Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
eighth leading cause of cancer mortality worldwide (1). As one of the most common types of
cancer in the head and neck, laryngeal squamous carcinoma (LSCC) is
a major type of HNSCC (2). The
incidence of LSCC in China has been progressively increasing,
particularly in the Northeast region. Despite advancements in local
control and overall life quality achieved with the use of combined
modality therapies, the survival rates for LSCC patients have not
improved significantly over the past two decades. With the
development of molecular biology, the use of biomarkers in the
diagnosis of LSCC in the future and the results from investigating
the molecular mechanisms of LSCC may improve treatment and survival
rates of LSCC patients (3).
S100A4 is a small (11.5 kDa) acidic calcium binding
protein that belongs to the S100 protein family. It has been
associated with numerous biological functions, for example,
protection of cells from proapoptotic stimuli and stimulation of
neurite outgrowth (4,5). Overexpression of S100A4 has been
reported in several types of cancer and is correlated with poor
patient prognosis (6–8). Previous studies suggested that S100A4
acts as a potential marker of metastasis by regulating cell growth,
motility and invasion (9,10). Our previous study revealed that
overexpression of S100A4 is associated with the progression of
laryngeal cancer, and it is therefore considered an important
molecular marker for laryngeal cancer (11). However, the exact role and
molecular mechanism of S100A4 in LSCC have not been well
characterized. Therefore, in the present study, the biological
effects of S100A4 suppression were examined by using the RNA
interference method to inhibit S100A4 expression in the laryngeal
cancer cell line Hep-2 in order to evaluate the importance of
S100A4 and to elucidate the possibilities for utilization of S100A4
in the treatment of LSCC.
Materials and methods
Cell culture and transfection
The human laryngeal cancer cell line Hep-2 was
purchased from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China) and originated from a
metastatic peidermoid carcinoma of the larynx (12). The cell was cultured in RPMI-1640
medium (Invitrogen Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal calf serum, 100 U/ml penicillin and 100
U/ml streptomycin at 37°C in a 5% CO2 incubator. Upon
reaching 60–70% confluence, the cells were divided into two new
dish or seeded in six-well plates at a density of 1×105
cells per well for further experiments. The Hep-2 cells were
transfected with short hairpin RNA (shRNA) control or S100A4 shRNA
expression plasmids. Hep-2-shRNA cells expressed an shRNA,
specifically targeting S100A4 mRNA, while Hep-2 negative control
(Hep-2-shNC) cells expressed an unrelated control sequence. shRNA
were transfected into Hep-2 cells using Lipofectamine 2000
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. Total RNA was prepared 24 h post transfection and the
results of gene knockdown were determined by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Cells were incubated for 72 h and then harvested for further
experiments.
RT-qPCR
The quantification of S100A4 expression levels was
performed using an SYBR-Green I-based real-time fluorescence
detection method (13). Total RNA
was isolated from Hep-2 cells using an mRNA isolation kit (Qiagen,
Valencia, CA, USA), and subsequently reverse transcribed using the
Reverse Transcription PCR kit kit (Takara Bio Inc., Shiga, Japan)
with Oligo-dT primers. cDNA was amplified using an SYBR Premix Ex
Taq kit (Takara Bio Inc.) on an ABI 7500 Real-time PCR system
(Applied Biosystems, Foster City, CA, USA). GAPDH served as a
reference gene and samples without cDNA or without oligonucleotides
were used as negative controls. The control GAPDH fragment was
amplified using the following primer sequences: GAPDH, forward
5′-ATCATCAGCAATGCCTCC-3′ and reverse 5′-CATCACGCCACAGTTTCC 3′;
S100A4, forward 5′-CCCTGGATGTGATGGTGTC-3′ and reverse
5′-CTCGTTGTCCCTGTTGCTG-3′. The 25 μl PCR included 0.5 μl cDNA,
SYBR® Premix EX Taq™ 12.5 and 2 μl of primers. The
reaction was run at 95°C for 1 min, followed by 45 cycles at 95°C
for 5 sec and 60°C for 20 sec. The Ct data was determined using
default threshold settings. The threshold cycle (Ct) was defined as
the fractional cycle number at which the fluorescence passes the
fixed threshold. All measurements were performed at least three
times.
Western blot analysis
To determine the levels of protein expression,
soluble proteins were isolated using radioimmunoprecipitation assay
buffer (50 mM Tris, 150 mM NaCl, 1 mM ethylenediaminetetraacetic
acid, 0.2% NP40) containing complete protease inhibitor followed by
centrifugation at 12,000 × g at 4°C. The protein concentration was
determined from the supernatant using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology, Haimen, China) and the
results were produced by an ELISA-plate reader (Sunrise RC; Tecan,
Theale, UK). Protein extracts were diluted with 5X sodium dodecyl
sulfate (SDS) loading buffer, boiled and resolved on a 12% w/v
SDS-PAGE. Protein extract (40 mg) was loaded. Following
electrophoresis, the proteins were transferred onto a
polyvinylidene fluoride membrane (Roche Diagnostics, Mannheim,
Germany) by semidry blotting. Detection was performed using rabbit
polyclonal anti-S100A4 antibody (Thermo Fisher Scientific, Waltham,
MA, USA; 1:1,000) and a mouse monoclonal anti-GAPDH antibody (Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA; 1:1,000) was used as
a loading control. Secondary antibodies anti-rabbit and anti-mouse
IgG coupled to horseradish peroxidase (Beyotime Institute of
Biotechnology) were used at dilutions of 1:2,000. Blots were
developed with Western Lightning Chemiluminescence Reagent Plus
(Thermo Fisher Scientific) and chemiluminescence detection film
(Beyotime Institute of Biotechnology).
