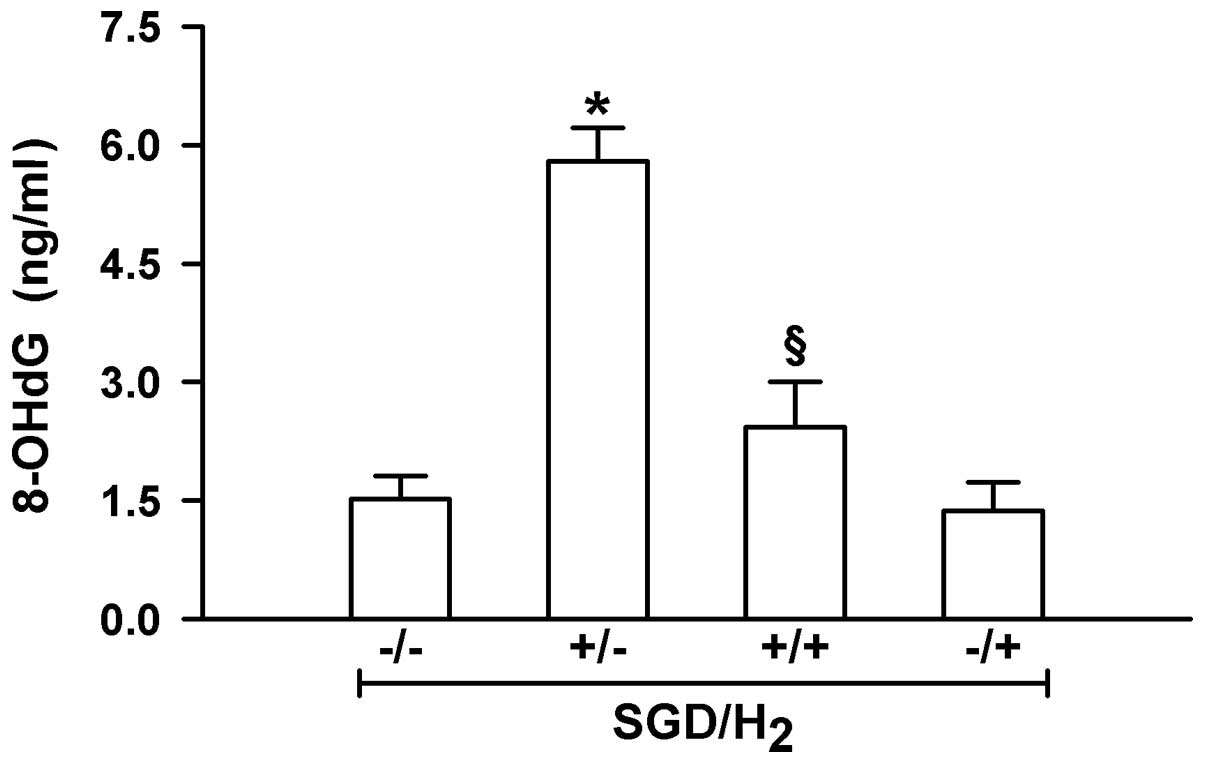
H2 gas-rich medium prevents
SGD-induced ·OH free radical generation
One mechanism underlying H2 protection is
ROS scavenging, particularly of ·OH free radicals. To investigate
whether H2 gas-mediated myocardial protection was
associated with scavenging ·OH free radicals, the ·OH levels in
H9c2 cells were measured using ELISA. As shown in Fig. 3, exposure of the cells to SGD
significantly enhanced intracellular ·OH levels (P<0.01).
However, this effectiveness was significantly inhibited by
incubation in H2 gas-rich medium (P<0.01). This
indicated that the elimination of ·OH free radicals may be an
important mechanism of myocardial protection by H2
gas.
