Introduction
Gliomas are the most common tumor types of the
central nervous system with a low 5-year survival rate and high
morbidity rate (1). The current
clinical treatments for glioma include surgery, radiotherapy and
chemotherapy (2). However,
accumulating evidence shows that the resistance of glioma cells to
conventional drugs is becoming a problem and it is imperative to
find novel and effective drugs against glioma (3).
Traditional Chinese Medicinal plants are a
significant source of drugs that serve as potential therapeutic
compounds for the treatment of cancer (4). The rhizome of Curcuma aromatica
Salisb. is widely used as a traditional herbal medicine for
anti-tumor therapy in China, Japan and other Asian countries
(5). Findings of recent studies
have demonstrated that germacrone, a major bioactive component of
Curcuma aromatica Salisb., possesses anti-tumor,
anti-inflammatory and neuroprotective properties. Claeson et
al (6) found that germacrone
had an obvious anti-inflammatory activity against
carrageenan-induced hind paw edema in rats. Matsuda et al
(7) and Morikawa et al
(8) reported that germacrone
exerted a potent protective effect on acute liver injury in mice
induced by D-galactosamine/lipopolysaccharide and tumor necrosis
factor-α. Another study showed that the treatment of HepG2 and
Bel7402 hepatoma cell lines with germacrone promoted apoptosis
associated with the upregulation of bax and the downregulation of
bcl-2. The upregulation of p53 and an increase in reactive oxygen
species were observed, which suggested that germacrone is a novel
potent drug candidate for liver cancer (9). Furthermore, germacrone was found to
inhibit the proliferation of breast cancer cell lines MCF-7 and
MDA-MB-231 by inducing cell cycle arrest in the G0/G1 and G2/M
phase as well as apoptosis through a mitochondria-mediated caspase
pathway (10). However, to the
best of our knowledge, this is the first study to investigate the
inhibitory effect of germacrone on human glioma cells. Therefore,
in the present study, the anti-proliferative effect of germacrone
on glioma cells and normal human astrocytes as well as the
mechanism of action of the anti-tumor activity were
investigated.
Materials and methods
Germacrone (>95%) was purchased from Sigma (St.
Louis, MO, USA). RPMI-1640 culture medium, Dulbecco’s medium
Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate-buffered
saline (PBS), penicillin-streptomycin (PS) and 0.25% (w/v)
trypsin/1 mM EDTA were purchased from Gibco (Grand Island, NY,
USA).
Cell lines and culture
The U87 and U251 human glioma cell lines were
obtained from the Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in DMEM
supplemented with 10% FBS, 2 mmol/l glutamine, penicillin (100
U/ml) and streptomycin (100 mg/ml), and maintained at 37°C and 5%
CO2 in a humid environment. Normal human astrocytes
(NHA) were obtained as part of the human astrocytes kit (Gibco) and
cultured in astrocyte medium.
MTT assay
Cell proliferation was evaluated by MTT assay.
Briefly, the cells were seeded into 96-well plates at a density of
3×104 (cells/well) and left to adhere overnight. The
cells were then incubated with or without 0–250 μmol/l germacrone
prior to addition of 10 ml of 5 mg/ml MTT and incubation in the
dark at 37°C for 2 h. Absorbance was determined at a wavelength of
492 nm.
Flow cytometric analysis
Cells were incubated with germacrone at different
concentrations (0–250 μmol/l) for 24 h. The cells were washed with
PBS, detached with trypsin and harvested. The cells were
resuspended in 1 ml Hoechst 33258 for 5 min and washed with PBS
three times. Apoptotic cells were detected by staining with annexin
V-FITC/PI according to the protocol of the annexin V-FITC cell
Apoptosis Detection Kit (BD Biosciences, Franklin Lakes, NJ
USA).
DNA fragmentation
For DNA laddering, following exposure to drug
treatment, 2×106 cells were suspended in PBS and
homogenized in buffer containing proteinase K and RNase.
Subsequently, cell lysates were incubated at 50°C for 30 min, and
isopropranol was added. DNA was precipitated and washed by 70%
ethanol. DNA was then analyzed using 1.2% agarose gel
electrophoresis.
Cell cycle
Cells were seeded at a density of 1.0×106
cells and incubated with germacrone at various concentrations
(0–250 μmol/l) for 24 h. Following two washes with PBS, the cells
were harvested and collected by centrifugation, followed by
fixation in ice-cold 70% ethanol at −20°C overnight. Cells were
then collected and stained with 100 μl PI solution for 30 min in
the dark followed by cell cycle analysis.
