Introduction
Neuronal cell apoptosis is associated with various
neurological damaging factors, including radiation (1). Studies on the molecular mechanism of
neuronal cell apoptosis following radiation have enriched the
number of protective therapeutic strategies against
radiation-induced neuronal cell death (2). Unlike inflammation, apoptosis is a
‘programmed cell death’ mechanism, whereby enzymatic reactions lead
to cell apoptosis and phagocytes remove the debris without
stimulating an inflammatory response. The most important enzymes
involved in apoptosis are caspases, which hydrolyze important
structural and functional proteins of the cell, ultimately leading
to apoptosis. Caspases are synthesized in the cell as inactive
zymogens and require to be activated to be functional. There are
two pathways that stimulate the activation of caspases; the
extrinsic and intrinsic cascades (3,4). The
extrinsic pathway is associated with membrane receptors and their
ligands, and the intrinsic pathway is dependent on mitochondria
(5). The extrinsic pathway is
triggered by binding of death ligands, such as tumor necrosis
factor (TNF)-α, to death receptors of the TNF family, which results
in the assembly of a receptor-associated complex. The central
element in the mitochondrial pathway is a specialised protein
complex, the apoptosome, which enables and facilitates the
activation of procaspase 9. Once activated by their respective
upstream signals, caspase-8 and -9 may cleave and activate
downstream executioner caspases -3 and -7, which, in turn, cleave a
plethora of target proteins, resulting in apoptotic death (6–8).
Radiation and other agents induce caspase activation
fundamentally via the mitochondrial pathway, which includes
mitochondrial integration of apoptotic signals and the subsequent
release of cytochrome c into the cytosol (5,9,10).
The inhibitor of apoptosis proteins (IAPs) inhibit apoptosis by
interacting with and then regulating the functions of caspase-8 or
caspase-9, -3 and -7 (9,11,12).
z-VAD-fmk
(N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone) is a
powerful, irreversible and cell permeable inhibitor of caspases,
and has been demonstrated to directly block the activity of
caspases (13).
As a result, the present study aimed to investigate
changes in the expression of X-linked IAP (XIAP) induced by
radiation injury, the activity and expression of caspase members
following radiation and the effect of caspase blockade. In the
present study, a model of the nucleus of abducens nerve was
established to examine this.
Materials and methods
Radiation mode
The rats were housed in groups of four to five per
cage at a temperature- (24±1°C) and light-controlled room (12 h
light/dark cycle with lights on at 07:00 h). Food and water were
provided ad libitum before and after treatment. The animal
care and all experimental procedures were carried out in accordance
with the Guide for the Care and Use of Laboratory Animals published
by the US National Institutes of Health (publication no. 85-23,
revised 1996). Male Sprague-Dawley rats weighing 200–220 g were
obtained from the Experimental Animal Research Center, Institute of
Radiation Medicine, Chinese Academy of Medical Sciences and Peking
Union Medical College (Tianjin, P.R. China) and randomly divided
into three groups (six rats/group): the irradiation group (IR
group), the irradiation with z-VAD-fmk group (IR + Z-VAD group) and
the control group (con group). Irradiation was performed at room
temperature at a dose of radiation (4 Gy) with a Cr 137 r-ray
(Atomic Energy of Canada Ltd., Mississauga, ON, Canada) at a dose
rate of 0.71 Gy/min. The animals in the control group did not
receive any radiation.
Intracerebroventricular administration of
z-VAD-fmk
With a rat brain stereotaxic apparatus (Stoelting
Co., Wood Dale, IL, USA), the animals were implanted with a cannula
(AP=−2.4 mm, L=−1.4 mm, H=−3.0 mm) intracerebroventricularly
(i.c.v.) via an osmotic micropump (Alzet® micropump,
1007D; Durect Corporation, Cupertino, CA, USA). Infusion of 2 μg
z-VAD-fmk (BioVision, Inc., Milpitas, CA, USA) in 10 μl volume was
conducted at a rate of 0.2 μg/h for 1 h. The drug vehicle was 0.5%
dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS). The
infusions were performed at the onset of radiation administration
(13). The non-radiation controls
received PBS and vehicle i.c.v. and the radiation controls received
z-VAD-fmk. The animals were sacrificed 24 h following
administration of diazepam for further investigations.
