Introduction
Cervical cancer is the second most prevalent type of
cancer in females and the fourth leading cause of cancer-related
mortality in developing countries (1,2).
Although recent advances in the clinical implementation of numerous
therapeutic strategies, overall 5-year survival rates remain
<40% and the molecular pathogenesis of cervical cancer is
unclear (3). Therefore,
investigating the mechanisms of tumor pathogenesis that contribute
to disease progression may facilitate the development of novel
effective therapies to prevent the occurrence and development of
cervical cancer.
MicroRNAs (miRNAs) are short, non-coding RNAs that
regulate gene expression at the posttranscriptional level by
complementary pairing in the mRNA 3′ untranslated region (3′UTR),
which leads to mRNA degradation and/or translational repression
(4,5). Previous studies have suggested that
miRNAs have an important role in numerous biological functions,
including differentiation, proliferation, metastasis and apoptosis
(6–9). Aberrant expression of miRNAs has been
observed in human cervical cancer and several of these miRNAs have
been proven as either oncogenes or tumor suppressors (10–13).
Among them, miR-143, miR-145 miR-196b and miR-34a have been
demonstrated to suppress cell growth, and miR-146a, miR-205,
miR-182-5p and miR-21 to promote cell proliferation (11,14–16).
Additionally, forced overexpression of miR-214 repressed cell
growth, induced apoptosis and enhanced sensitivity to cisplatin by
targeting Bcl2-l2 in human cervical cancer cells (17). However, high miR-375 expression
resulted in acquired paclitaxel resistance in cervical tissues
(18).
Although previous studies have determined the
biological functions of certain miRNAs, the sophisticated mechanism
of miR-26a remains largely unknown in cervical cancer. The aim of
the present study was to examine the expression of miR-26a in the
cervical cancer tissues compared with the paired adjacent tissues.
In addition, the biological functions were detected by ectopically
expressing miR-26a, in order to identify its effect on the protein
tyrosine phosphatase type IVA 1 (PRL-1) and on the activity of the
MAPK pathway.
Materials and methods
Cell lines and clinical samples
Human cervical cancer cell lines (ME-180, CaSki,
HeLa, SiHa), an immortalized cervical epithelial cell line (NC104),
and a human embryonic kidney cell line (HEK293T) were cultured in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
(v/v) fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml
streptomycin under a humidified atmosphere of 5% CO2 at
37°C.
Fresh paired human cervical tissues and adjacent
normal tissues were collected from 64 patients who were undergoing
surgery for cervical carcinoma between 24th July 2005 and 13th
November 2008 in the Women’s Hospital, School of Medicine, Fudan
University (Shanghai, China). Informed consent was obtained from
each patient. The study was approved and supervised by the Ethics
Committee of Fudan University (Shanghai, China) and it was
performed in compliance with the Helsinki Declaration. All tissue
samples were snap-frozen in liquid nitrogen and stored at −80°C
until total RNA was extracted.
RNA extraction and reverse transcription
quantitative polymerase chain reaction (RT-qPCR) analyses
Total RNA was obtained from tissue samples and cells
using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. The relative
level of miR-26a was determined by RT-qPCR using the miRCURY LNA™
microRNA PCR system (Exiqon, Woburn, MA, USA). Briefly, 10 ng of
total RNA was reverse-transcribed with the miRNA corresponding RT
Primer (Exiqon, Woburn, MA, USA) and Transcriptor Reverse
Transcriptase (Exiqon), and the cDNA was used as a template for the
qPCR reaction using the miRNA specific LNA™ PCR primer (Exiqon) and
Universal PCR primer (Exiqon). ΔΔCt values were normalized with the
endogenous U6 small nuclear RNA.
For analysis of the expression of PRL-1 mRNA, 500 ng
of the total RNA was reverse transcribed using a Transcriptor First
Strand cDNA Synthesis kit (Takara, Dalian, China) with random
primers under standard conditions. The primers used were as
follows: Sense, 5′-CACCATCTTCCAGG-AGCGAG-3′ and antisense,
5′-TCACGCCACAGTTTCCCGGA-3′ for GAPDH; and sense,
5′-AGGGACAAGCCTACCCCTC-3′ and antisense, 5′-CTCATCTCCCGTCAGTTGGT-3′
for PRL-1. GAPDH was used as the endogenous control. qPCR was
performed in triplicate on the ABI Prism Sequence Detection system
7900HT (Applied Biosystems, Foster City, CA, USA). The gene
expression was normalized to the internal controls and the
fold-changes were calculated using relative quantification. All of
the experiments were performed three times with three technical
replicates.
Plasmid constructs and luciferase
assay
To construct the miR-26a overexpression vector, a
DNA fragment encoding the miR-26a pre-miRNA was amplified by PCR
using the following oligonucleotide primers: Sense: 5′-GGCGAA
TTCCCCACTGCTGACCCATTC-3′ and antisense: 5′-TATGG
ATCCCCACAAGACTCCTCGTTGC-3′. The PCR product was
TOPO®-cloned into the pCR®4-TOPO®
vector (Invitrogen Life Technologies). The construct was sequenced
and the pre-hsa-miR-26a fragment was sub-cloned into
pcDNA4/myc-HisA to generate pcDNA4/miR-26a.