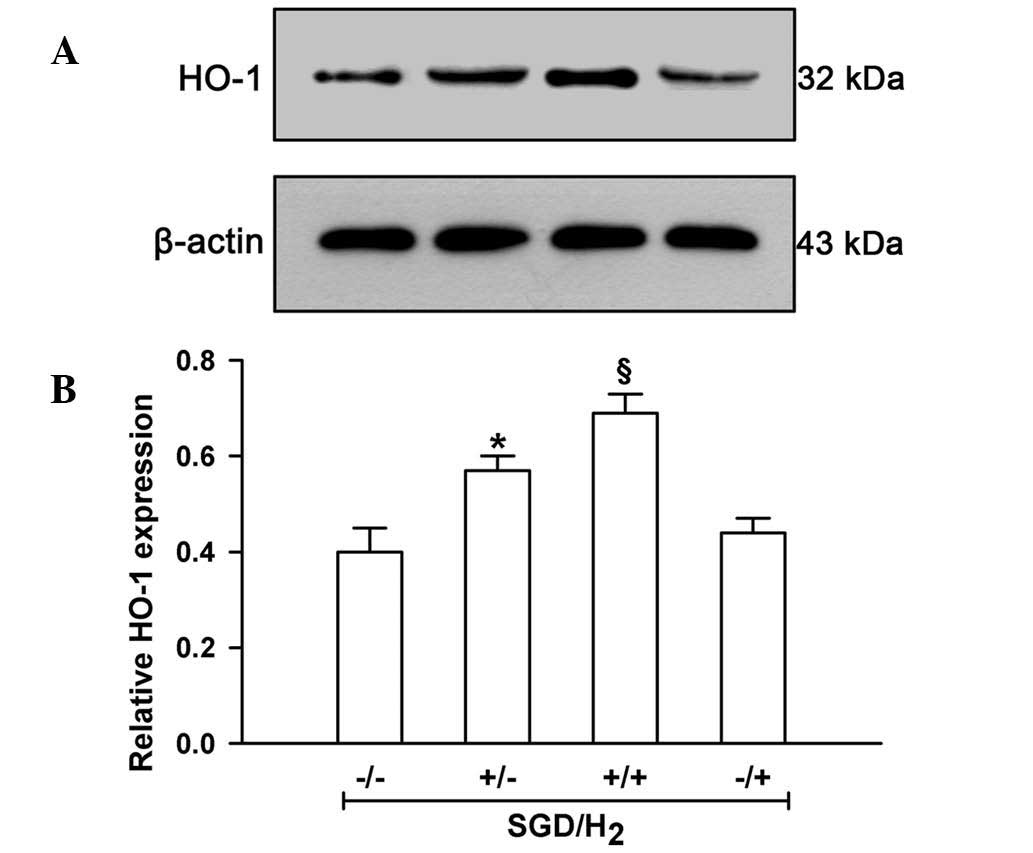
Adaptive HO-1 induction contributes to
H2 gas inhibition of SGD-induced injury in H9c2
cells
HO-1 is endogenously produced, functioning as an
antioxidation enzyme. In order to examine the role of HO-1 in
SGD-induced myocardial injury, experiments to detect HO-1 levels
were performed. The data in Fig. 4
show that exposure of H9c2 cells to SGD significantly upregulated
HO-1 levels when compared with the cells under normal conditions
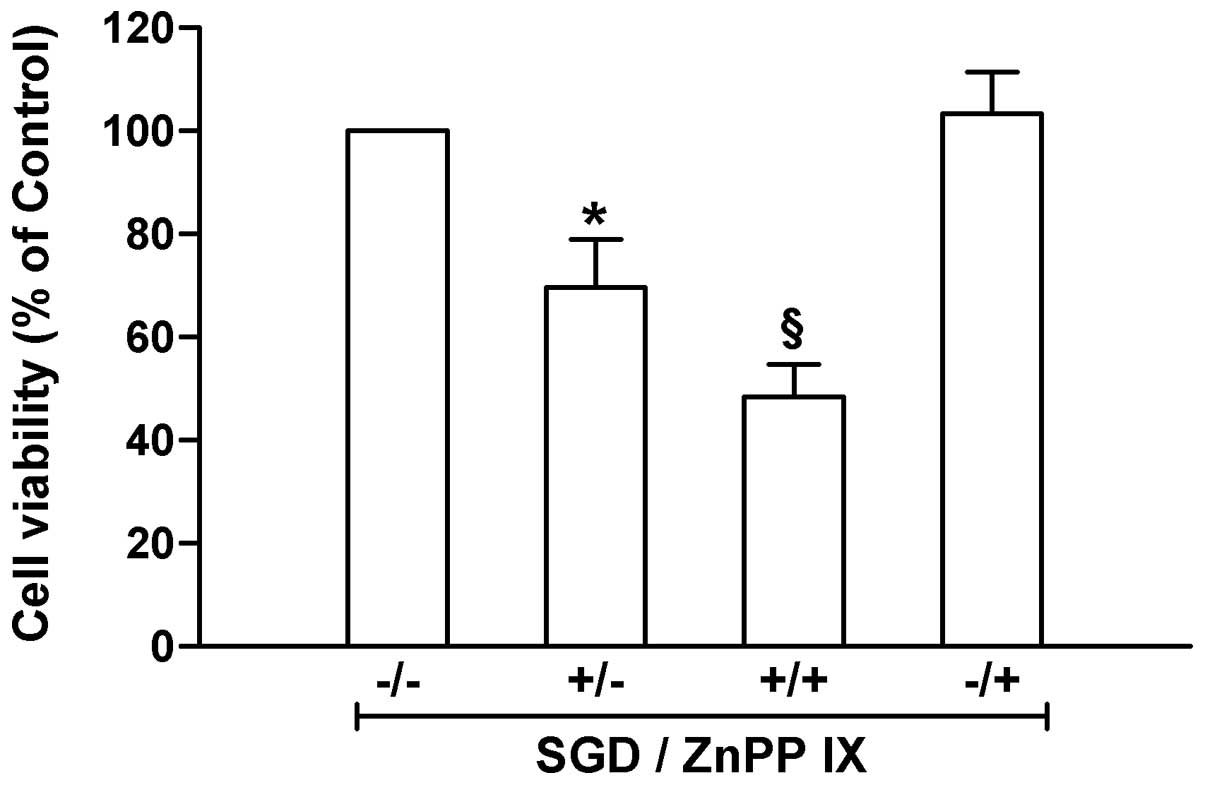
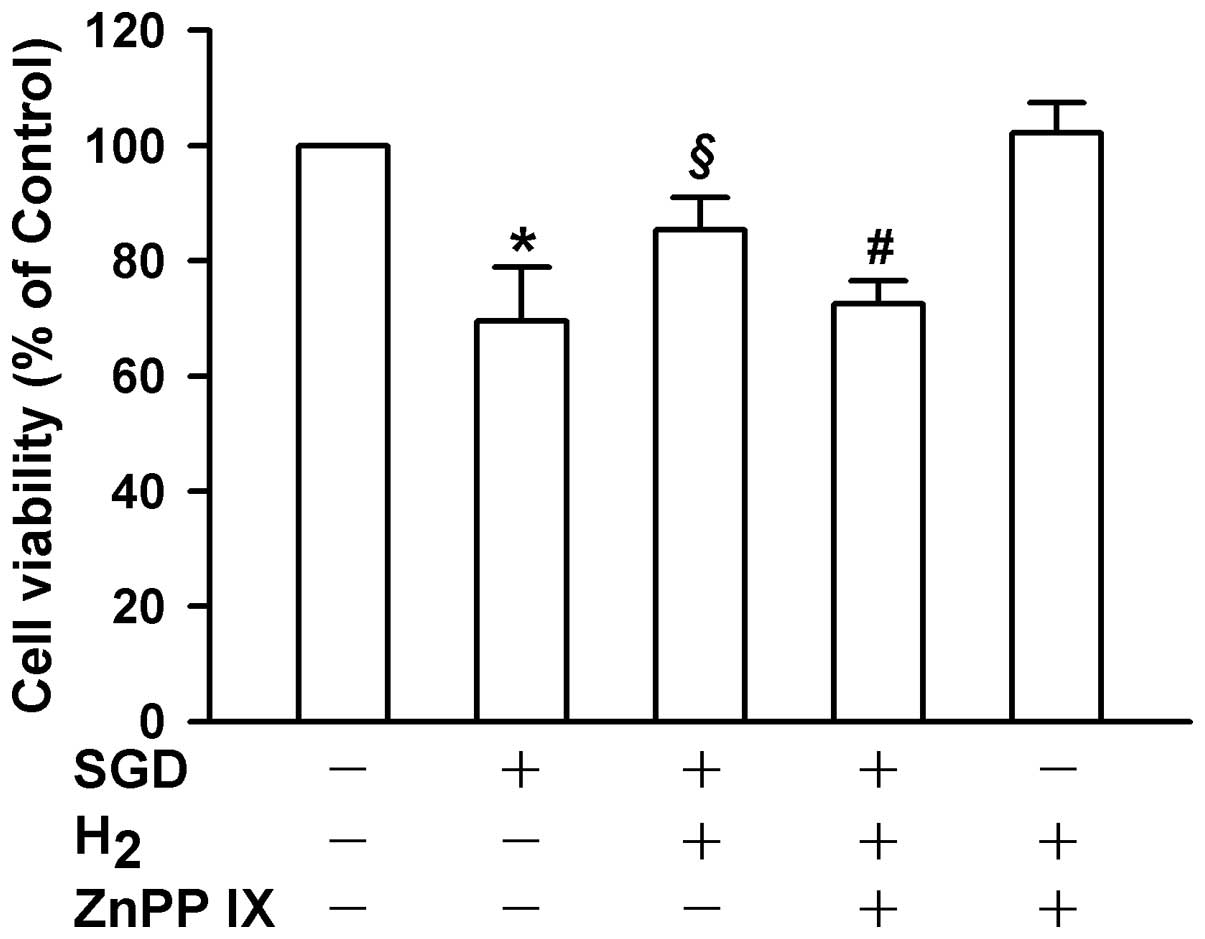
(P<0.01). When the HO-1 inhibitor ZnPP IX was administered,
SGD-induced cellular injury was found to be significantly
aggravated (P<0.05; Fig. 5).
These results suggested that HO-1 was beneficial in SGD-induced
injury in H9c2 cells.
Upregulation of HO-1 is involved in
H2 gas-induced myocardial protection
H2 gas-induced protection is not only
associated with simple elimination of ·OH free radicals, but also
with induction of endogenous genes, for instance, HO-1 (20,21).
To clarify whether HO-1 is involved in H2 gas-induced
myocardial protection, experiments were conducted to observe the
effect of H2 gas on SGD-triggered HO-1 upregulation and
the effect of HO-1 inhibition on H2 gas-induced
protection of H9c2 cells. The data in Fig. 4 reveal that exogenously applied
H2 gas significantly enhanced the upregulation elicited
by SGD in H9c2 cells (P<0.05). Notably, inhibition of HO-1 with
10 μM ZnPP IX significantly reduced the H2 gas-induced
increase in cellular viability following SGD treatment (P<0.05;
Fig. 6). The results suggested
that HO-1 at least partially mediated the protection from ischemia
provided by H2 gas in H9c2 cells.
H2 gas facilitates
nuclear Nrf2 expression induced by SGD in H9c2 cells.