Immunohistology and terminal
deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
staining
Brains were harvested and immediately frozen in
2-methylbutane (−30°C). The brainstem was cut into sections (12-μm
thick) with a Leica CM 3000 cryostat (Leica Microsystems, Wetzlar,
Germany) at the level of the nucleus of the abducens nerve
(14), and then stored at −80°C
until further use. Coronal sections were air dried for 15 min,
post-fixed in 10% formalin for 15 min, washed twice in PBS and then
processed for immunohistology with rabbit anti-XIAP (1:1,500
dilution; Abcam, Cambridge, MA, USA). The avidin-biotin-peroxidase
complex method was conducted as previously described (15, 16). For detection of DNA fragmentation,
the fluorescein-based TUNEL assay (Roche Molecular Biochemicals,
Indianapolis, IN, USA) was used. TUNEL staining was conducted
according to the manufacturer’s instructions. TUNEL cell counts
were performed on n=3 brain sections from the nuclei of the
abducens nerves. Images were visualized using a Leica microscope
(DMI3000B; Leica Microsystems, Wetzlar, Germany) under
excitation/emission wavelengths of 500/550 nm (green), captured
using an Optronics DEI-750 three-chip camera equipped with a BQ
8000 sVGA frame grabber and analyzed using Bioquant software
(Bioquant Image Analysis Corporation, Nashville, TN, USA).
Generation of cytosolic fraction
Twenty-four hours subsequent to irradiation, the
rats from each group were anesthetized with 10% chloral hydrate (30
mg/kg body weight) by intraperitoneal anesthesia and the brainstems
containing the nuclei of the abducens nerves were obtained. The
cytosolic fraction was performed as previously described (16).
Western blot analysis
The protein concentration of the supernatant
homogenate was determined using a Bio-Rad kit (Bio-Rad, Hercules,
CA, USA) at an absorbance of 595 nm with the Bradford method
(17). The samples (80 μg) were
transferred to polyvinylidene difluoride membranes and incubated
with the following primary antibodies: Rabbit polyclonal anti-XIAP
from Abcam (dilution, 1:500), rabbit anti-β-actin (dilution,
1:1,500; Sangon Biotech, Shanghai, China) and goat anti-rabbit
immunoglubulin G-conjugated to horseradish peroxidase (dilution,
1:800; ZSGB Biotechnology, Co., Ltd., Beijing, China).
RNA extraction, cDNA synthesis and
quantitative polymerase chain reaction (qPCR)
Total RNA was purified and extracted as conducted
previously by our laboratory (18). The expression of a target gene was
calculated by the comparative CT method [fold-change =
2(−ΔΔCT)]. The PCR primers for caspase-3, -8 and -9 as
well as the housekeeping gene GAPDH were obtained from Sangon
Biotech. The specific primer pairs used were: CASP3,
5′-ATCACAGCAAAAGGAGCAGTTT-3′ (forward) and
5′-ACACCACTGTCTGTCTCAATGC-3′ (reverse); CASP8,
5′-TAGGGACAGGAATGGAACACA-3′ (forward) and
5′-TGGGAGAGGATACAGCAGATG-3′ (reverse); CASP9,
5′-TCTGGAGGATTTGGTGATGTC-3′ (forward) and
5′-CATTTTCTTGGCAGTCAGGTC-3′ (reverse); GAPDH,
5′-ATGACATCAAGAAGGTGGTG-3′ (forward) and 5′-CATACCAGGAAATGAGCTTG-3′
(reverse).