The human PRL-1 3′UTR luciferase reporter,
containing putative binding sites for miR-26a, was generated by
cloning the PRL-1 mRNA 3′UTR sequence into the downstream of the
luciferase gene of the pGL3-control vector (Promega Corporation,
Madison, WI, USA) using the following primers: Sense: 5′-GGC
TCTAGAGGGCCTACAGGAGGGGTTA-3′ and antisense:
5′-GGCTCTAGATGTGATTAAAGTAAAATGCA ATTCA-3′. The plasmids was termed
PRL-1-UTR–WT and site-directed mutagenesis of the miR-26a target
site in the PRL-1 3′UTR was performed using the Quick-change
mutagenesis kit (Stratagene, La Jolla, CA, USA) to generate the
PRL-1-UTR–MUT plasmids. Correct vector construction was verified by
direct sequencing. For the mutated construct, the miR-26a target
site UACUUGAC was substituted with a UAGAACAC fragment.
To silence the PRL-1 expression, lentivirus vector
shPRL-1 was constructed to establish PRL-1 silencing as described
previously (19) using the
following primer sequences: Sense:
5′-CCGGTTCTTGCTGTCAGCATATAAACTCGA GTTTATATGCTGACAGCAAGAATTTTTG-3′
and antisense: 5′-AATTCAAAAATTCTTGCTGTCAGCATATAAA
CTCGAGTTTATATGCTGACAGCAAGAA-3′.
A dual luciferase assay was conducted by
co-transfecting HEK293T cells with 20 ng pcDNA4/miR-26a or empty
lentiviral vector, along with 100 ng of firefly luciferase reporter
comprising wild type or mutant 3′UTR of PRL-1 gene and 10 ng pRL-TK
vector, using Lipofectamine™ 2000 (Invitrogen Life Technologies)
per well, according to the manufacturer’s instructions in a 24-well
plate format. The cells were harvested 48 h following transfection
for luciferase assay using a luciferase assay kit (Promega
Corporation) and Renilla luciferase activity was used for
transfection variation normalization according to the
manufacturer’s instructions. Each experiment was repeated three
times.
Cell proliferation assays
The cells were seeded at a density of 2,000 cells
per well in a 96-well plate containing 100 μl DMEM culture media
with 10% FBS. A total of 10 μl cell counting kit (CCK)-8 solution
was added to each well and the cells were incubated at 37°C for 2
h. The absorbance values were measured at 450 nm every 24 h
following the manufacturer’s instructions. Triplicate wells were
measured in each treatment group.
Matrigel invasion assay
A total of 4×104 cells in 100 μl serum
free media were seeded into the upper chamber coated with 150 μg of
Matrigel (BD Biosciences, Bedford, MD, USA), and NIH 3T3 fibroblast
conditioned medium was added to the lower chamber. After the cells
were incubated for 36 h, noninvasive cells were removed from the
upper surface of the membrane with a cotton swab, and the invaded
cells on the lower membrane surface were fixed and stained with
hematoxylin for 30 min. Following drying, the invasive cells were
captured using a microscope in five random fields using a DP
controller (Olympus, Tokyo, Japan).
Colony formation assay
The cells were placed in 6-well plates at a density
of 500 cells per well in normal culture medium as stated above and
incubated for two weeks, the medium was replaced every four days.
Next the cells were washed twice with PBS, fixed with 4%
polyoxymethylene and stained with 1% crystal violet for 30 min. The
number of colonies was counted and a single clone contained >50
cells. Each assay was performed in triplicate.
Western blot analysis
The cells were washed twice with PBS and then lysed
using RIPA buffer supplemented with protease inhibitor cocktail
(Roche, Basel, Switzerland) and sonicated (Shengyan, Shanghai,
China) with one 10 sec burst. Whole cell extracts containing equal
quantities of proteins (50 μg) were separated by 10%
SDS-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to
0.45-μm polyvinylidene difluoride membrane sheets (Millipore,
Billerica, MA, USA). The membrane was blocked with 5% skimmed milk
at room temperature for 1 h and probed with the following
antibodies: Anti-PRL-1 antibody (ab3523, Abcam, Cambridge, UK) and
anti-GAPDH antibody (sc-25778; Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). Following an overnight incubation, the
membrane was washed and incubated with the appropriate goat
anti-rabbit horseradish peroxidase-conjugated secondary antibody
(Kangcheng, Shanghai, China) at room temperature for 1 h. The
protein bands were subjected to a chemiluminescence detection assay
(chemiluminescence kit; Tiangen, Beijing, China).
Nude mouse tumor xenograft model
The present study was conducted in accordance with
the Care and Use of Laboratory Animals of the National Institutes
of Health. All of the experimental procedures were approved by the
Committee on the Care and Use of Laboratory Animals of the Fudan
University. Female nude mice (age, 3–5 weeks) were purchased from
Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). The
mice were housed under humidity- and temperature-controlled
conditions with a 12 h light/dark cycle, and were fed a normal
diet. Xenografts were established by subcutaneous injection of
5×106 Hela cells with vector control or overexpression
miR-26a per mouse in the right flank area in a volume of 200 μl
FBS-free DMEM medium. The animals were sacrificed by joint
dislocation following six weeks and the tumor volume was measured
using a caliper every seven days, using the following formula:
Volume = (width)2 × length/2. Four mice were used in
each group and the experiment was repeated three times
independently.
Statistical analysis
The experimental data were demonstrated as the mean
± standard deviation for each group. Student’s t-test (two-tailed)
was performed to analyze the data using GraphPad Prism software (La
Jolla, CA, USA). P<0.05 were considered to indicate a
statistically significant difference.