Cell proliferation assay
S100A4-shRNA Hep-2 cells and control cells between
12 h and 5 days after transfection, were seeded in a 96-well
flat-bottomed plate at a density of 2×103 cells in 190
μl of the medium per well. Cell proliferation was determined using
the Cell Counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology) according to the manufacturer’s instructions. After
10 μl CCK-8 was added to each well, the cells were incubated at
37°C for 2 h and the absorbance was monitored using a microplate
reader (Sunrise RC) at a wavelength of 490 nm. Each experiment was
performed in triplicate and repeated three times. Data are
presented as the mean ± standard deviation.
Anchorage-independent growth assay
The anchorage-independent growth of cells was
determined by assaying colony formation in soft agar. Following
transfection, single cell suspension of transfected Hep-2 cells was
prepared by trypsinization and homogenization. Cells were plated
onto each well of a six-well plate at a density of 1,000 cells/well
in RPMI-1640 containing 10% fetal bovine serum and 0.3% low melting
point agarose (Amresco, Solon, OH, USA) on a base layer of 0.6% low
melting point agarose. The number of colonies >50 μm was counted
and images were captured following 2 weeks of incubation at 37°C.
The experiments were performed three times independently.
Apoptosis assay
The Hep-2-shRNA transfected cells and control cells
were harvested, fixed and resuspended in phosphate-buffered saline.
Apoptotic cells were measured using an annexin V-phycoerythrin
(PE)/7-aminoactinomycin D (7AAD) kit (Nanjing KeyGen Biotech., Co.,
Ltd., Nanjing, China) according to the manufacturer’s instructions.
Briefly, annexin V-PE and 7AAD were added to a mixture containing
100 μl of cell resuspension in binding buffer (BD Biosciences,
Franklin Lakes, NJ, USA). The cells were vortexed and incubated for
15 min at room temperature in the dark and 400 μl of binding buffer
was added to the mixture for flow cytometric analysis using a
Becton Dickinson FACScan Flow Cytometer (FACScan; Becton Dickinson,
Franklin Lakes, NJ, USA).
Caspase-3, -8 and -9 activity assay
The activity of caspase-3, -8 and -9 was determined
using the caspase-3, -8 and -9 activity kit (Beyotime Institute of
Biotechnology). To evaluate the activity of caspase-3, -8 and -9,
Hep-2-shRNA transfected and control Hep-2 cells were
homogenized in 100 ml reaction buffer [1% NP-40, 20 mM Tris-HCl (pH
7.5), 137 mM Nicotinamide adenine dinucleotide and 10% glycerol]
containing 10 ml caspase-3, -8 and -9 substrate (Ac-DEVD-pNA; 2 mM)
following all treatments. Lysates were incubated at 37°C for 2 h.
Samples were measured with an ELISA reader (Sunrise RC) at an
absorbance of 405 nm.
Subcutaneous xenografts in nude mice
All the animals (Vital River Laboratory Animal
Technology Co., Ltd., Beijing, China) in the present study were
housed in a pathogen-free facility and the animal experiments were
performed in accordance with the institutional animal welfare
guideline of The Center for Laboratory Animal Research, China
Medical University (Shenyang, Liaoning, China). Cells were injected
subcutaneously into BALB/c nude mice (6–8 weeks). The tumor volume
(TV) was calculated using the following formula: TV
(mm3) = (Length × Width2) / 2.