Caspase activation assay
The activity of caspase-3, -8 and -9, was analyzed
using a fluorogenic caspase assay with
Ac-DEVD-amido-trifluoromethylcoumarin (AFC), Ac-IETD-AFC,
Ac-LEHD-AFC (BD Pharmingen) as the substrate, respectively. The
results are expressed as the fold-change compared with the control
as previously described (18).
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed using one-way analysis of variance with a post-hoc
test (multiple comparison test), which was used to determine the
significance of the differences between the groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of XIAP and TUNEL-positive
cells within the nuclei of abducens nerves
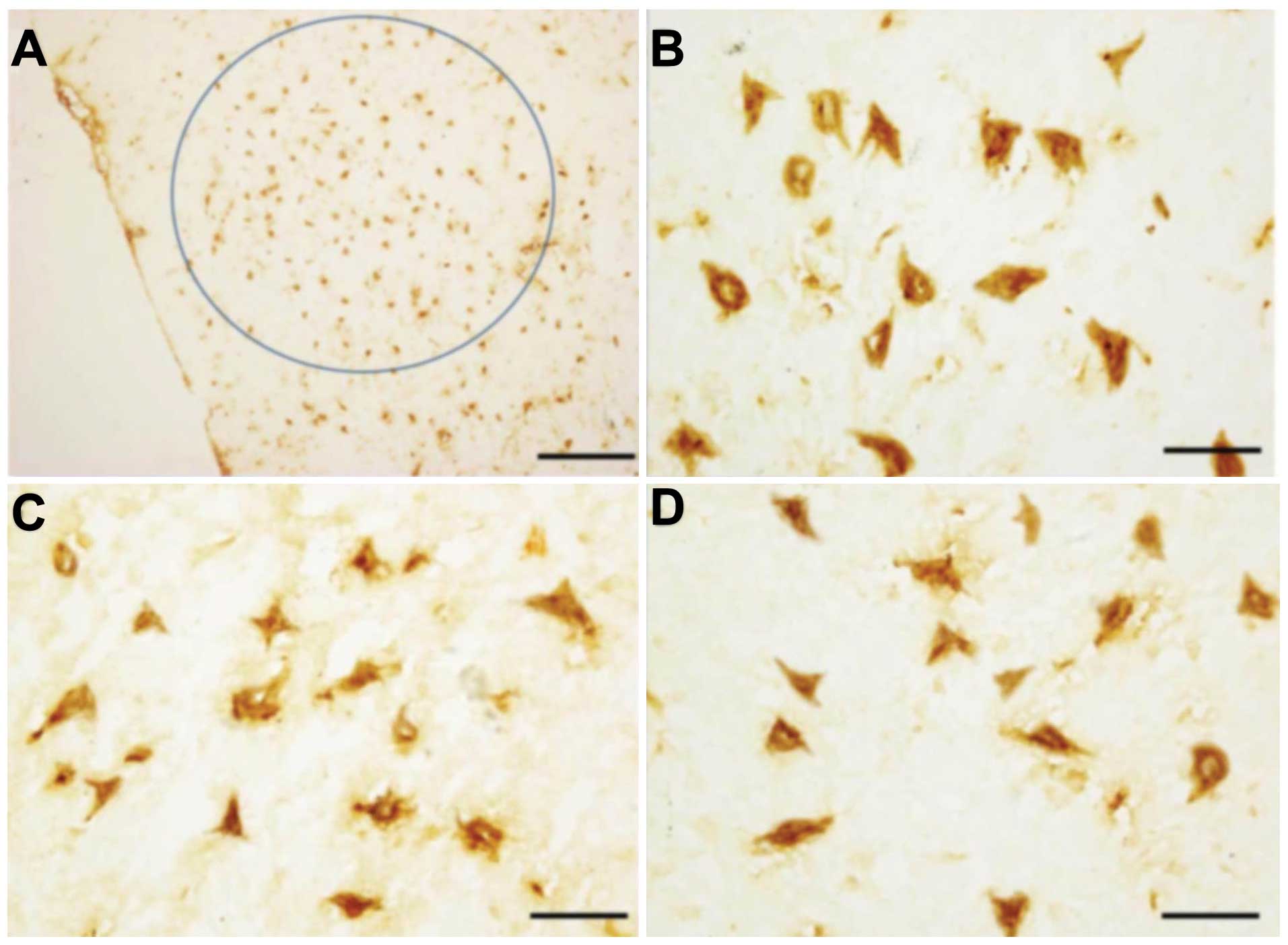
XIAP was predominantly expressed in the cytoplasm as
indicated by positive yellow-brown staining, at high magnification
of the brown granulate (Fig. 1).