Results
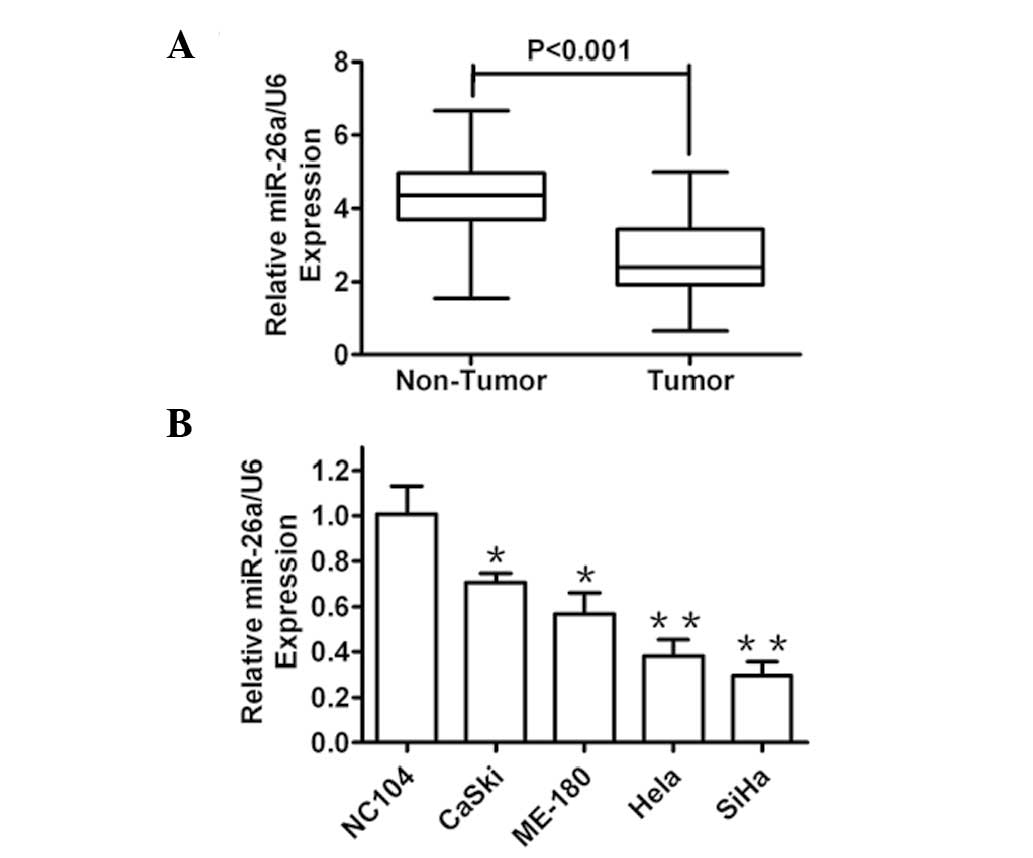
miR-26a is significantly downregulated in
primary cervical cancer tissues and cell lines
In order to assess the roles of miR-26a in human
cervical cancer development, the miR-26a expression levels in
cervical cancer tissues (n=64) and paired adjacent normal tissues
were compared by RT-qPCR. It was identified that miR-26a expression
was markedly reduced in tumor tissues as compared with that in
adjacent normal tissues (Fig. 1A).
Furthermore, the expression level of miR-26a in several human
cervical cancer cell lines and the NC104 human normal immortalized
cervical epithelial cell line was detected. As expected, the
expression of miR-26a was decreased in all of the cancer cell lines
compared with that in the normal cells (Fig. 1B). These results indicated that
miR-26a was significantly downregulated in clinical human cervical
cancer tissues and cells, and therefore, may be involved in human
cervical cancer development.
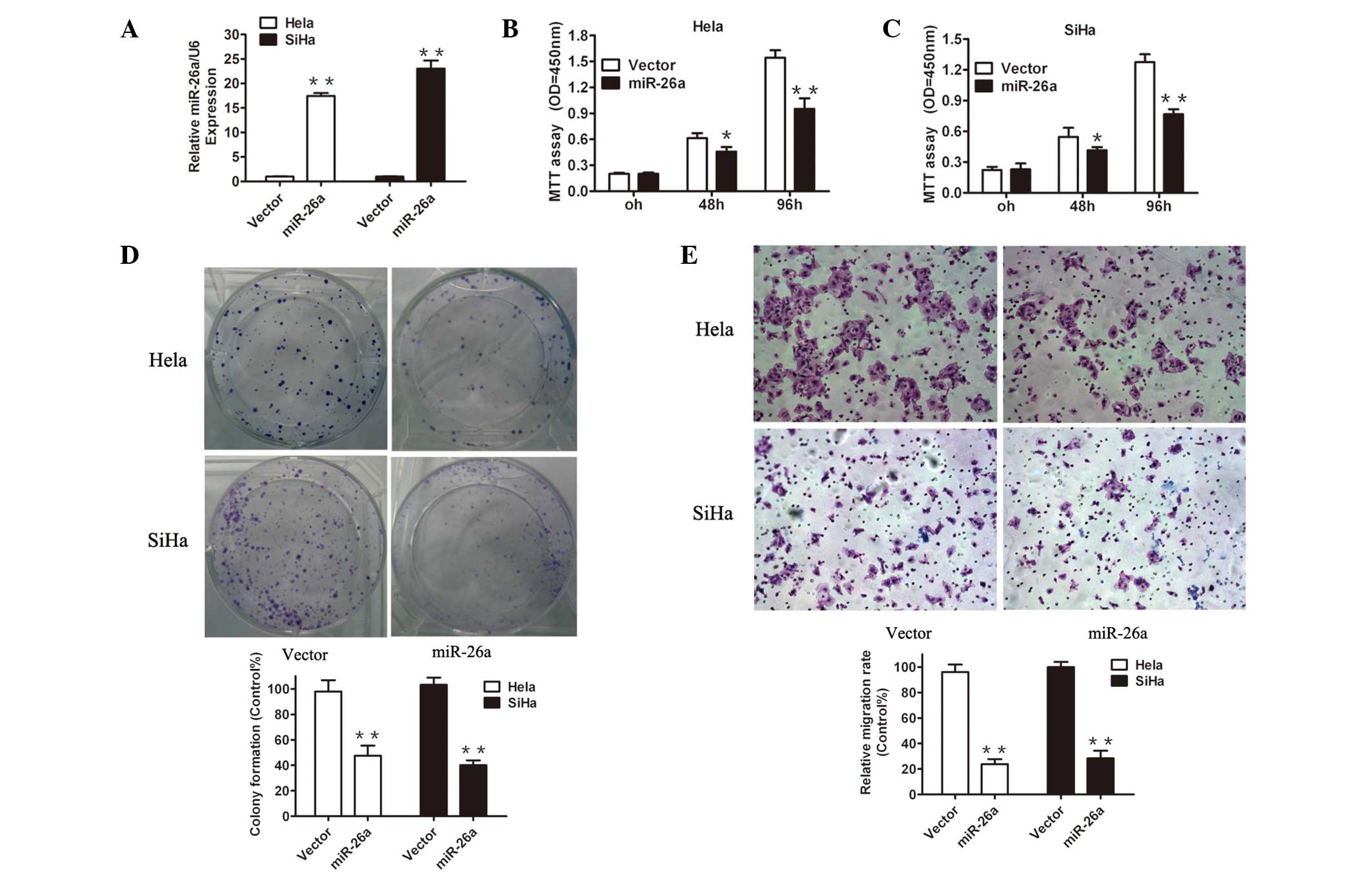
miR-26a overexpression suppresses
proliferation and invasion of cervical cancer cells
To elucidate the potential mechanisms underlying the
effects of miR-26a in cervical cancer cells, miR-26a was restored
in the Hela and SiHa cell lines by a lentiviral vector. miR-26a
transduction significantly increased the miR-26a expression in Hela
and SiHa cells compared with the vector cells, as demonstrated by
RT-qPCR analysis (Fig. 2A),
reflecting efficient overexpression of miR-26a in these cervical