Statistical analysis
Unless otherwise stated, each experiment was
performed a minimum of three times. Data were subjected to
statistical analysis using Statistical Package for the Social
Sciences software (version 13.0; SPSS, Inc., Chicago, IL, USA) and
presented as the mean ± standard error of the mean. The independent
Student’s t-test or analysis of variance was used to compare the
continuous variables among groups. The Kaplan-Meier estimate was
used for survival analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
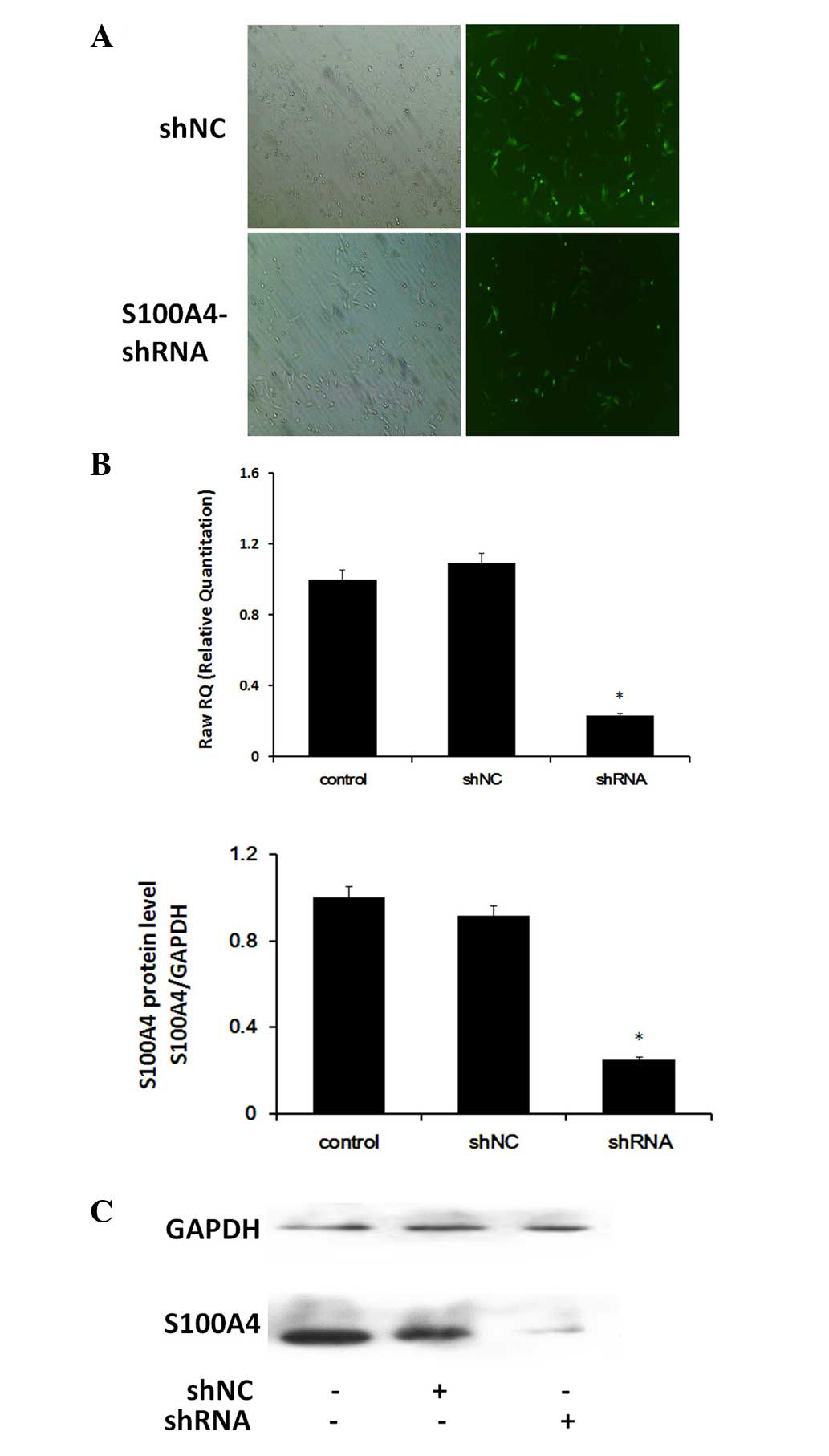
Expression of S100A4 following shRNA
transfection in Hep-2 cells
The mRNA and protein expression of S100A4 in
cultured Hep-2 cells 72 h after shRNA transfection was detected by
RT-qPCR and western blot analysis. Hep-2-shNC cells were also
transfected as a control (Fig.
1A). The results of the analysis of S100A4 are shown in
Fig. 1. Following transfection
with S100A4 shRNA, the Hep-2 cells showed significant
downregulation of the S100A4 gene at the mRNA and protein
levels (Fig. 1B and C;
P<0.05).