Western blot analysis
Following incubation with germacrone at various
concentrations (0–250 μmol/l), total cell lysates were prepared and
subjected to SDS-PAGE. For western blot analysis, primary
antibodies used included anti-p53, B-cell lymphoma 2 (bcl-2), bcl-2
associated X (bax), cyclin D1 and cyclin D kinase 2 (CDK2) (Cell
Signaling, Beverly, MA, USA) as well as p21 and GAPDH (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). Anti-rabbit or
anti-mouse secondary antibody conjugated with horseradish
peroxidase was also used (Pierce Chromatography Cartridges,
Rockford, IL, USA). Immunoreactive bands were detected using an
enhanced chemiluminescence (ECL) kit for western blot analysis and
the ChemiGenius bioimaging system (Syngene, Cambridge, UK).
Statistical analysis
Data were presented as the mean ± standard deviation
and processed for statistical analysis by the SPSS program (SPSS,
Inc., Chicago, IL, USA). A comparison between groups was made using
analysis of variance (ANOVA), and a statistically significant
difference between values was defined as P<0.05.
Results
Germacrone inhibits the proliferation of
human glioma cells
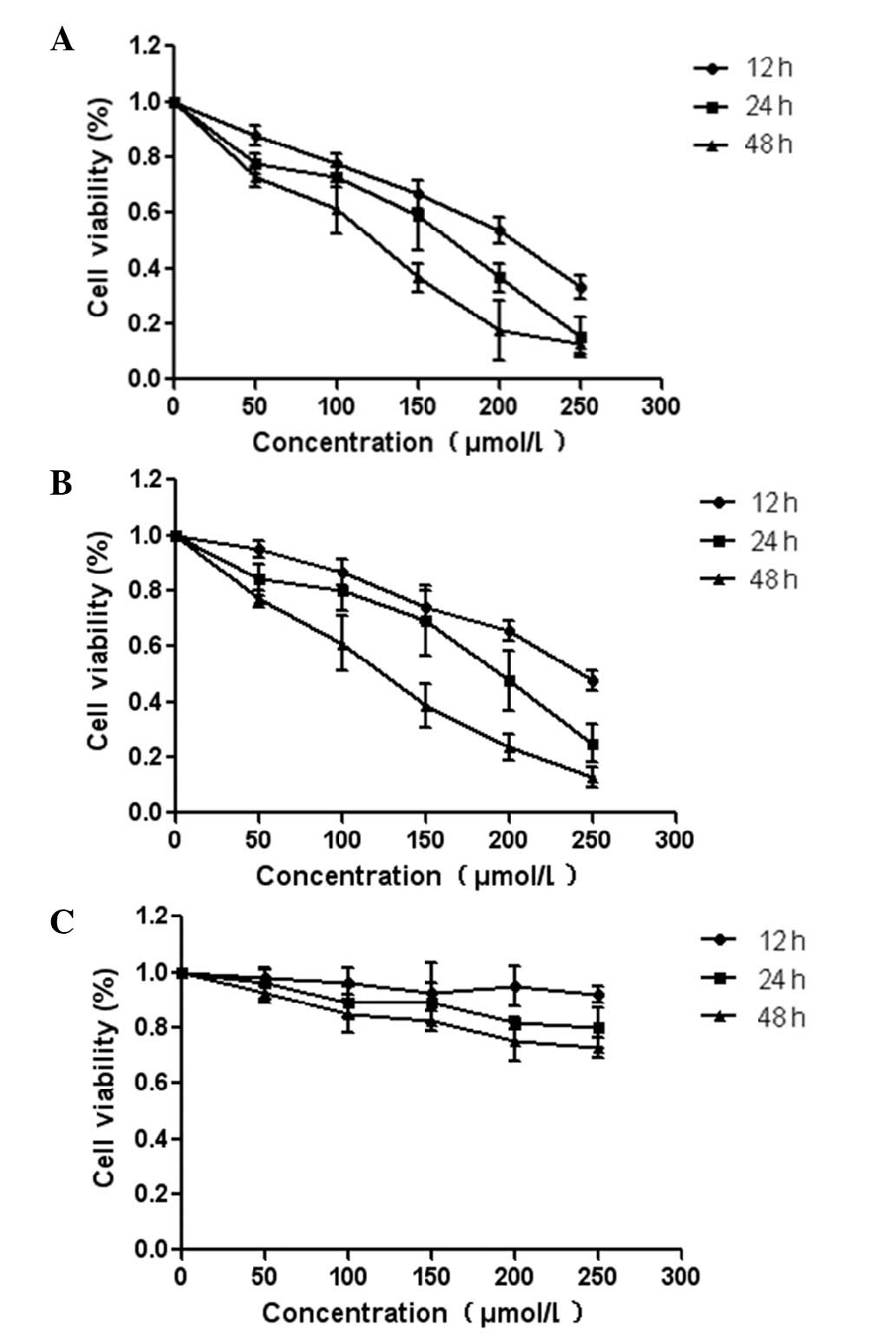
To investigate the effects of germacrone on the
proliferation of glioma cells, U87 and U251 cells were incubated
with 0–250 μmol/l of germacrone for different time periods using
the MTT assay. As shown in Fig. 1,
germacrone significantly inhibited the proliferation of U87 and
U251 cells in a time- and dose-dependent manner. Compared with
glioma cells, exposure of normal human astrocytes to germacrone
exerted no obvious inhibitory effect as shown in Fig. 1C.