cancer cells. Next, several functional analyses were performed. A
CCK-8 assay demonstrated that forced expression of miR-26a
significantly reduced cell proliferation in the two cervical cancer
cell lines (Fig. 2B and C). To
further evaluate the proliferation ability, the effects of the
restoration of miR-26a on colony formation were examined. As
demonstrated in Fig. 2D, there
were notably fewer and smaller colonies of the cells overexpressing
miR-26a compared with the vector transductions.
Invasion is the key element of tumor metastasis,
thus it was investigated whether reintroducing miR-26a affects cell
invasion by utilizing transwell migration assays. As exhibited in
Fig. 2E, miR-26a significantly
reduced the number of Hela and SiHa cells invading through the
Matrigel basement membrane. Together, these observations suggested
that the loss of miR-26a in cervical cancer may, at least
partially, contribute to tumor growth and invasive capacity.
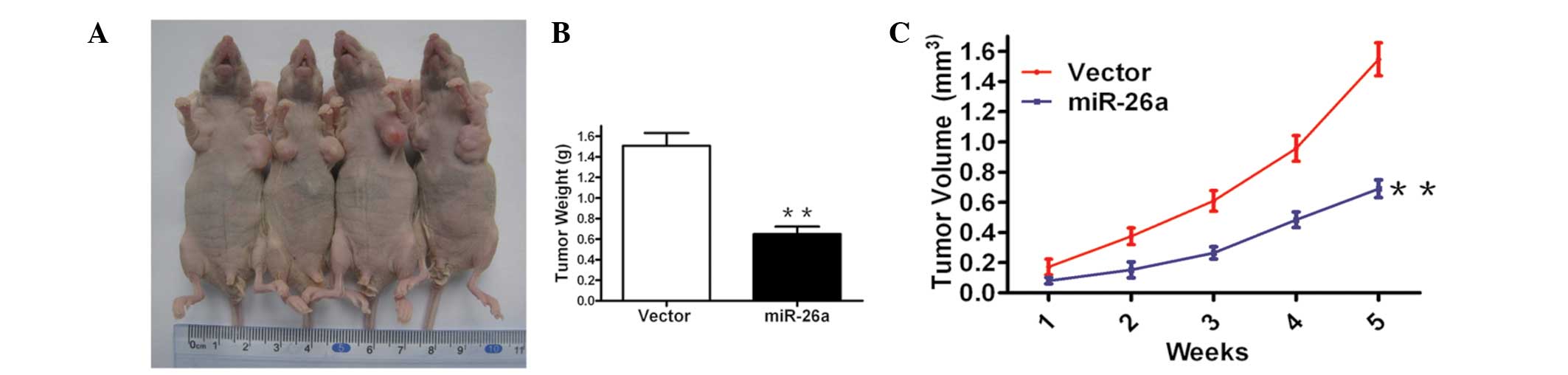
miR-26a inhibits tumor growth in an
allograft murine model
Considering the aforementioned results that
demonstrated that forced expression of miR-26a inhibited cell
growth in cervical cancer cells in vitro, an in vivo
model was used to examine the effect of miR-26a restoration on
tumor growth. It was identified that miR-26a restoration markedly
inhibited tumor formation and reduced the tumor size and weight
after five weeks compared with the vector control (Fig. 3A and B). These data indicate that
overexpression of miR-26a reduces tumor growth in Hela cells in
vivo.