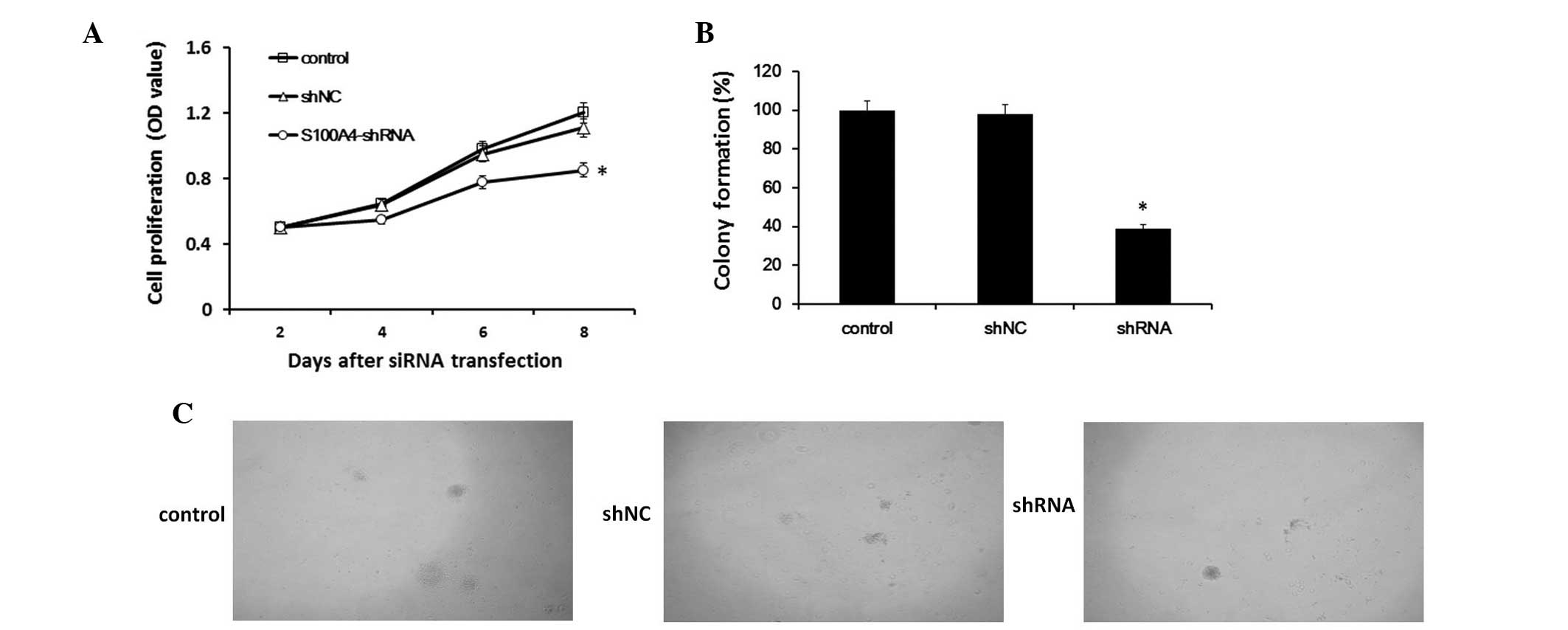
Downregulation of S100A4 expression
decreases the proliferation of Hep-2 cells
As determined by the CCK-8 assay, inhibition of
S100A4 in Hep-2 cells resulted in a significant decrease in
cellular proliferation at 2, 4, 6 and 8 days after transfection
(P<0.05). The results indicated that S100A4 suppression
correlated with decreased proliferation in Hep-2 cells (Fig. 2A).
Effect of S100A4 inhibition on
anchorage-independent growth
The anchorage-independent growth of cells was
determined by assaying colony formation in soft agar. Our results
demonstrated that, compared with Hep-2-shNC cells, the colonies in
the Hep-2-shRNA cells were significantly decreased in number and
notably smaller in size (Fig. 2B and
C).
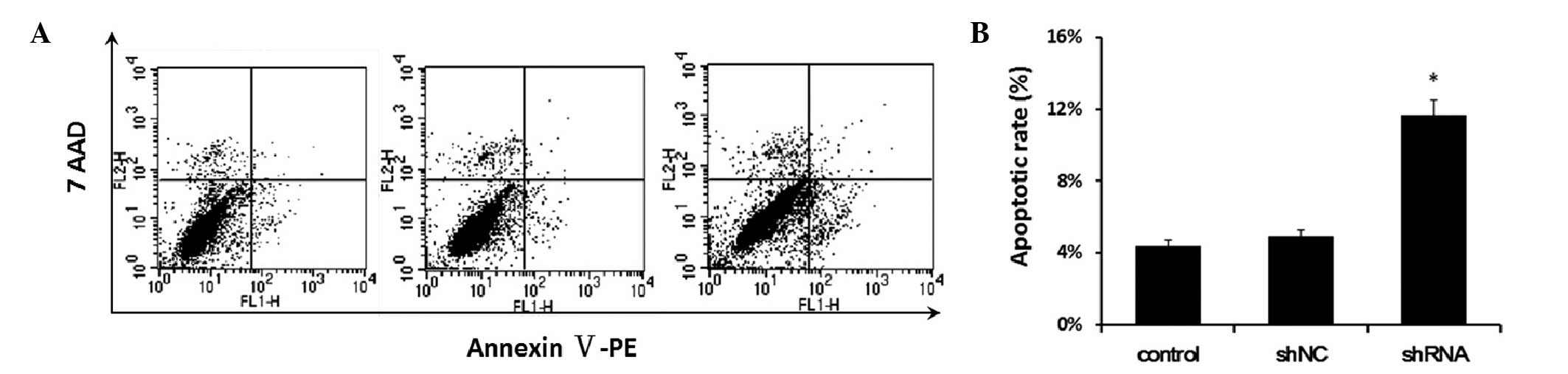
Downregulation of S100A4 expression
increases apoptosis of Hep-2 cells
Apoptosis of Hep-2-shNC and Hep-2-shRNA cells was
examined by flow cytometry. As shown in Fig. 3, after 3 days, 4.4 and 4.92% of the
control cells and Hep-2-shNC cells were apoptotic, while 11.7% of
Hep-2-shRNA cells were apoptotic.