Germacrone induces apoptosis in human
glioma cells
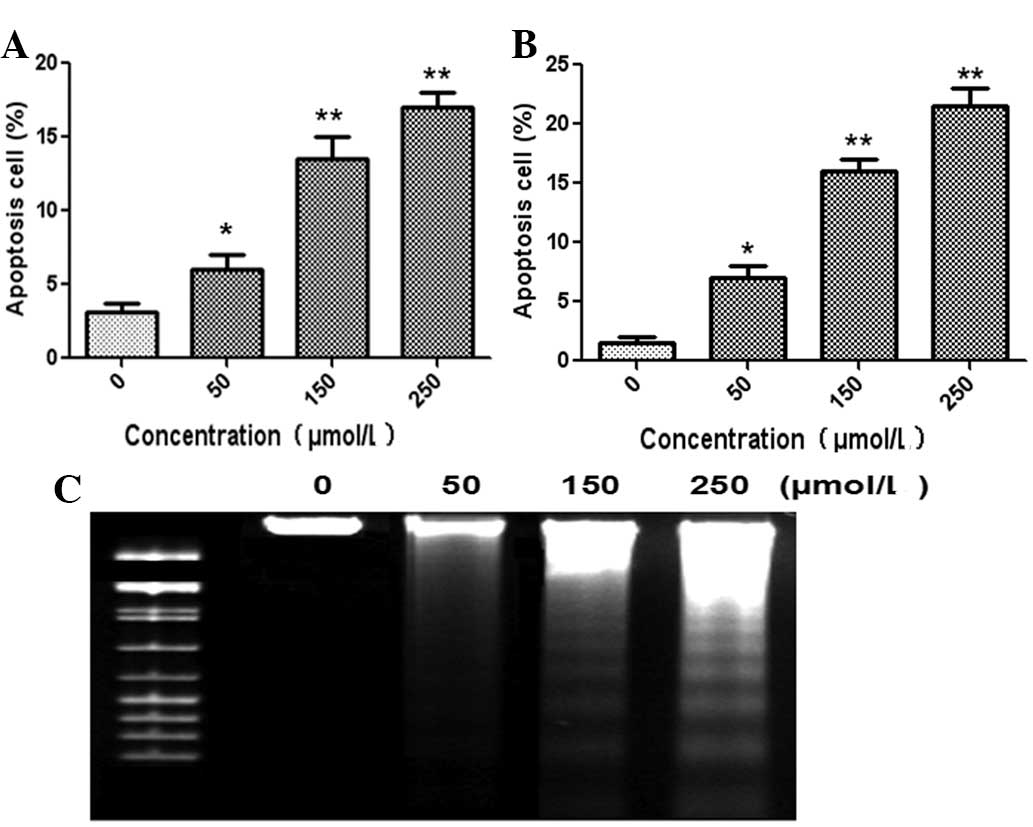
The apoptotic effect of germacrone on human glioma
cells was investigated by flow cytometry. The U87 and U251 cell
lines were incubated with germacrone at different concentrations
for 24 h and the apoptotic rate was analyzed using annexin V and PI
staining. As shown in Fig. 2,
treatment with germacrone (50, 150 and 250 μmol/l) for 24 h
significantly increased the apoptotic rates of U87 (Fig. 2A) and U251 (Fig. 2B) compared with the control. The
DNA fragmentation experiment also showed that germacrone induced
the apoptosis of U87 cells following incubation for 24 h at
different concentrations (Fig.