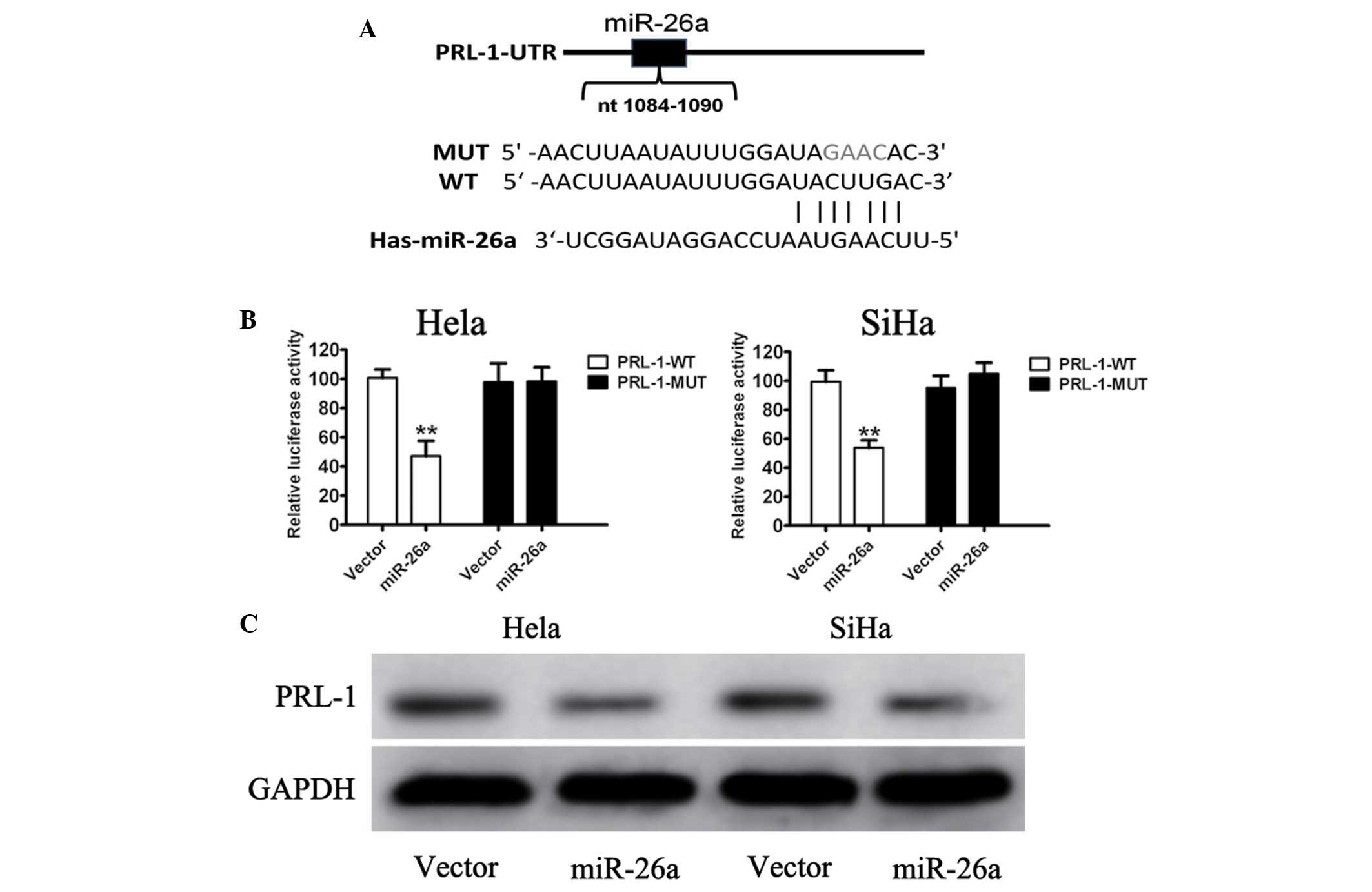
miR-26a directly targets PRL-1 in
cervical cancer cells
To examine the mechanisms through which miR-26a
regulated cervical cancer cell growth, two publicly available
algorithms were used to facilitate predicting the miR-143 targets.
A bioinformatic prediction (Targetscan and Pictar) for putative
targets of miR-26a was used and it was identified that PRL-1 was a
potential target of miR-34a. Increased expression of PRL-1 has been
reported and implicated in tumor progression and angiogenesis in
human lung cancer and colon cancer (20). The present study focused on the
possible regulation of PRL-1 by miR-26a. Sequence analyses revealed
that the 3′-UTR of PRL-1 mRNA was partially complementary to
miR-26a (Fig. 4A). To
experimentally validate the direct miR-26a-PRL-1 interaction,
PRL-1-UTR-WT and PRL-1-UTR-MUT plasmids were constructed.
Upregulation of miR-26a led to a significant decrease in luciferase
activity when the reporter contained a wild-type sequence, but no
change in the luciferase activity of the mutant sequence (Fig. 4B). Furthermore, the endogenous
expression of PRL-1 in Hela and SiHa cells upon miR-26a forced
expression was examined, and it was identified that the endogenous
PRL-1 protein level was markedly reduced by miR-26a (Fig. 4C). These results demonstrated a
direct interaction between miR-26a and PRL-1 in cervical cancer
cell lines.