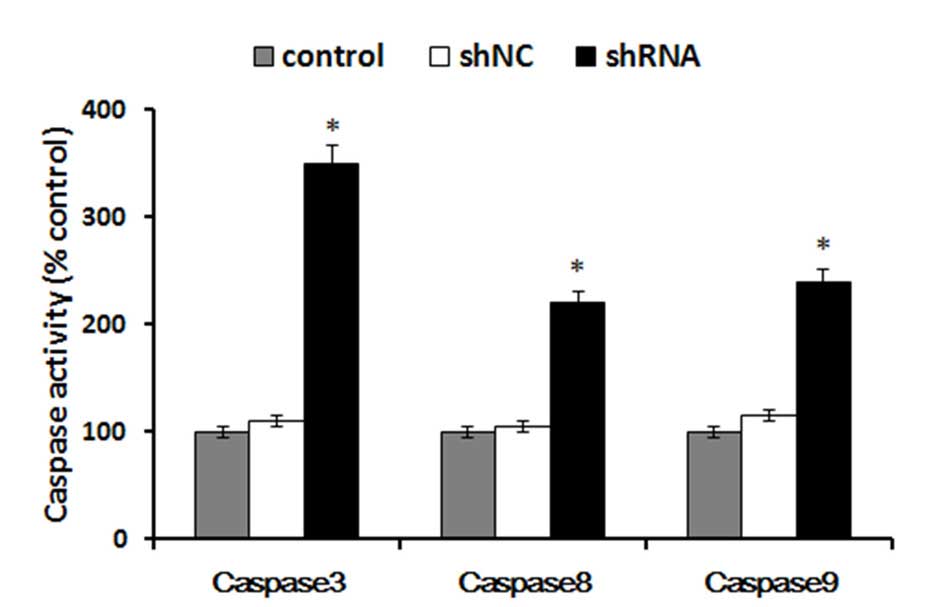
Downregulation of S100A4 increases the
activity of caspase-3, -8 and -9
Caspase proteins are cysteine proteases that act
downstream of the B-cell lymphoma 2 family by initiating cellular
breakdown during apoptosis. Among the effector caspases, caspase-3
is the most frequently involved in apoptosis. To determine whether
caspase-3, -8 and -9 are activated following S100A4 shRNA
treatment, caspase-3, -8 and -9 activity was measured by the
caspase-3, -8 and -9 activity kit. In the present study, the
results demonstrated that compared with the control cells,
treatment of cells with S100A4 shRNA caused a significant increase
in caspase-3, caspase-8 and caspase-9 activation (P<0.05). The
activity of caspase-3, caspase-8 and caspase-9 in cells treated
with S100A4 shRNA increased >12% (P<0.05), compared with
cells treated with control shRNA (Fig.
4). These results suggested that the downregulation of S100A4
was able to significantly increase the activity of caspase-3,
caspase-8 and caspase-9 in Hep-2 cells.
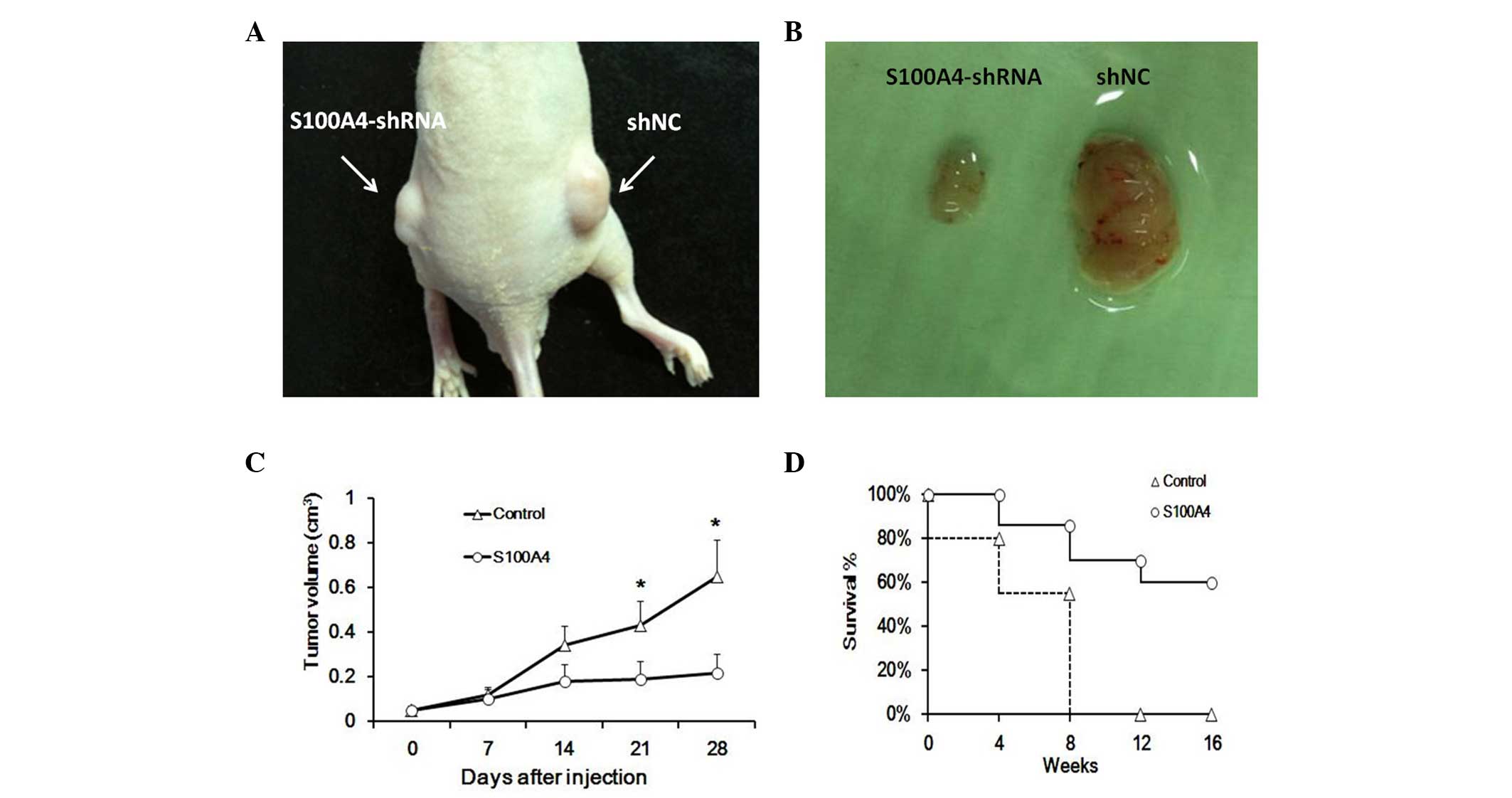
Intratumoral injection of S100A4
suppresses the development of human Hep-2 cells in nude mice
Since inhibition of S100A4 reduced Hep-2 cell
proliferation in vitro, whether these in vitro data
have in vivo relevance was examined. To accomplish this
goal, Hep-2 cells generated tumors when 2×105 cells were
injected into the flank of nude mice. Visible tumors had developed
at the injection sites within 2 weeks (Fig. 5A and B). The S100A4 knockdown Hep-2
cells significantly reduced the tumor volumes and prolonged the
survival rate of nude mice (Fig. 5C
and D). Our data indicated that downregulation of S100A4
decreased the tumorigenicity of laryngeal cancer in
vivo.
Discussion
Previous studies have demonstrated that S100A4
expression is correlated with a poor prognosis in cancer patients
(8,14,15).
S100A4 is involved in tumor metastasis by affecting cell growth,