Nrf2 is a transcription factor responsible for HO-1
expression. To address the role of Nrf2 in H2
gas-induced myocardial protection, the effect of H2
gas-rich medium on the changes of Nrf2 levels induced by SGD was
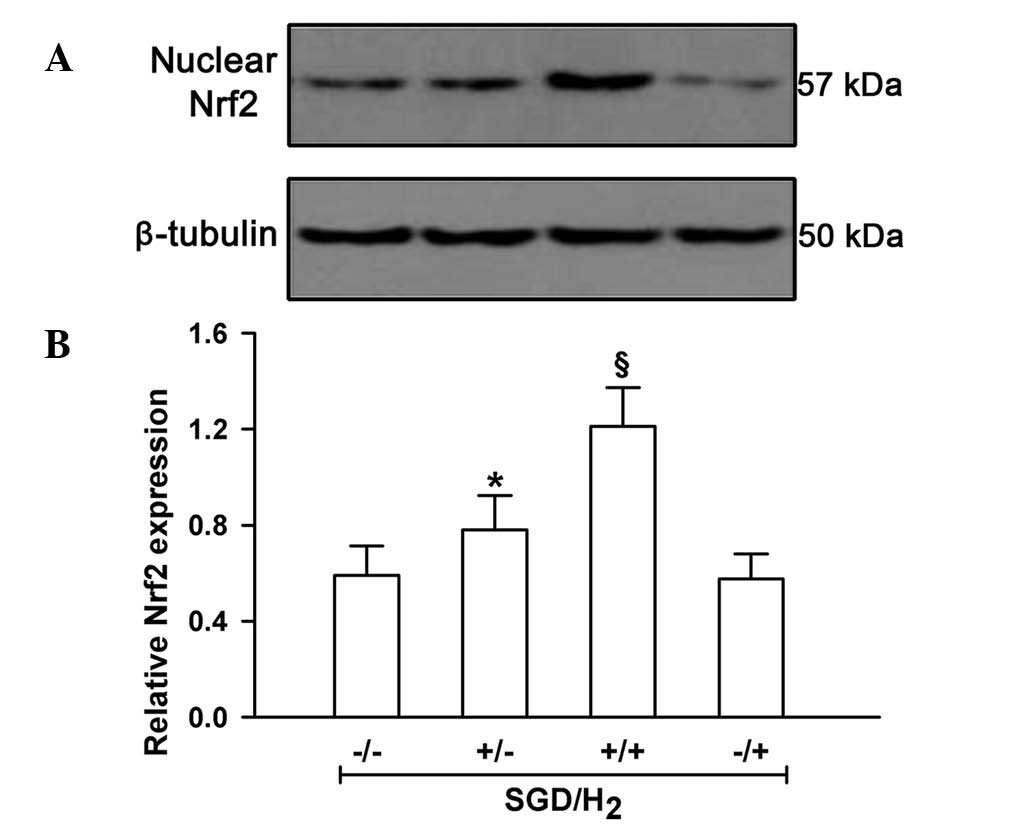
investigated. As shown in Fig. 7,
the SGD challenge resulted in a significant increase in nuclear
Nrf2 expression levels, indicating its activation under SGD
conditions in H9c2 cells (P<0.05). Notably, H2 gas
application following SGD exposure induced a significant increase
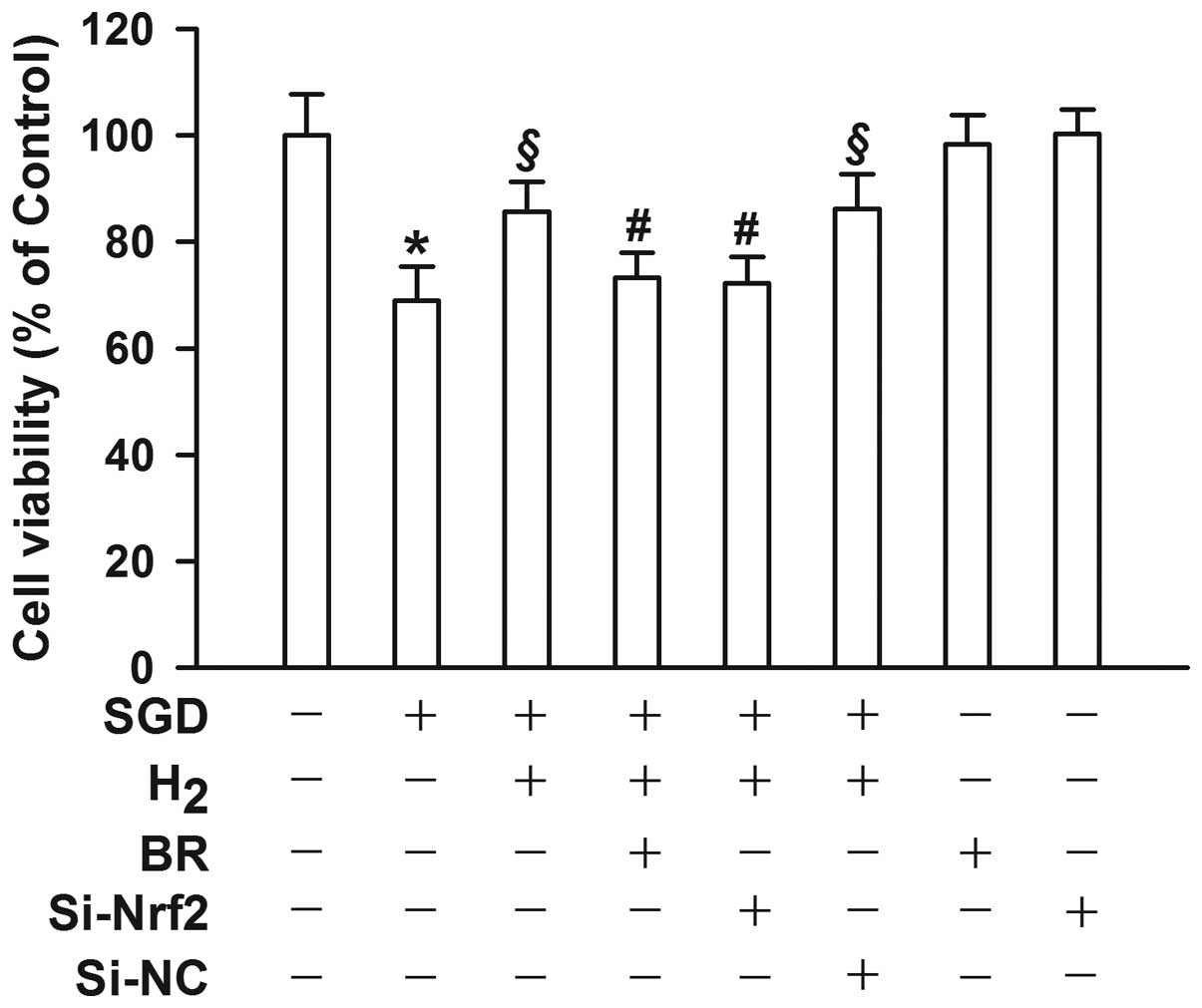
in nuclear Nrf2 expression levels (P<0.05). Inhibition of Nrf2
with 10 μM BR significantly reduced myocardial protection by
H2 gas (P<0.05, Fig.
8).