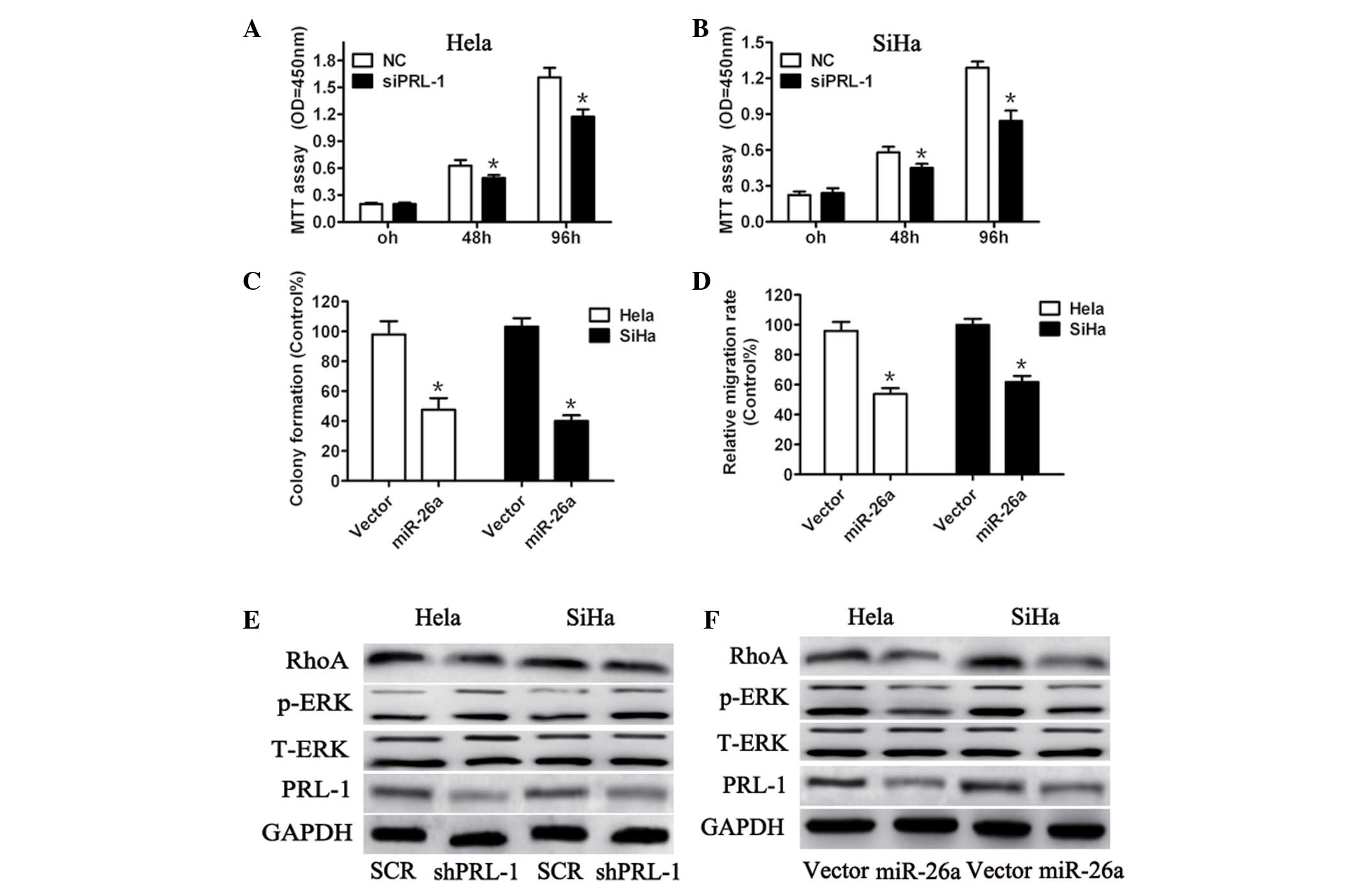
Knockdown of PRL-1 expression phenocopies
the effects of miR-26a restoration in cervical cancer cells
To evaluate whether PRL-1 is potentially involved in
miR-26a-regulated cell proliferation and invasiveness, shPRL-1
efficiently knocked down endogenous PRL-1 expression (Fig. 5). Compared with the negative
control, silencing PRL-1 markedly suppressed cell proliferation
(Fig. 5A and B), colony formation
(Fig. 5C) and invasive ability
(Fig. 5D) of Hela and SiHa cells.
As PRL-1, a direct target of miR-26a, has an important role in
extracellular signal regulated kinases (ERK)1/2 activation
(21), the expression of ERK1/2
and the downstream effectors following PRL-1 silencing were
detected. As expected, PRL-1 knockdown reduced ERK1/2 and RhoA
activation in the cervical cancer cells (Fig. 5E). To further confirm that PRL-1 is
a target gene protein of miR-26a, it was investigated whether
miR-26a affected the expression of ERK1/2 and RhoA through
suppression of PRL-1 expression. Consistent with the results of
PRL-1 silencing, miR-26a overexpression significantly decreased the
expression of ERK1/2 and RhoA (Fig.
5F). These results provide evidence suggesting that PRL-1 is
involved in miR-26a-regulated cervical cancer cell growth and
invasiveness.