2C). Similar DNA ladders were also observed in U251 cells (data
not shown).
Germacrone induces cell cycle arrest in
G1 phase in glioma cells
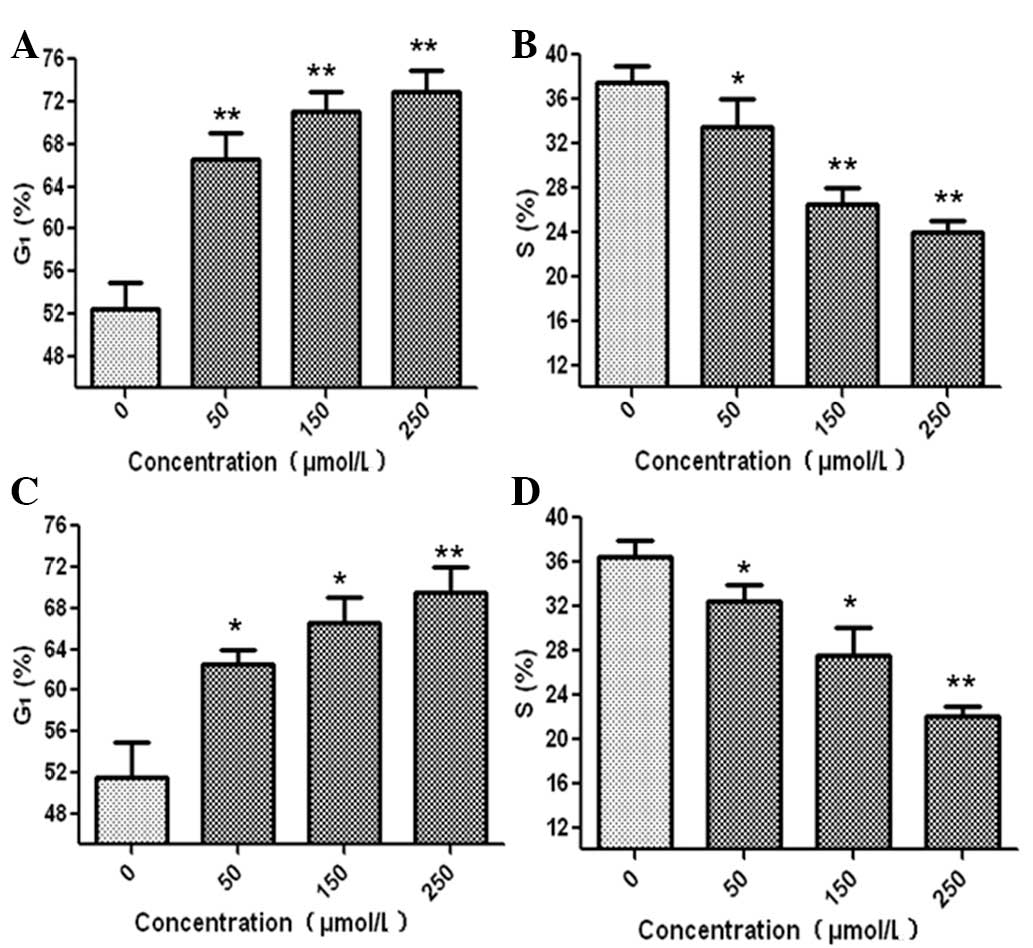
Human glioma cells were treated with germacrone at
different concentrations for 24 h to investigate its effect on the
cell cycle distribution. In the U87 human glioma cell line,
germacrone significantly increased the proportion of cells in G1
phase as compared with the control cells. The number of cells in S
phase decreased compared with the levels in the controls (Fig. 3A and B). Similar results were
observed in U251 cells following treatment with germacrone
(Fig. 3C and D). These results
demonstrated that germacrone induced cell cycle arrest in G1 phase
in human glioma cells.