In the normal brain, XIAP was predominantly expressed in the
perinuclear region of neurons (Fig. 1A
and B). Similar levels of XIAP were present in the brainstems
following radiation (Fig. 1C and
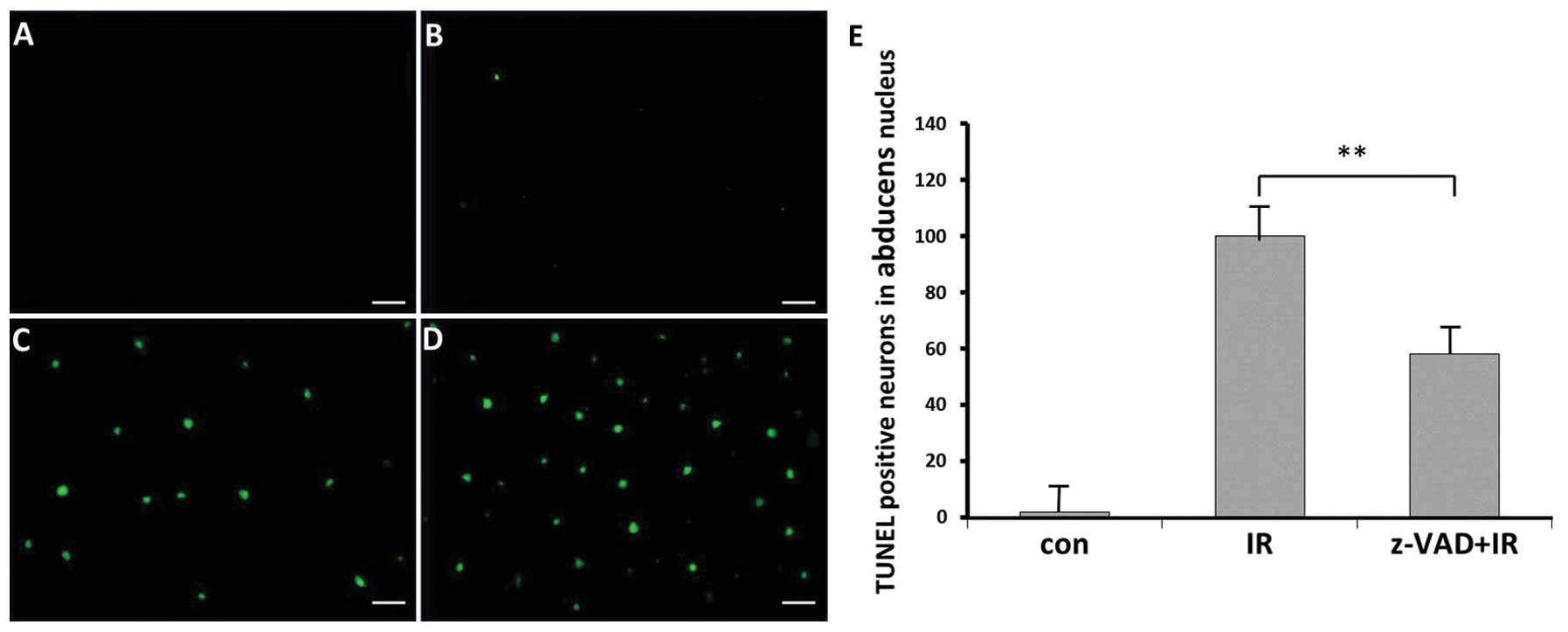
D). TUNEL-positive cells appeared mainly in the nuclei of
abducens nerves of the radiation groups IR and IR+z-VAD (Fig. 2C and D). By contrast, few
TUNEL-positive cells were detected in the control rats (Fig. 2B).