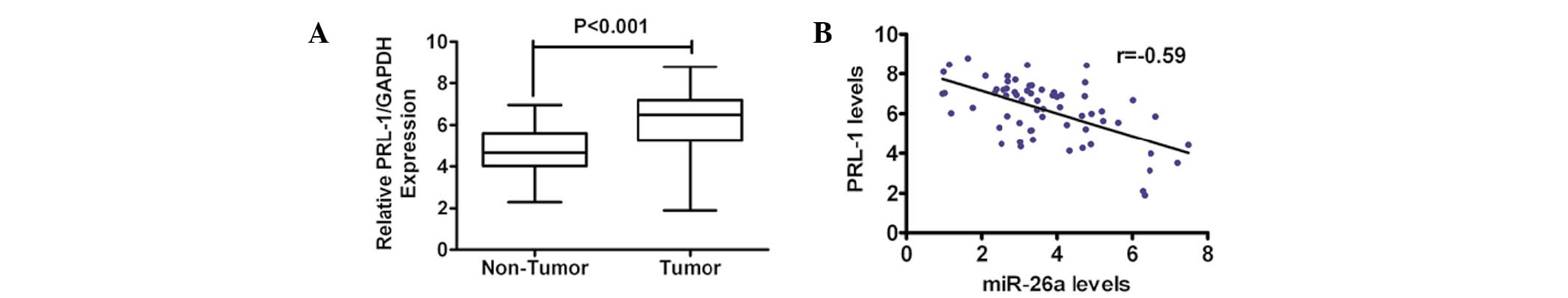
Upregulation of PRL-1 expression is
inversely correlated with miR-26a in primary cervical cancer
tissues
To further determine the correlation between miR-26a
and PRL-1, the mRNA level of PRL-1 in primary human cervical cancer
tissues was detected and compared with that in the normal adjacent
tissue in the same patient cohort that was used for measuring the
miR-26a levels. The expression of PRL-1 was markedly higher in the
tumor tissue compared with the adjacent normal tissue (Fig. 6A). Notably, there was an inverse
correlation between the expression level of miR-26a and PRL-1 in
the cervical cancer tissues, as determined by Pearson’s correlation
coefficient (Fig. 6B).
Collectively, these data supported the evidence for a reciprocal
correlation between the levels of miR-26a and PRL-1 in human
cervical cancer.
Discussion
Previous studies have reported that aberrant
expression of miR-26a is a common feature of a wide spectrum of
human malignancies and is associated with poor prognosis in certain
cancer types, including breast cancer (22), nasopharyngeal carcinoma (23), hepatocellular carcinoma (24), breast cancer (25), gliomagenesis (26), cholangiocarcinoma (27) and lung cancer (28). miR-26a may act as a tumor
suppressor or oncogene in different tumor types, which may owe to
unique genetic backgrounds and/or it not being conserved in certain
types of cancer cells. miR-26a has been reported to be reduced in
human cervical cancer tissues, compared with normal cervical
tissues or adjacent benign cervical tissues. In the present study,
the action of miR-26a in cervical cancer lines was examined.
In the present study, miR-26a was identified as a
tumor suppressor in cervical cancer through directly targeting
PRL-1, which is known to upregulate ERK1/2 and RhoA pathways
(21). First the expression of
miR-26a was determined and it was identified that miR-26a was
significantly lower in cervical cancer tissues than in the
corresponding adjacent tissues. As is consistent with the results
in the tissue samples, the miR-26a expression demonstrated a marked
attenuation in the cervical cancer cell lines, particularly in Hela
and SiHa cells. Furthermore, forced expression of miR-26a inhibited
the proliferation and invasion of cervical cancer cells in
vitro. These results are consistent with previous studies that
have demonstrated that miR-26a was downregulated in several cancer
tissues and was identified as a tumor suppressor (12,23–25).
Systemic administration of miR-26a AAV by tail vein injection into
mice led to tumor suppression without any toxic effects when
assessed three weeks later (29).
The present results indicated that miR-26a may be an attractive
therapeutic agent for cervical cancer treatment.
With use of bioinformatics prediction and sequence
analyses, PRL-1 was considered to be direct target of miR-26a in
cervical cancer. In luciferase reporter assays, miR-26a was able to
suppress luciferase activity in the PRL-1 WT but had no effect in
the mutant construct. Exogenous overexpression of miR-26a confirmed
the decrease in PRL-1 expression. Upregulation of PRL-1 expression
has been reported in several distinct cancer types, including
hepatocellular carcinoma (30),
colorectal carcinoma (20) and
lung cancer (31). However, the
expression and roles of PRL-1 in cervical cancer remain unclear.
Higher expression of PRL-1 in cervical cancer tissue relative to
the normal adjacent cervical tissue was observed, suggesting that
upregulation of PRL-1 expression in cervical cancer may be
implicated in processes that are essential for tumor cell growth
and metastasis due to their ability to affect cell migration.
Silencing PRL-1, by downregulating its expression, decreased the
cell proliferation and invasion with decreased expression of p-ERK
and inactivation of RhoA. Consistent with PRL-1 knockdown, miR-26a
overexpression also reduced the expression of p-ERK and
inactivation of RhoA. Additionally, miR-26a expression was
inversely correlated with the PRL-1 mRNA expression in cervical
cancer tissues.
In the present study, it was identified that miR-26a
was reduced in cervical cancer tissues and cervical cancer cells.
Furthermore, the overexpression of miR-26a suppressed cervical
cancer cell growth in vitro and in vivo. Although the
different biological functions of miR-26a have been previously
reported, its function in cervical cancer remained unclear. To the
best of our knowledge, the present study is the first to
demonstrate the role of miR-26a in cervical cancer. Together with
previous studies, the present results confirm that miR-26a is a
tumor suppressor that has a key role in the initiation and
progression of human cervical cancer by affecting the expression of
PRL-1. Further investigation of the association between miR-26a
with the clinicopathological parameters of cervical cancer is
required to address the clinical applications of these data. The
determination of the functions of miR-26a may aid the development
of future therapeutics for cervical cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81272877) and the Cancer
Foundation of China. The funders had no role in the study design,
data collection, analysis, decision to publish or preparation of
the manuscript.