Effect of germacrone on the expression of
apoptosis- and cell cycle-associated proteins
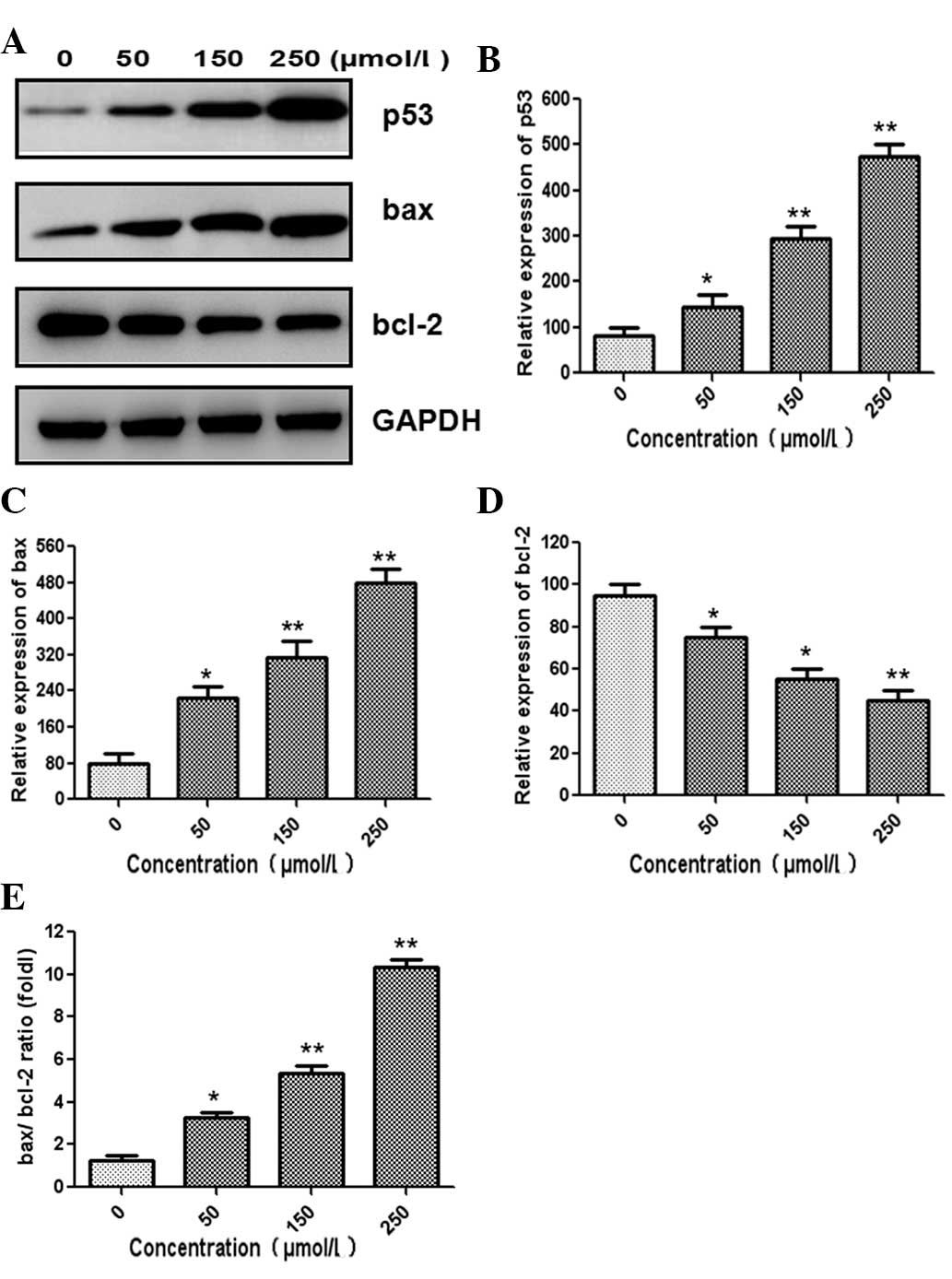
The expression levels of apoptosis- and cell
cycle-associated proteins were assessed using western blot
analysis. The expression of tumor suppressor gene p53 was
significantly increased (Fig. 4A and
B). As shown in Fig. 4C and D,
cells treated with germacrone showed an increase in bax expression
and a decrease in bcl-2 expression in a dose-dependent manner.
Densitometric analysis revelaed that the bax/bcl-2 ratio was
significantly increased in a dose-dependent manner following
treatment with germacrone (Fig.