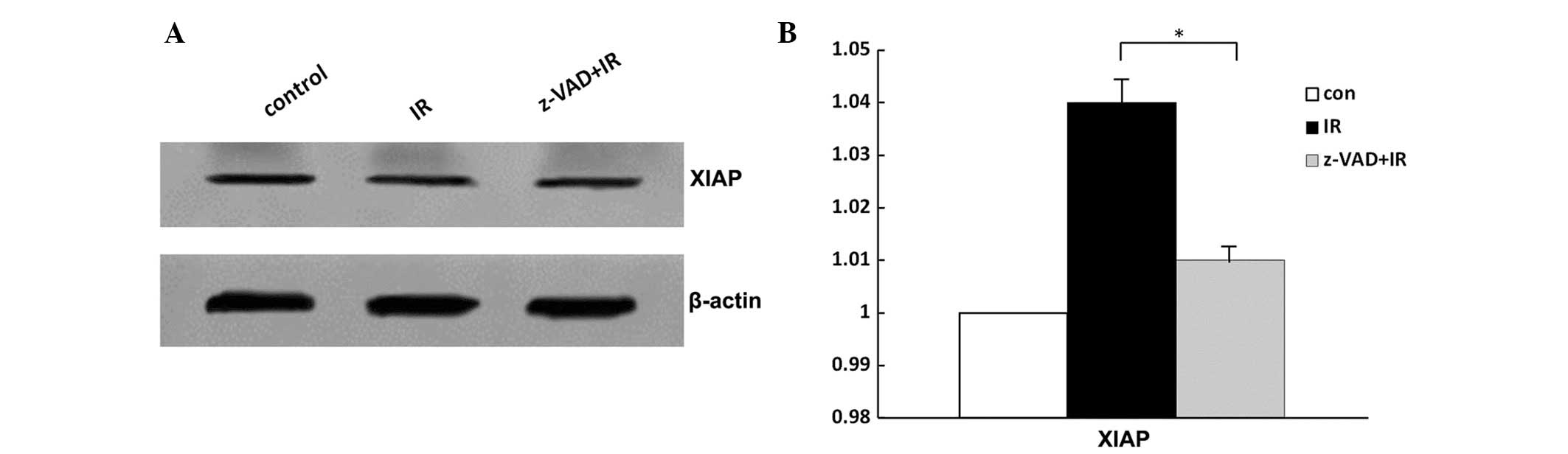
Western blot analysis of XIAP following
radiation
There was no difference in XIAP expression in the
groups of radiated rats, z-VAD-fmk-treated rats and vehicle-treated
rats following radiation (Fig.
3B–D). No significant change was identified in the expression
of XIAP following radiation (P>0.05; Fig. 3).
Neuroprotective effects of the
pan-caspase inhibitor z-VAD-fmk in vivo
Compared with the radiation alone group, the number
of TUNEL-positive neurons was reduced in the z-VAD-fmk-treated
animals following radiation (P<0.01; Fig. 2E).