invasion and motility (16,17).
Our previous study reported a significant correlation between
S100A4 expression levels and LSCC (11). However, the role of S100A4 in
laryngeal cancer remains to be elucidated. To address the question
of whether S100A4 was able to serve as a therapeutic target for
laryngeal cancer, the RNA interference method was employed in an
attempt to reduce the expression of S100A4 in cultured Hep-2 cells.
As shown in Fig. 1, significant
suppression of S100A4 expression was observed using RT-qPCR and
western blotting.
S100A4 is important in a variety of cellular events,
including cell growth and apoptosis (18,19).
However, previous discrepancy studies suggest the effect of S100A4
on cell growth and apoptosis depends on the cell type. For
instance, the suppression of S100A4 reduces the proliferative
potential and induction of apoptosis in certain cancer cells,
including pancreatic cancer cells (20) and gastric cancer cells (21) in vitro and in vivo.
Whereas another study demonstrated that in human osteosarcoma
cells, in vitro cell proliferation was unaltered by
anti-S100A4 ribozyme (22).
Compared with anti-S100A4 ribozyme transfected counterpart II-11b
cells, the S100A4-secreting human osteosarcoma cell line OHS was
more sensitive to IFN-γ-mediated apoptosis in a previous study, and
the S100A4/IFN-γ-mediated induction of apoptosis was demonstrated
to be independent of caspase activation (23). The in vitro growth
suppression of laryngeal cancer Hep-2 cells was analyzed. The
present study demonstrated that the growth of Hep-2 cells was
inhibited by transfection with S100A4 shRNA. Furthermore,
anchorage-independent growth refers to the ability of cells to
survive and proliferate in the absence of attachment to the
extracellular matrix, which is modeled in vitro by colony
formation in soft agar. Our data demonstrated that, compared with
shNC and control group cells, colony formation of Hep-2-shRNA cells
was profoundly suppressed.