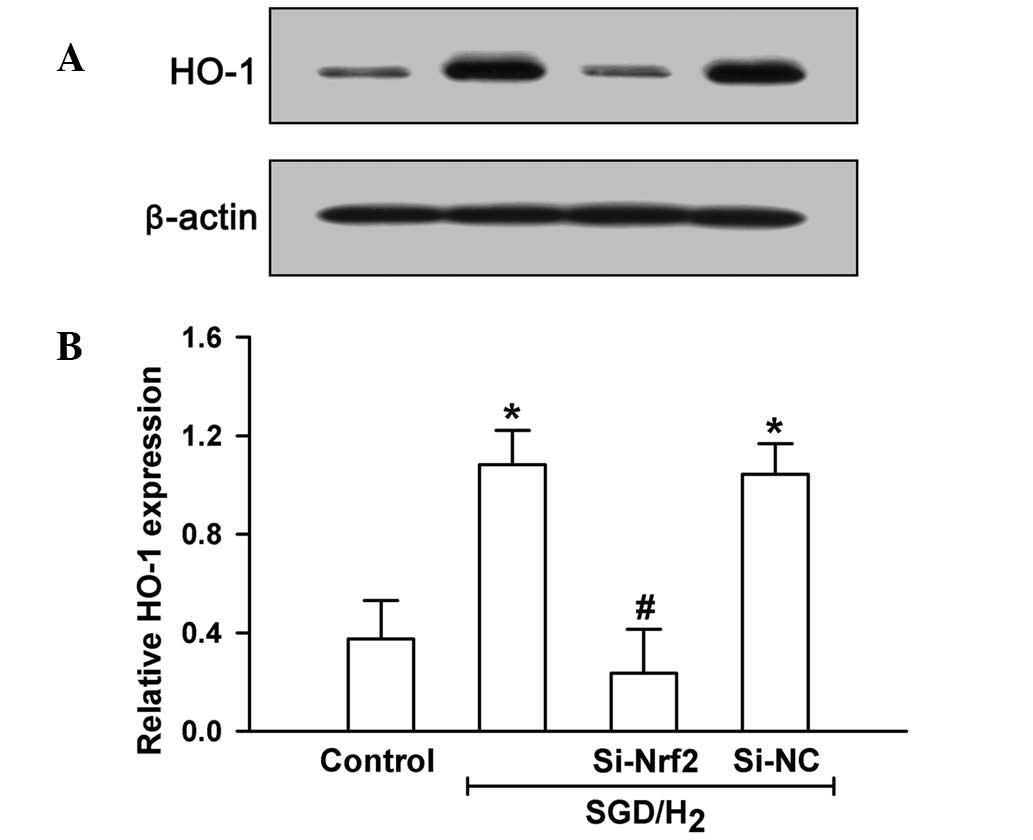
In addition, genetic silencing of Nrf2 by RNAi
(Si-Nrf2) also significantly inhibited H2 gas-elicited
HO-1 induction (P<0.05, Fig. 9)
and myocardial protective action (P<0.05, Fig. 8). These results indicated that the
H2 gas protection from SGD-induced injury in H9c2 cells
was at least in part mediated through the Nrf2/HO-1 signaling
pathway.
Discussion
The results of the present study suggested that
H2 gas exhibited myocardial protection against
ischemia-induced injury in H9c2 cells in vitro through
elimination of ·OH free radicals and activation of the Nrf2/HO-1
signaling pathway. These findings provide further evidence of
H2 gas protection and deepen the understanding of the
molecular mechanisms involved.