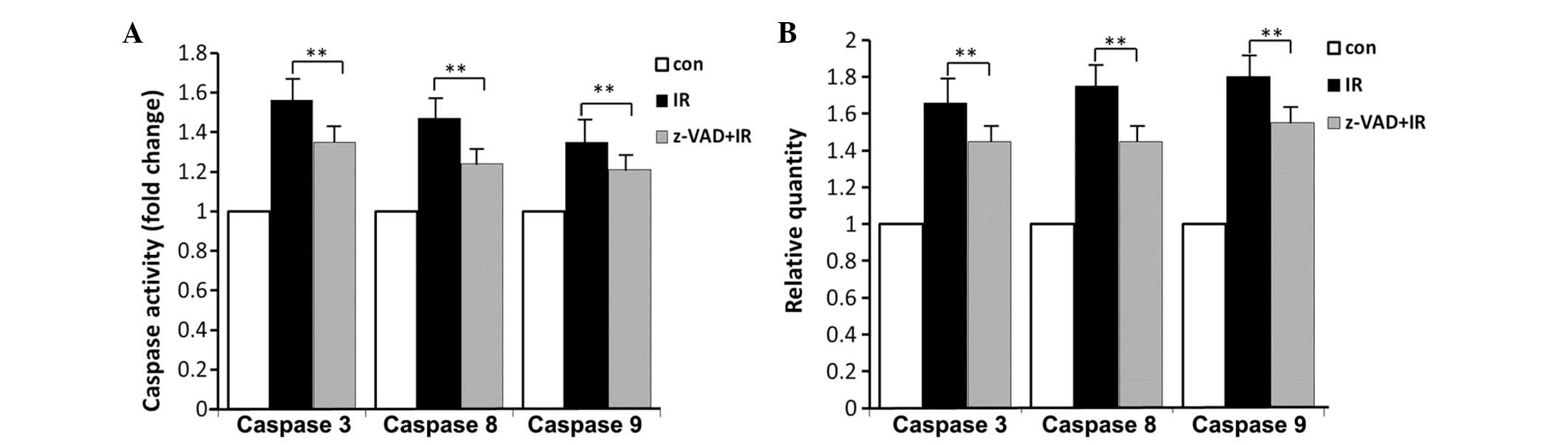
Caspase expression and activity
The mRNA expression of caspase-3, -8 and -9 was
measured. In the brainstem, radiation alone treatment increased the
mRNA expression of caspase-3, -8 or -9 by 1.65-, 1.75- and
1.80-fold and enhanced their activity by 1.56-, 1.47- and
1.35-fold, respectively. Combined treatment caused a significant
decrease in the mRNA expression of caspase-3, -8 and -9 by 1.45-,
1.45- and 1.55-fold and reduced their activity by 1.35-, 1.24- and
1.21-fold, respectively (Fig. 4A and
B).
Discussion
To completely elucidate the role of caspase in the
radiation injury model of the abducens nerve, i.c.v. injection of
Sprague-Dawley rats with z-VAD-fmk, a cell-permeable pan-caspase
inhibitor, was performed. z-VAD-fmk reduced the number of
TUNEL-positive cells within the nucleus of the abducens nerve. The
results demonstrated that inhibition of caspase induced by
z-VAD-fmk reduced the expression and activation of caspase-3, -8
and -9, indicating that intervention in the caspase cascade may
have applications as a potential protective treatment of brain
radiation injury and may represent a therapeutic target.
Evidence has demonstrated that IAP family members
are involved in the regulation of caspase activation (19). IAPs inhibit apoptosis by
interacting with and then controlling the functions of caspase-8 or
caspase-9, -3 and -7 (9). Cellular
(c)-IAP1, c-IAP2 and XIAP are three significant members of the IAP
family, particularly XIAP, which has numerous domains interacting
with different caspases, including caspase-3, -7 and -9 (20,21)
and its BIR2 domain inhibits caspase-7 in a non-competitive manner
(22). XIAP blocks apoptosis at
the effector phase, a point where multiple signaling pathways
converge (23,24). The majority of the current studies
of XIAP have concentrated on its role in cancer or cerebral
ischemia reperfusion injury (11,19).
By contrast, the effect of XIAP following brain injury induced by
radiation remains elusive. In the present study, no significant
change was detected in the expression of XIAP when compared with
the control. These results indicated that XIAP did not have an
important role as an antiapoptotic agent following irradiation.