To elucidate the growth and colony formation
suppression, FACS analyses was performed 3 days after transfection
of the shRNA and the results indicated that the shRNA-mediated
knockdown of S100A4 led to apoptosis of the laryngeal cancer cells.
It was hypothesized that the increased apoptosis in Hep-2 cells
transfected with S100A4-shRNA was able to contribute to the
reduction of cell numbers. Previous studies demonstrated that
S100A4 interacts with the tumor suppressor p53 to affect cell
growth and p53-dependent apoptosis (24). It was suggested that the control
functions of p53 will be abrogated at the G1-S checkpoint when the
complex of p53 with S100A4 and the sequestration of p53 stimulate
the cell enter to the S phase. The p53-dependent transactivation
target genes were related with the state of apoptosis. S100A4
knockdown leads to p53-dependent cell cycle arrest and increased
cisplatin-induced apoptosis (25).
Apoptosis is a type of cell death that may occur in
multicellular organisms. It represents highly programmed mechanisms
by activated proteolytic caspases. Exploration of the underlying
mechanisms of apoptosis by elucidating the patterns of these
factors in the apoptosis may be critical. It is understood that
caspase-8 is important in transduction of the death-receptor
pathway while caspase-9 is involved in the mitochondrial pathway
(26). Once activated, caspase-8
and caspase-9 activate downstream caspase-3, which is a key
executioner caspase for triggering cell apoptosis (27). However, a previous study suggested
that caspase-8 is not always activated early in the context of Fas
signaling, in certain cells, caspase-3 in turn activates caspase-6,
which was revealed to be required for the activation of downstream
caspase-8 (28). The present study
demonstrated that the activity of caspase-3, -8 and -9 was higher
in response to S100A4-shRNA, suggesting that apoptosis proceeded at
least via the mitochondrial pathway. Overall, future research in
order to delineate the details of how S100A4 is involved in
apoptosis is required.
Following determining the functions of S100A4 in
Hep-2 cells in vitro, the present study next sought to
determine whether downregulation of S100A4 expression reduces the
tumor-forming ability of Hep-2 cells in vivo. The present
study aimed to address this question by using S100A4 shRNA in a
xenograft laryngeal cancer model. Knockdown of S100A4 in Hep-2
cells significantly reduced the tumor volumes and prolonged the
survival rate of nude mice. Shi et al (29) obtained similar results in which
S100A4 knockdown was able to inhibit the formation and growth of
thyroid cancer. Lo et al (30) identified that knockdown of S100A4
affected epithelial-mesenchymal transition-calcium-embryonic stem
cells associated genes, including TP53, Notch2, phosphatase and
tensin homolog (PTEN) and phosphoinositide 3-kinase (PI3K). The
Notch and PTEN/PI3K/Akt signaling pathways have been demonstrated
to regulate the tumorigenicity of cancer (30). All these results suggest the
importance of aberrant expression of S100A4 in the cancer process
and intratumoral injections of S100A4-shRNA exert a strong
antitumor effect in vivo.
In conclusion, the present study demonstrated that
inhibition of S100A4 through RNA interference resulted in
significantly decreased cell proliferation and increased apoptosis
in laryngeal cancer in vitro and in vivo. The present
study also provided evidence that S100A4 promoted apoptosis through
released caspase-3, -8 and -9. Whether apoptosis occurred via the
mitochondrial pathway or the death receptor-mediated pathway and
which factors were involved in this process requires further
investigation. The present study showed that S100A4 exhibits a
major role in Hep-2 cell proliferation and apoptosis and targeting
of the S100A4 signaling may be a potential therapeutic target for
laryngeal cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81301766).
References
|
1
|
Ragin CC, Modugno F and Gollin SM: The
epidemiology and risk factors of head and neck cancer: a focus on
human papillomavirus. J Dent Res. 86:104–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mahon PC, Baril P, Bhakta V, et al: S100A4
contributes to the suppression of BNIP3 expression,
chemoresistance, and inhibition of apoptosis in pancreatic cancer.
Cancer Res. 67:6786–6795. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pedersen MV, Køhler LB, Grigorian M, et
al: The Mts1/S100A4 protein is a neuroprotectant. J Neurosci Res.
77:777–786. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sekine H, Chen N, Sato K, et al: S100A4,
frequently overexpressed in various human cancers, accelerates cell
motility in pancreatic cancer cells. Biochem Biophys Res Commun.
429:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boye K, Nesland JM, Sandstad B, Haugland
Haugen M, Mælandsmo GM and Flatmark K: EMMPRIN is associated with
S100A4 and predicts patient outcome in colorectal cancer. Br J
Cancer. 107:667–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Liu Z, Xu C, et al: Overexpression
of S100A4 is closely associated with the progression and prognosis
of gastric cancer in young patients. Oncol Lett. 5:1485–1490.
2013.PubMed/NCBI
|
|
9
|
Klingelhöfer J, Grum-Schwensen B, Beck MK,
et al: Anti-S100A4 antibody suppresses metastasis formation by
blocking stroma cell invasion. Neoplasia. 14:1260–1268.