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Parkin DM: Global cancer statistics in the
year 2000. Lancet Oncol. 2:533–543. 2001.PubMed/NCBI
|
|
3
|
Saslow D, Solomon D, Lawson HW, et al:
American Cancer Society, American Society for Colposcopy and
Cervical Pathology, and American Society for Clinical Pathology
screening guidelines for the prevention and early detection of
cervical cancer. Am J Clin Pathol. 137:516–542. 2012. View Article : Google Scholar
|
|
4
|
Farh KK, Grimson A, Jan C, et al: The
widespread impact of mammalian MicroRNAs on mRNA repression and
evolution. Science. 310:1817–1821. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marzi MJ, Puggioni EM, Dall’Olio V, et al:
Differentiation-associated microRNAs antagonize the Rb-E2F pathway
to restrict proliferation. J Cell Biol. 199:77–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kane NM, Howard L, Descamps B, et al: Role
of microRNAs 99b, 181a, and 181b in the differentiation of human
embryonic stem cells to vascular endothelial cells. Stem Cells.
30:643–654. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu XC, Dong QZ, Zhang XF, et al:
microRNA-29a suppresses cell proliferation by targeting SPARC in
hepatocellular carcinoma. Int J Mol Med. 30:1321–1326.
2012.PubMed/NCBI
|
|
9
|
Cheng AM, Byrom MW, Shelton J and Ford LP:
Antisense inhibition of human miRNAs and indications for an
involvement of miRNA in cell growth and apoptosis. Nucleic Acids
Res. 33:1290–1297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee JW, Choi CH, Choi JJ, et al: Altered
MicroRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Tang S, Le SY, et al: Aberrant
expression of oncogenic and tumor-suppressive microRNAs in cervical
cancer is required for cancer cell growth. PLoS One. 3:e25572008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pereira PM, Marques JP, Soares AR, Carreto
L and Santos MA: MicroRNA expression variability in human cervical
tissues. PLoS One. 5:e117802010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gocze K, Gombos K, Juhasz K, et al: Unique
microRNA expression profiles in cervical cancer. Anticancer Res.
33:2561–2567. 2013.PubMed/NCBI
|
|
14
|
Hirata H, Ueno K, Shahryari V, et al:
Oncogenic miRNA-182-5p targets Smad4 and RECK in human bladder
cancer. PLoS One. 7:e510562012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xie H, Zhao Y, Caramuta S, Larsson C and
Lui WO: miR-205 expression promotes cell proliferation and
migration of human cervical cancer cells. PLoS One. 7:e469902012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wang HK, McCoy JP, et al:
Oncogenic HPV infection interrupts the expression of
tumor-suppressive miR-34a through viral oncoprotein E6. RNA.
15:637–647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. Febs Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen Y, Wang P, Li Y, et al: miR-375 is
upregulated in acquired paclitaxel resistance in cervical cancer.
Br J Cancer. 109:92–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dong J, Cheng M and Sun H: Function of
inducible nitric oxide synthase in the regulation of cervical
cancer cell proliferation and the expression of vascular
endothelial growth factor. Mol Med Rep. 9:583–589. 2014.PubMed/NCBI
|
|
20
|
Fiordalisi JJ, Keller PJ and Cox AD: PRL
tyrosine phosphatases regulate rho family GTPases to promote
invasion and motility. Cancer Res. 66:3153–3161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bai Y, Luo Y, Liu S, et al: PRL-1 protein
promotes ERK1/2 and RhoA protein activation through a non-canonical
interaction with the Src homology 3 domain of p115 Rho
GTPase-activating protein. J Biol Chem. 286:42316–42324. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen HC, Chen GH, Chen YH, et al: MicroRNA
deregulation and pathway alterations in nasopharyngeal carcinoma.
Br J Cancer. 100:1002–1011. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu J, He ML, Wang L, et al: MiR-26a
inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma
through repression of EZH2. Cancer Res. 71:225–233. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Liang L, Zhang XF, et al:
MicroRNA-26a suppresses tumor growth and metastasis of human
hepatocellular carcinoma by targeting interleukin-6-Stat3 pathway.
Hepatology. 58:158–170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang B, Liu XX, He JR, et al:
Pathologically decreased miR-26a antagonizes apoptosis and
facilitates carcinogenesis by targeting MTDH and EZH2 in breast
cancer. Carcinogenesis. 32:2–9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huse JT, Brennan C, Hambardzumyan D, et
al: The PTEN-regulating microRNA miR-26a is amplified in high-grade
glioma and facilitates gliomagenesis in vivo. Genes Dev.
23:1327–1337. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang J, Han C and Wu T: MicroRNA-26a
promotes cholangiocarcinoma growth by activating beta-catenin.
Gastroenterology. 143:246–256. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu B, Wu X, Liu B, et al: MiR-26a
enhances metastasis potential of lung cancer cells via AKT pathway
by targeting PTEN. Biochim Biophys Acta. 1822:1692–1704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kota J, Chivukula RR, O’Donnell KA, et al:
Therapeutic microRNA delivery suppresses tumorigenesis in a murine
liver cancer model. Cell. 137:1005–1017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu JW, Chang JG, Yeh KT, et al: Increased
expression of PRL-1 protein correlates with shortened patient
survival in human hepatocellular carcinoma. Clin Transl Oncol.
14:287–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Achiwa H and Lazo JS: PRL-1 tyrosine
phosphatase regulates c-Src levels, adherence, and invasion in
human lung cancer cells. Cancer Res. 67:643–650. 2007. View Article : Google Scholar : PubMed/NCBI
|