The nucleus of the abducens nerve has a relatively
large volume in the brainstem, and the distribution of neurons is
predominantly balanced (25);
therefore, locating the nucleus is comparatively simple (Fig. 1A). Since z-VAD-fmk does not
penetrate the blood-brain barrier (13), it was applied
intracerebroventricularly as a bolus injection to overcome this
limitation. The injection was administered into the cerebrospinal
fluid circulating through the fourth ventricle, allowing z-VAD-fmk
to permeate to the neurons through the process of osmosis. This may
act as a useful model of radiation injury, providing visual
information on the morphology of the apoptotic nucleus. The
abducens nucleus contains a large number of mitochondria (26), which was highly useful for the
assays conducted in the present study. Changes in the abducens
nuleus in an animal model established by exposure to radiation were
examined. To the best of our knowledge, the use of this method to
study radiation damage and protection has not been reported
previously. It is important to note that the suspension was
extracted from the cells of the brainstem corresponding to the
nucleus of the abducens nerve section. Further studies are required
to investigate the effects of radiation in other nuclei, for which
novel models will be developed.
In conclusion, z-VAD-fmk effectively prevented
radiation-induced apoptosis, and the caspase cascade may be a
potential therapeutic target in the treatment of brain radiation
injury. The nucleus of the abducens nerve suitable as a radiation
injury model, providing visual information and data on the
apoptotic morphology of nuclei.
Acknowledgements
The present study was supported by the Special
Foundation of the Ministry of Health (no. 201002009), the National
Natural Science Foundation of China (nos. 31170804, 31240052 and
31200634), the Natural Science Foundation of Tianjin (nos.
13JCYBJC23500, 13JCQNJC11600, 11ZCGYSY02400, 12JCYBJC15300 and
12JCYBJC32900) and the PUMC Youth Fund and Fundamental Research
Funds for the Central Universities (no. 2012G01,2012J05).
References
|
1
|
Bladen CL, Kozlowski DJ and Dynan WS:
Effects of low-dose ionizing radiation and menadione, an inducer of
oxidative stress, alone and in combination in a vertebrate embryo
model. Radiat Res. 178:499–503. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loftis GK, Collins S and McDowell M:
Anesthesia-induced neuronal apoptosis during synaptogenesis: a
review of the literature. AANA J. 80:291–298. 2012.PubMed/NCBI
|
|
3
|
Lisi S, Sisto M, Lofrumento D, Frassanito
MA, Caprio S, Romano ML, Mitolo V and D’Amore M: Regulation of mRNA
caspase-8 levels by anti-nuclear autoantibodies. Clin Exp Med.
10:199–203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yao X, Tan G, He C, Gao Y, Pan S, Jiang H,
Zhang Y and Sun X: Hydrogen sulfide protects cardiomyocytes from
myocardial ischemia-reperfusion injury by enhancing phosphorylation
of apoptosis repressor with caspase recruitment domain. Tohoku J
Exp Med. 226:275–285. 2012. View Article : Google Scholar
|
|
5
|
Thornberry NA and Lazebnik Y: Caspases:
enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Canbay A, Taimr P, Torok N, Higuchi H,
Friedman S and Gores GJ: Apoptotic body engulfment by a human
stellate cell line is profibrogenic. Lab Invest. 83:655–663. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mouw G, Zechel JL, Zhou Y, Lust WD, Selman
WR and Ratcheson RA: Caspase-9 inhibition after focal cerebral
ischemia improves outcome following reversible focal ischemia.
Metab Brain Dis. 17:143–151. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Srinivasula SM, Hegde R, Saleh A, Datta P,
Shiozaki E, Chai J, Lee RA, Robbins PD, Fernandes-Alnemri T, Shi Y
and Alnemri ES: A conserved XIAP-interaction motif in caspase-9 and
Smac/DIABLO regulates caspase activity and apoptosis. Nature.
410:112–116. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verhagen AM, Ekert PG, Pakusch M, Silke J,
Connolly LM, Reid GE, Moritz RL, Simpson RJ and Vaux DL:
Identification of DIABLO, a mammalian protein that promotes
apoptosis by binding to and antagonizing IAP proteins. Cell.