H2 gas is a gas with novel medical
application, in addition to NO, CO and H2S, whose
cytoprotective effects have gradually gained attention (15). The cytoprotective effect of
H2 gas has been investigated in the nervous,
cardiovascular and digestive systems (11–14).
In the present study, myocardial protection by H2 gas
was investigated in two distinct models: Chemical hypoxia-induced
injury and SGD-induced injury in H9c2 cells. Treatment with
chemical hypoxia-mimicking agent CoCl2, at 400–1,000 μM
for 24 h, reduced cell viability in a concentration-dependent
manner, although no effect of H2 gas on
CoCl2-induced injury was observed. The effect of
H2 gas on SGD-induced injury was then analyzed. Notably,
H2 gas exerted marked myocardial protection, since its
application impeded SGD-induced injury in H9c2 cells. These
findings were in accordance with a study by Sun et al
(29) on cardiac
ischemia/reperfusion injury in a rat model. However, the findings
of the present study also indicated that although CoCl2
(25,26) and SGD (27) are frequently used in
hypoxia/ischemia in vitro models, they may possess markedly
different underlying injury mechanisms. In addition, as
H2 gas is a water- and lipid-soluble and simple
molecule, H2 gas may exhibit a greater clinical
antioxidative value than the water-soluble vitamin C and
lipid-soluble vitamin E.
Oxidative stress is critical in myocardial ischemic
damage through overproduction of ROS (2). ROS in mammals include
O2•−, H2O2 and ·OH.
O2•−, H2O2 ROS are
eliminated by corresponding enzymes. For example,
O2•− may be catalyzed into O2 and
H2O2 via dismutation (28), and H2O2, one
of the most powerful oxidizers, may be converted into
H2O by catalase or guaiacol peroxidase, but may also be
converted into ·OH. Although ·OH free radicals are toxic to cells,
enzymes responsible for ·OH elimination remain to be identified.
Therefore, it is important for antioxidants to eliminate ·OH free
radicals and/or inhibit the production of ·OH. In the present
study, exposure of H9c2 cells to SGD was found to significantly
increase intracellular ·OH free radical levels as identified
through assessment of 8-OHdG. The ·OH levels after SGD stimulation
were significantly reduced during cell cultivation in H2
gas-rich medium, in concurrence with previous reports (15,16,29).
Increasing evidence suggested that the
cytoprotective effect of H2 gas is not only associated
with simple elimination of ·OH free radicals, but also with
numerous signaling molecules (20,21).
A study on plants demonstrated that pretreatment with H2
gas enhanced the salt tolerance of arabidopsis through zinc-finger
transcription factor ZAT10/12 (30). Additionally, the HO-1 signaling
pathway has been observed to be involved in H2 gas
protection against paraquat-induced oxidative injury (21). In animals, Cai et al
(31) revealed that H2
gas treatment alleviated tumor necrosis factor-alpha-induced rat
osteoblast inflammatory injury via upregulation of SOD activity.
Furthermore, evidence has indicated that HO-1 induction mediated
H2 gas mitigation of rat lung injury resulting from
transplantation (20). In the
present study, SGD exposure was found to markedly increase HO-1
expression. The mechanism of HO-1 in SGD-induced myocardial insult
was further elucidated using a selective HO-1 inhibitor, ZnPP IX.
Since the data indicated that the addition of ZnPP IX markedly
aggravated the SGD-induced insult, HO-1 may exhibit a protective
action against ischemia, a hypothesis which is supported by the
results of a study by Hwa et al (32). Notably, exogenously applied
H2 gas was found to result in a further increase in HO-1
expression; application of ZnPP IX partially abolished this
H2 gas-triggered myocardial protection. Therefore,
H2 gas protection may be partially mediated by HO-1
induction. One study suggested that inhalation of H2 gas
combined with CO, a product of HO-1, enhanced its therapeutic
efficacy in ischemia/reperfusion-induced myocardial injury
(33). These findings, alongside
those of the present study, support the hypothesis that the
mechanisms underlying H2 gas-induced myocardial
protection are not limited to the elimination of the ·OH free
radical and may also include upregulation of protective genes.
Nrf2 belongs to the NF-E2 superfamily of nuclear
basic leucine zipper transcription factors. Under conditions of
oxidative stress or pharmacological stimuli, Nrf2, as an adaptive
response, regulates phase II gene expression of numerous enzymes
that serve to detoxify pro-oxidative stressors (34). In the promoter region of certain
genes, such as HO-1 and SOD, Nrf2 binds to the cis-acting
regulatory element or enhancer sequence and induces gene expression
(35). In the present study, SGD
exposure markedly induced nuclear Nrf2 expression, which was
further enhanced by H2 gas administration. When the
action of Nrf2 was inhibited by BR, H2 gas-induced
myocardial protection was significantly attenuated. Studies have
indicated that BR may inhibit the Nrf2 signaling pathway (36,37).
In addition, genetic silencing of Nrf2 may also impede
H2-induced HO-1 expression and increases in cell
viability. These data suggested that the activation of Nrf2 is
involved in H2 gas protection and HO-1 induction. One
study observed that another medical gas, H2S, protected
cardiomyocytes against ischemia-induced injury by activation of the
Nrf2 signaling pathway (38).