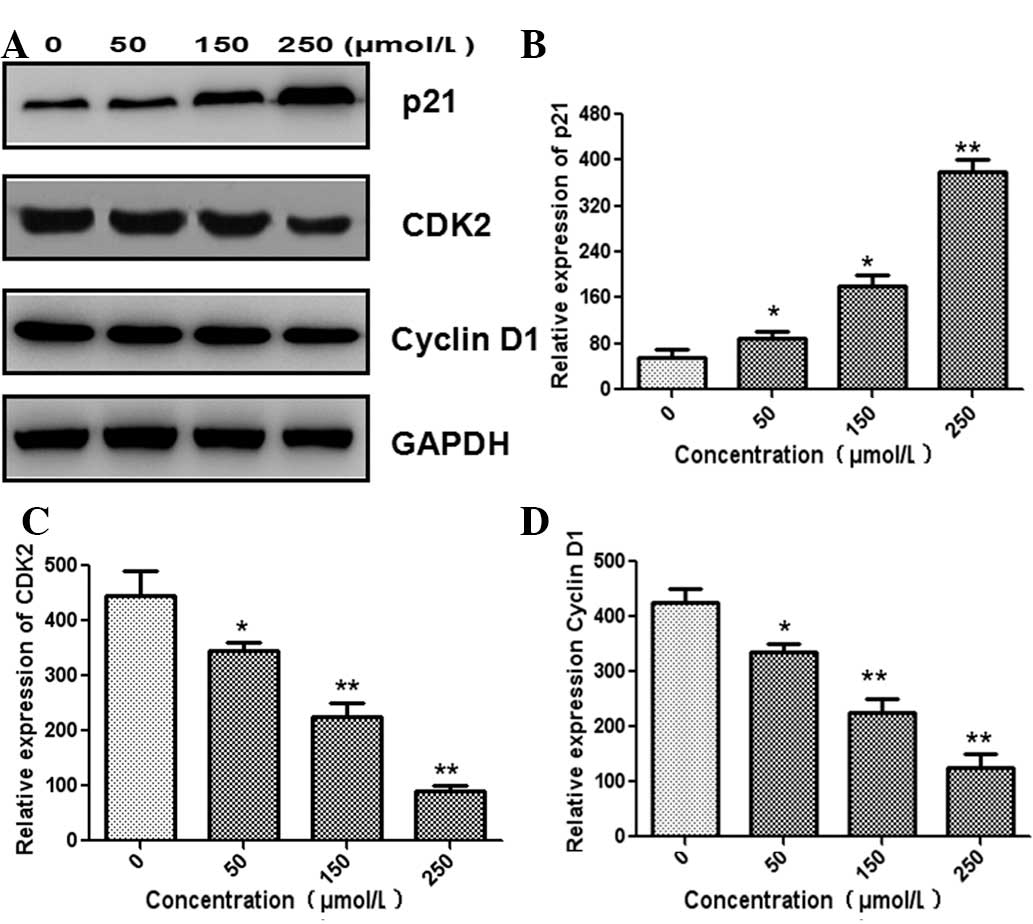
4E). The expression of p21 was significantly increased
following treatment with germacrone (Fig. 5A and B). The expression of G1-phase
regulatory proteins, including CDK2 and cyclin D1, significantly
decreased in a dose-dependent manner following treatment with
germacrone for 24 h (Fig. 5C and
D).
Discussion
Germacrone is a sesquiterpene derivative and has
been shown to be one of the most promising agents against several
types of cancer, including liver and breast cancer (9,10).
However, to the best of our knowledge, its effect on glioma and the
underlying molecular mechanism has not been previously explored. In
the present study, it was demonstrated for the first time that
germacrone inhibited the proliferation of glioma cells by inducing
cell cycle arrest in G1-phase and promoted apoptosis.
The MTT assay results revealed that germacrone
exerted a significant inhibitory effect on the growth of the human
glioma cell lines U87 and U251. However, no obvious effect on
normal human astrocytes was observed following treatment with
germacrone. Taken together, these data showed that germacrone
inhibited the proliferation of glioma cells.
Apoptosis is the process of programmed cell death
characterized by typical cellular and molecular features, including
shrinkage, externalization of phosphatidylserine and condensation
of chromatin (11). The present
study has demonstrated that germacrone treatment promoted apoptosis
of U87 and U251 as shown by flow cytometry, as well as DNA
fragmentation. Cell cycle is a regulated process controlled by cell
cycle checkpoints which ensure the fidelity of cell division in
various types of cells (12,13).
However, cell cycle arrest is able to be triggered by various
exogenous and endogenous stimulating factors and results in the
breakdown of cell division, cell death and/or apoptosis (14). In the present study, germacrone
induced cell cycle arrest in G1 phase, indicating another possible
mechanism by which germacrone inhibits the proliferation of glioma
cells.