102:43–53. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dean EJ, Ranson M, Blackhall F and Dive C:
X-linked inhibitor of apoptosis protein as a therapeutic target.
Expert Opin Ther Targets. 11:1459–1471. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vanden Berghe T, van Loo G, Saelens X, Van
Gurp M, Brouckaert G, Kalai M, Declercq W and Vandenabeele P:
Differential signaling to apoptotic and necrotic cell death by
Fas-associated death domain protein FADD. J Biol Chem.
279:7925–7933. 2004.PubMed/NCBI
|
|
13
|
Wiessner C, Sauer D, Alaimo D and
Allegrini PR: Protective effect of a caspase inhibitor in models
for cerebral ischemia in vitro and in vivo. Cell Mol Biol
(Noisy-le-grand). 46:53–62. 2000.PubMed/NCBI
|
|
14
|
Graeber MB, López-Redondo F, Ikoma E,
Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW and Kohsaka S: The
microglia/macrophage response in the neonatal rat facial nucleus
following axotomy. Brain Res. 813:241–253. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lotocki G, Alonso OF, Frydel B, Dietrich
WD and Keane RW: Monoubiquitination and cellular distribution of
XIAP in neurons after traumatic brain injury. J Cereb Blood Flow
Metab. 23:1129–1136. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vellanki SH, Grabrucker A, Liebau S,
Proepper C, Eramo A, Braun V, Boeckers T, Debatin KM and Fulda S:
Small-molecule XIAP inhibitors enhance gamma-irradiation-induced
apoptosis in glioblastoma. Neoplasia. 11:743–752. 2009.PubMed/NCBI
|
|
17
|
Giagkousiklidis S, Vellanki SH, Debatin KM
and Fulda S: Sensitization of pancreatic carcinoma cells for
gamma-irradiation-induced apoptosis by XIAP inhibition. Oncogene.
26:7006–7016. 2007. View Article : Google Scholar
|
|
18
|
Chen F, Xu C, Du L, Wang Y, Cao J, Fu Y,
Guo Y, Liu Q and Fan F: Tat-SmacN7 induces radiosensitization in
cancer cells through the activation of caspases and induction of
apoptosis. Int J Oncol. 42:985–992. 2013.PubMed/NCBI
|
|
19
|
Saito A, Hayashi T, Okuno S, Ferrand-Drake
M and Chan PH: Interaction between XIAP and Smac/DIABLO in the
mouse brain after transient focal cerebral ischemia. J Cereb Blood
Flow Metab. 23:1010–1019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chai J, Shiozaki E, Srinivasula SM, Wu Q,
Datta P, Alnemri ES and Shi Y: Structural basis of caspase-7
inhibition by XIAP. Cell. 104:769–780. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riedl SJ, Renatus M, Schwarzenbacher R,
Zhou Q, Sun C, Fesik SW, Liddington RC and Salvesen GS: Structural
basis for the inhibition of caspase-3 by XIAP. Cell. 104:791–800.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guijin H, QiYong G, XiaoDan Z, DaWei J, Xi
G, ChunLai P, Liu W and XianWei D: Effect of 103Pd radioactive
stent on caspase-9, cholangiocarcinoma cell growth and its
radiosensitivity. Surg Oncol. 20:247–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rudel T: Caspase inhibitors in prevention
of apoptosis. Herz. 24:236–241. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC
and Takahashi R: X-linked inhibitor of apoptosis protein (XIAP)
inhibits caspase-3 and -7 in distinct modes. J Biol Chem.
276:27058–27063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stahl JS and Thumser ZC: Dynamics of
abducens nucleus neurons in the awake mouse. J Neurophysiol.
108:2509–2523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Loo G, Saelens X, van Gurp M,
MacFarlane M, Martin SJ and Vandenabeele P: The role of
mitochondrial factors in apoptosis: a Russian roulette with more
than one bullet. Cell Death Differ. 9:1031–1042. 2002.PubMed/NCBI
|