Nrf2-mediated myocardial protection has also been reported
regarding a number of other compounds (39,40).
For instance, in the nervous system, curcumin protected rat brains
against focal ischemia via upregulation of the Nrf2/HO-1 signaling
pathway (41). Therefore, the
upregulation of the Nrf2/HO-1 signaling pathway is considered to be
a general antioxidative mechanism of numerous drugs and compounds,
and this signaling pathway may represent a novel molecular target
of drug design.
In conclusion, the present study suggested that
H2 gas application not only directly scavenged ·OH free
radicals but also enhanced the expression of proteins of the
Nrf2/HO-1 signaling pathway in SGD-stimulated H9c2 cells. These
findings provided basic information for the development of novel
treatments of ischemic myocardial injury with H2
gas.
Acknowledgements
This study was supported by Science & Technology
Planning Project of Guangdong Province in China (no.
2012A030400033).
References
|
1
|
Shibata R, Sato K, Pimentel DR, et al:
Adiponectin protects against myocardial ischemia-reperfusion injury
through AMPK- and COX-2-dependent mechanisms. Nat Med.
11:1096–1103. 2005. View
Article : Google Scholar
|
|
2
|
Yang XY, Zhao N, Liu YY, et al: Inhibition
of NADPH oxidase mediates protective effect of cardiotonic pills
against rat heart ischemia/reperfusion injury. Evid Based
Complement Alternat Med. 2013:7280202013.PubMed/NCBI
|
|
3
|
Dickinson BC and Chang CJ: Chemistry and
biology of reactive oxygen species in signaling or stress
responses. Nat Chem Biol. 7:504–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devasagayam TP, Tilak JC, Boloor KK, Sane
KS, Ghaskadbi SS and Lele RD: Free radicals and antioxidants in
human health: current status and future prospects. J Assoc
Physicians India. 52:794–804. 2004.PubMed/NCBI
|
|
5
|
Martinez-Outschoorn UE, Balliet RM,
Rivadeneira DB, et al: Oxidative stress in cancer associated
fibroblasts drives tumor-stroma co-evolution: A new paradigm for
understanding tumor metabolism, the field effect and genomic
instability in cancer cells. Cell Cycle. 9:3256–3276. 2010.
View Article : Google Scholar
|
|
6
|
Ryter SW, Kim HP, Hoetzel A, et al:
Mechanisms of cell death in oxidative stress. Antioxid Redox
Signal. 9:49–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jha P, Flather M, Lonn E, Farkouh M and
Yusuf S: The antioxidant vitamins and cardiovascular disease. A
critical review of epidemiologic and clinical trial data. Ann
Intern Med. 123:860–872. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hamilton KL: Antioxidants and
cardioprotection. Med Sci Sports Exerc. 39:1544–1553. 2007.
View Article : Google Scholar
|
|
9
|
Padayatty SJ, Katz A, Wang Y, et al:
Vitamin C as an antioxidant: evaluation of its role in disease
prevention. J Am Coll Nutr. 22:18–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ingold KU, Webb AC, Witter D, Burton GW,
Metcalfe TA and Muller DP: Vitamin E remains the major
lipid-soluble, chain-breaking antioxidant in human plasma even in
individuals suffering severe vitamin E deficiency. Arch Biochem
Biophys. 259:224–225. 1987. View Article : Google Scholar
|
|
11
|
Wang C, Li J, Liu Q, et al: Hydrogen-rich
saline reduces oxidative stress and inflammation by inhibit of JNK
and NF-κB activation in a rat model of amyloid-beta-induced
Alzheimer’s disease. Neurosci Lett. 491:127–132. 2011.PubMed/NCBI
|
|
12
|
Zheng X, Mao Y, Cai J, et al:
Hydrogen-rich saline protects against intestinal
ischemia/reperfusion injury in rats. Free Radic Res. 43:478–484.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Q, Shen WF, Sun HY, et al:
Hydrogen-rich saline protects against liver injury in rats with
obstructive jaundice. Liver Int. 30:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu P, Wang Z, Sun X, et al: Hydrogen-rich
medium protects human skin fibroblasts from high glucose or
mannitol induced oxidative damage. Biochem Biophys Res Commun.
409:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohsawa I, Ishikawa M, Takahashi K, et al:
Hydrogen acts as a therapeutic antioxidant by selectively reducing
cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar
|
|
16
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar
|
|
17
|
Ambrosio G, Becker LC, Hutchins GM,
Weisman HF and Weisfeldt ML: Reduction in experimental infarct size
by recombinant human superoxide dismutase: insights into the
pathophysiology of reperfusion injury. Circulation. 74:1424–1433.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang X, Xiao Z, Yao J, Zhao G, Fa X and
Niu J: Participation of protein kinase C in the activation of Nrf2
signaling by ischemic preconditioning in the isolated rabbit heart.