2012.PubMed/NCBI
|
|
10
|
Wang Z and Griffin M: The role of TG2 in
regulating S100A4-mediated mammary tumour cell migration. PLoS One.
8:e570172013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu J, Guo Y, Fu S, Yang M, Sun KL and Fu
WN: Hypomethylation-induced expression of S100A4 increases the
invasiveness of laryngeal squamous cell carcinoma. Oncol Rep.
23:1101–1107. 2010.PubMed/NCBI
|
|
12
|
Toolan HW: Transplantable human neoplasms
maintained in cortisone-treated laboratory animals: H.S. No. 1; HEp
No. 1; HEp No. 2; HEp No. 3; and HEmbRh No. 1. Cancer Res.
14:660–666. 1954.PubMed/NCBI
|
|
13
|
Boehm D, Herold S, Kuechler A, Liehr T and
Laccone F: Rapid detection of subtelomeric deletion/duplication by
novel real-time quantitative PCR using SYBR-green dye. Hum Mutat.
23:368–378. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Zhang T and Wang Q: S100
calcium-binding protein A4 is a novel independent prognostic factor
for the poor prognosis of gastric carcinomas. Oncol Rep.
30:111–118. 2013.
|
|
15
|
Roh J, Knight S, Chung JY, et al: S100A4
expression is a prognostic indicator in small intestine
adenocarcinoma. J Clin Pathol. 67:216–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Berge G, Pettersen S, Grotterød I, Bettum
IJ, Boye K and Mælandsmo GM: Osteopontin - an important downstream
effector of S100A4-mediated invasion and metastasis. Int J Cancer.
129:780–790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sack U, Walther W, Scudiero D, et al:
S100A4-induced cell motility and metastasis is restricted by the
Wnt/beta-catenin pathway inhibitor calcimycin in colon cancer
cells. Mol Biol Cell. 22:3344–3354. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Luther G, Zhang W, et al: The E-F
hand calcium-binding protein S100A4 regulates the proliferation,
survival and differentiation potential of human osteosarcoma cells.
Cell Physiol Biochem. 32:1083–1096. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang XC, Wang X, Luo L, et al: RNA
interference suppression of A100A4 reduces the growth and
metastatic phenotype of human renal cancer cells via
NF-kB-dependent MMP-2 and bcl-2 pathway. Eur Rev Med Pharmacol Sci.
17:1669–1680. 2013.PubMed/NCBI
|
|
20
|
Tabata T, Tsukamoto N, Fooladi AA, et al:
RNA interference targeting against S100A4 suppresses cell growth
and motility and induces apoptosis in human pancreatic cancer
cells. Biochem Biophys Res Commun. 390:475–480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hua J, Chen D, Fu H, et al: Short hairpin
RNA-mediated inhibition of S100A4 promotes apoptosis and suppresses
proliferation of BGC823 gastric cancer cells in vitro and in vivo.
Cancer Lett. 292:41–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maelandsmo GM, Hovig E, Skrede M, et al:
Reversal of the in vivo metastatic phenotype of human tumor cells
by an anti-CAPL (mts1) ribozyme. Cancer Res. 56:5490–5498.
1996.PubMed/NCBI
|
|
23
|
Pedersen KB, Andersen K, Fodstad Ø and
Maelandsmo GM: Sensitization of interferon-gamma induced apoptosis
in human osteosarcoma cells by extracellular S100A4. BMC Cancer.
4:522004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grigorian M, Andresen S, Tulchinsky E, et
al: Tumor suppressor p53 protein is a new target for the
metastasis-associated Mts1/S100A4 protein: functional consequences
of their interaction. J Biol Chem. 276:22699–22708. 2001.
View Article : Google Scholar
|
|
25
|
Orre LM, Panizza E, Kaminskyy VO, et al:
S100A4 interacts with p53 in the nucleus and promotes p53
degradation. Oncogene. 32:5531–5540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cho SG and Choi EJ: Apoptotic signaling
pathways: caspases and stress-activated protein kinases. J Biochem
Mol Biol. 35:24–27. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin
(Shanghai). 37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Slee EA, Harte MT, Kluck RM, et al:
Ordering the cytochrome c-initiated caspase cascade: hierarchical
activation of caspases-2, -3, -6, -7, -8, and -10 in a
caspase-9-dependent manner. J Cell Biol. 144:281–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shi Y, Zou M, Collison K, et al:
Ribonucleic acid interference targeting S100A4 (Mts1) suppresses
tumor growth and metastasis of anaplastic thyroid carcinoma in a
mouse model. J Clin Endocrinol Metab. 91:2373–2379. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lo JF, Yu CC, Chiou SH, et al: The
epithelial-mesenchymal transition mediator S100A4 maintains
cancer-initiating cells in head and neck cancers. Cancer Res.
71:1912–1923. 2011. View Article : Google Scholar : PubMed/NCBI
|