The molecular mechanism of G1 cell cycle arrest and
apoptosis induced by germacrone in human glioma cells was further
investigated. It is well known that the protein p53, encoded by the
TP53 gene, has a crucial role in multi-cellular organisms, where it
regulates apoptosis and the cell cycle (15). When a cell is exposed to damage,
p53 is activated and inhibits various downstream target genes
involved in apoptosis and cell cycle arrest, including bax,
p53-upregulated modulator of apoptosis (PUMA), bcl-2 and p21
(16). Bax is a p53
primary-response gene, which is involved in a p53-regulated pathway
for the induction of apoptosis. p53 directly activates the
proapoptotic protein bax, which permeabilizes mitochondria and
engages in the apoptotic program (17). p53 also accumulates in the cytosol
to activate the expression of bax and subsequently triggers
apoptosis (18). The
anti-apoptotic protein bcl-2 has been shown to prevent the
disruption of mitochondrial physiology and block the release of
cytochrome C from mitochondria, which is a response gene of p53 and
involved in p53-regulated apoptosis (19). In the present study, germacrone
treatment resulted in an increase in p53 and bax expression and a
decrease in bcl-2 in a dose-dependent manner. The bax/bcl-2 ratio
was significantly increased in a dose-dependent manner following
treatment with germacrone. These results showed that germacrone
promoted apoptosis of glioma cells.
The protein p21 is a tumor suppressor and acts as an
inhibitor of cell cycle progression. It negatively regulates the
G1/S phase transition and its up-regulation results in cell cycle
arrest in G1 phase (20). In
association with CDK2 complexes, it inhibits kinase activity and
blocks cell cycle progression through G1/S arrest (21). CDK2 is a member of the
cyclin-dependent kinase family of Ser/Thr protein kinases and is
essential for the G1/S transition (22). The central role of CDK2 in the
regulation of the cell cycle makes it a promising indicator for
studying inhibitory molecules that are able to modify the degree of
cell proliferation. CDK2 has been shown to be a crucial regulator
of S-phase progression and was evaluated as an anticancer drug
target (23). Cyclin D1 promotes
progression through the G1-S phase of the cell cycle and the
over-expression of cyclin D1 has a pivotal role in the development
of numerous cancers, including breast, colon and prostate cancer,
as well as melanoma (24,25). The present study has shown that the
protein expression levels of cyclin D1 and CDK2 were significantly
decreased, while p21 expression was significantly increased,
suggesting that germacrone treatment induced cell cycle arrest in
G1 phase.
In conclusion, results of the present study have
demonstrated that germacrone inhibited the proliferation of glioma
cells by regulating the expression of proteins associated with cell
cycle arrest in G1 phase and apoptosis. Therefore, germacrone may
be a novel potential drug for the treatment of gliomas in the
future.
References
|
1
|
Wang Y, Zhou Z, Luo H, et al: Combination
of tamoxifen and antisense human telomerase RNA inhibits glioma
cell proliferation and anti-apoptosis via suppression of telomerase
activity. Mol Med Rep. 3:935–940. 2010.PubMed/NCBI
|
|
2
|
Krakstad C and Chekenya M: Survival
signalling and apoptosis resistance in glioblastomas: opportunities
for targeted therapeutics. Mol Cancer. 9:1352010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lefranc F, Facchini V and Kiss R:
Proautophagic drugs: a novel means to combat apoptosis-resistant
cancers, with a special emphasis on glioblastomas. Oncologist.
12:1395–1403. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu YH, Li ML, Hsu MY, et al: Effects of a
Chinese Herbal Medicine, Guan-Jen-Huang (Aeginetia indica
Linn.), on renal cancer cell growth and metastasis. Evid Based
Complement Alternat Med. 2012:9358602012.PubMed/NCBI
|
|
5
|
Bamba Y, Yun YS, Kunugi A and Inoue H:
Compounds isolated from Curcuma aromatica Salisb. inhibit
human P450 enzymes. J Nat Med. 65:583–587. 2011.
|
|
6
|
Claeson P, Panthong A, Tuchinda P, et al:
Three non-phenolic diarylheptanoids with anti-inflammatory activity
from Curcuma xanthorrhiza. Planta Med. 59:451–454. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsuda H, Ninomiya K, Morikawa T and
Yoshikawa M: Inhibitory effect and action mechanism of
sesquiterpenes from Zedoariae Rhizoma on
D-galactosamine/lipopolysaccharide-induced liver injury. Bioorg Med
Chem Lett. 8:339–344. 1998. View Article : Google Scholar
|
|
8
|
Morikawa T, Matsuda H, Ninomiya K and
Yoshikawa M: Medicinal foodstuffs. XXIX Potent protective effects
of sesquiterpenes and curcumin from Zedoariae Rhizoma on liver
injury induced by D-galactosamine/lipopolysaccharide or tumor
necrosis factor-alpha. Biol Pharm Bull. 25:627–631. 2002.