Mol Cell Biochem. 372:169–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hui Y, Zhao Y, Ma N, et al: M3-mAChR
stimulation exerts anti-apoptotic effect via activating the
HIF-1α/HO-1/VEGF signaling pathway in H9c2 rat ventricular cells. J
Cardiovasc Pharmacol. 60:474–482. 2012.PubMed/NCBI
|
|
20
|
Kawamura T, Wakabayashi N, Shigemura N, et
al: Hydrogen gas reduces hyperoxic lung injury via the Nrf2 pathway
in vivo. Am J Physiol Lung Cell Mol Physiol. 304:L646–L656. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jin Q, Zhu K, Cui W, Xie Y, Han B and Shen
W: Hydrogen gas acts as a novel bioactive molecule in enhancing
plant tolerance to paraquat-induced oxidative stress via the
modulation of heme oxygenase-1 signalling system. Plant Cell
Environ. 36:956–969. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Branco AF, Pereira SL, Moreira AC, Holy J,
Sardão VA and Oliveira PJ: Isoproterenol cytotoxicity is dependent
on the differentiation state of the cardiomyoblast H9c2 cell line.
Cardiovasc Toxicol. 11:191–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang G, Zhou J, Zhan W, et al: The
neuroprotective effects of intraperitoneal injection of hydrogen in
rabbits with cardiac arrest. Resuscitation. 84:690–695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang C, Yang Z, Zhang M, et al: Hydrogen
sulfide protects against chemical hypoxia-induced cytotoxicity and
inflammation in HaCaT cells through inhibition of ROS/NF-κB/COX-2
pathway. PLoS One. 6:e219712011.PubMed/NCBI
|
|
25
|
Chen SL, Yang CT, Yang ZL, et al: Hydrogen
sulphide protects H9c2 cells against chemical hypoxia-induced
injury. Clin Exp Pharmacol Physiol. 37:316–321. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang Z, Yang C, Xiao L, et al: Novel
insights into the role of HSP90 in cytoprotection of H2S against
chemical hypoxia-induced injury in H9c2 cardiac myocytes. Int J Mol
Med. 28:397–403. 2011.PubMed/NCBI
|
|
27
|
Yao LL, Wang YG, Cai WJ, Yao T and Zhu YC:
Survivin mediates the anti-apoptotic effect of delta-opioid
receptor stimulation in cardiomyocytes. J Cell Sci. 120:895–907.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Elchuri S, Oberley TD, Qi W, et al:
CuZnSOD deficiency leads to persistent and widespread oxidative
damage and hepatocarcinogenesis later in life. Oncogene.
24:367–380. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Q, Kang Z, Cai J, et al: Hydrogen-rich
saline protects myocardium against ischemia/reperfusion injury in
rats. Exp Biol Med (Maywood). 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie Y, Mao Y, Lai D, Zhang W and Shen W:
H2 enhances arabidopsis salt tolerance by manipulating
ZAT10/12-mediated antioxidant defence and controlling sodium
exclusion. PLoS One. 7:e498002012.
|
|
31
|
Cai WW, Zhang MH, Yu YS and Cai JH:
Treatment with hydrogen molecule alleviates TNFα-induced cell
injury in osteoblast. Mol Cell Biochem. 373:1–9. 2013.PubMed/NCBI
|
|
32
|
Hwa JS, Jin YC, Lee YS, et al:
2-methoxycinnamaldehyde from Cinnamomum cassia reduces rat
myocardial ischemia and reperfusion injury in vivo due to HO-1
induction. J Ethnopharmacol. 139:605–615. 2012.
|
|
33
|
Nakao A, Kaczorowski DJ, Wang Y, et al:
Amelioration of rat cardiac cold ischemia/reperfusion injury with
inhaled hydrogen or carbon monoxide, or both. J Heart Lung
Transplant. 29:544–553. 2010. View Article : Google Scholar
|
|
34
|
Fisher CD, Augustine LM, Maher JM, et al:
Induction of drug-metabolizing enzymes by garlic and allyl sulfide
compounds via activation of constitutive androstane receptor and
nuclear factor E2-related factor 2. Drug Metab Dispos. 35:995–1000.
2007. View Article : Google Scholar
|
|
35
|
Kang KW, Lee SJ and Kim SG: Molecular
mechanism of nrf2 activation by oxidative stress. Antioxid Redox
Signal. 7:1664–1673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauer AK, Hill T III and Alexander CM: The
involvement of NRF2 in lung cancer. Oxid Med Cell Longev.
2013:7464322013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ren D, Villeneuve NF, Jiang T, et al:
Brusatol enhances the efficacy of chemotherapy by inhibiting the
Nrf2-mediated defense mechanism. Proc Natl Acad Sci USA.
108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Calvert JW, Jha S, Gundewar S, et al:
Hydrogen sulfide mediates cardioprotection through Nrf2 signaling.
Circ Res. 105:365–374. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Deng C, Sun Z, Tong G, et al: α-Lipoic
acid reduces infarct size and preserves cardiac function in rat
myocardial ischemia/reperfusion injury through activation of
PI3K/Akt/Nrf2 pathway. PLoS One. 8:e583712013.
|
|
40
|
Cui G, Shan L, Hung M, et al: A novel
Danshensu derivative confers cardioprotection via PI3K/Akt and Nrf2
pathways. Int J Cardiol. 168:1349–1359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang C, Zhang X, Fan H and Liu Y: Curcumin
upregulates transcription factor Nrf2, HO-1 expression and protects
rat brains against focal ischemia. Brain Res. 1282:133–141. 2009.
View Article : Google Scholar : PubMed/NCBI
|