View Article : Google Scholar
|
|
9
|
Liu Y, Wang W, Fang B, et al: Anti-tumor
effect of germacrone on human hepatoma cell lines through inducing
G2/M cell cycle arrest and promoting apoptosis. Eur J Pharmacol.
698:95–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong Z, Chen X, Tan W, et al: Germacrone
inhibits the proliferation of breast cancer cell lines by inducing
cell cycle arrest and promoting apoptosis. Eur J Pharmacol.
667:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pang M, Wang ZL, Gao CL, Qu P and Li HD:
Characterization of apoptotic changes induced by yessotoxin in the
Bel7402 human hepatoma cell line. Mol Med Rep. 4:547–552.
2011.PubMed/NCBI
|
|
12
|
Gutierrez GJ, Tsuji T, Cross JV, et al:
JNK-mediated phosphorylation of Cdc25C regulates cell cycle entry
and G(2)/M DNA damage checkpoint. J Biol Chem. 285:14217–14228.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weinert T and Hopper AK: tRNA traffic
meets a cell-cycle checkpoint. Cell. 131:838–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanaka H, Arakawa H, Yamaguchi T, et al: A
ribonucleotide reductase gene involved in a p53-dependent
cell-cycle checkpoint for DNA damage. Nature. 404:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu W, Ge Y, Ojcius DM, et al: p53
signalling controls cell cycle arrest and caspase-independent
apoptosis in macrophages infected with pathogenic Leptospira
species. Cell Microbiol. 15:1642–1659. 2013.
|
|
16
|
Yan X, Feng J, Ye Z, Miao X, Li W and Yang
D: p53 siRNA inhibits apoptosis of U2OS cells treated with azurin.
Mol Med Rep. 4:1089–1094. 2011.PubMed/NCBI
|
|
17
|
Deng Y and Wu X: Peg3/Pw1 promotes
p53-mediated apoptosis by inducing Bax translocation from cytosol
to mitochondria. Proc Natl Acad Sci USA. 97:12050–12055. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gogada R, Prabhu V, Amadori M, Scott R,
Hashmi S and Chandra D: Resveratrol induces p53-independent,
X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein
oligomerization on mitochondria to initiate cytochrome c release
and caspase activation. J Biol Chem. 286:28749–28760. 2011.
View Article : Google Scholar
|
|
19
|
Tsutsui S, Yasuda K, Suzuki K, et al:
Bcl-2 protein expression is associated with p27 and p53 protein
expressions and MIB-1 counts in breast cancer. BMC Cancer.
6:1872006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pirngruber J and Johnsen SA: Induced G1
cell-cycle arrest controls replication-dependent histone mRNA 3′
end processing through p21, NPAT and CDK9. Oncogene. 29:2853–2863.
2010.PubMed/NCBI
|
|
21
|
Satyanarayana A, Hilton MB and Kaldis P:
p21 Inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase
DNA damage checkpoint. Mol Biol Cell. 19:65–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Kotoshiba S, Berthet C, Hilton MB
and Kaldis P: Rb/Cdk2/Cdk4 triple mutant mice elicit an alternative
mechanism for regulation of the G1/S transition. Proc Natl Acad Sci
USA. 106:486–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen XZ, Cao ZY, Chen TS, et al: Water
extract of Hedyotis Diffusa Willd suppresses proliferation of human
HepG2 cells and potentiates the anticancer efficacy of low-dose
5-fluorouracil by inhibiting the CDK2-E2F1 pathway. Oncol Rep.
28:742–748. 2012.
|
|
24
|
Fang Y, Cao Z, Hou Q, et al: Cyclin D1
downregulation contributes to anticancer rffect of isorhapontigenin
on human bladder cancer cells. Mol Cancer Ther. 12:1492–1503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grillo M, Bott MJ, Khandke N, et al:
Validation of cyclin D1/CDK4 as an anticancer drug target in MCF-7
breast cancer cells: Effect of regulated overexpression of cyclin
D1 and siRNA-mediated inhibition of endogenous cyclin D1 and CDK4
expression. Breast Cancer Res Treat. 95:185–194. 2006. View Article : Google Scholar : PubMed/NCBI
|