Introduction
Subarachnoid hemorrhage (SAH), one of the serious
and life-threatening types of stroke incurred by bleeding into the
space surrounding the brain, occurs when brains are deprived of
oxygen by various factors, particularly an interruption to the
blood supply or a ruptured aneurysm (1). Approximately 5–10% of all strokes are
induced by SAH resulting from ruptured berry aneurysms. There are
~200,000 new SAH patients in China each year, which account for
~6–8% of all stroke diagnosis (1).
Due to the poor prognoses resulting from SAH, the mortality rate of
SAH patients is ~50%, which accounts for 22–35% of the mortalities
of all cerebral vasospasm (CVS) patients. Only 20–35% SAH patients
recover their full health (1).
CVS is one of the most common complications of SAH
patients. In 1959, Beard et al (2) first discovered narrowing of the
arterial diameter in patients that had experienced SAH. Ecker and
Riemenschnieder described clinical characteristics of CVS in detail
in an analysis of cerebral angiography images in 1951.
Subsequently, Ecker and Riemenschneider (4) were the first to outline the concept
of CVS and described the clinical features mentioned above, which
attracted the interest of the neurosurgical community. It is
generally accepted that cerebral ischemia and cerebral infarction
induced by CVS are the main causes of mortality and disability, and
15–30% patients suffer from delayed ischemic neurological deficits.
Although the pathogenesis of CVS has been studied for the past 50
years, the mechanism remains unclear (4).
Currently, the treatments for SAH involve prevention
of vasospasm, relief of blood pressure in the brain, cessation of
bleeding, and restoration of normal blood flow. SAH is mainly
diagnosed using urgent computed tomography (CT) scanning without
contrast, combined with radiology. However, a lumbar puncture is
used following a negative CT scan. Therefore, ~99% SAH can be ruled
out by non-contrast CT and CT angiography in the brain (1). More effective and accurate diagnosis
and treatment methods, and criteria for clinical practices are
required (1).
Platelet-derived growth factor (PDGF) is a mitogenic
growth factor located in platelets (5). Borel et al (6) observed that the levels of PDGF-β
increased following SAH in human patients, which may be associated
with peripheral thrombosis, and therefore contribute to CVS.
Increased levels of PDGF were also detected in ischemic brain
injury and brain puncture wounds in recent studies (5,6).
However, the expression of PDGF in patients that experience CVS
following SAH remains unknown.
In the present study, post-SAH CVS rat models were
created using endovascular puncture and employed to analyze the
expression patterns of PDGF by enzyme-linked immunosorbent assay
(ELISA) and immunohistochemistry (IHC).
Materials and methods
Reagents
A PDGF-β IHC kit was purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). A PDGF-β ELISA kit was
purchased from BD Biosciences (San Jose, CA, USA).
Animals
A total of 66 healthy New Zealand rabbits were
provided by the Animal Center of the Medical College of Soochow
University (Suzhou, China). For experiments involving animals,
approval was obtained from the Institutional Review Committee of
Soochow University. Animals were randomly divided into three
groups: The normal control group; the sham surgery group (a guide
wire was inserted into arteria carotis interna without
piercing the vessel); and the SAH group. SAH subgroups were
prepared at time points of 3 and 12 h, and 1, 2, 3, 7 and 14 days
following SAH, with each subgroup containing 5 rabbits. The normal
control group and the sham surgery group included 5 rabbits each.
Throughout the experiment, 21 rabbits died due to the procdedure
performed in the present study.
CVS rabbit models
Post-SAH CVS rabbit models were created using the
endovascular puncture method. Briefly, experimental rabbits were
anesthetized by a 3% pentobarbital (2.0–3.0 ml/kg) injection
through veins in the ears. The rabbits were oriented in the supine
position under racks with their heads and limbs fixed. The heads of
the rabbits were straightened out. Rabbits were placed on DSA
machine tool (innova310; General Electric, Fairfield, CT, USA) and
the fur of the inguinal region was shaved. Rabbits were disinfected
with iodophors and placed on aseptic towel sheets. A cut of 1.5–2.0
cm was made to the top of the right inguinal artery, and the
arteria femoralis was separated. The catheter insertion was
performed according to the method previously described in the study
by Gokal et al (7). A Y
valve was inserted into the upper segment of the carotid artery at
the junction of the internal and external carotid arteries, by
replacing the catheter guide wire using fluoroscopic
surveillance.
CT examination
Unenhanced CT scanning of the rabbit heads was
conducted in the various groups prior to surgery. Scanning
parameters were as follows: Depth of stratum, 2.5 mm; spacing, 2.5
mm; field of view, 80 mm and 16 layers. Quantity evaluation of SAH
was performed as described by the Fisher grading method (1). Unclear definition of the subarachnoid
space along with a pointed-shaped high density image was designated
as class I (1). Annular high
density image of the subarachnoid space along with multiple levels
was designated as class II (1).
High density image of the subarachnoid space along with multiple
levels was designated as class III (1). Unenhanced CT scanning of the rabbit
heads was conducted in the different time point groups prior to
execution in order to evaluate SAH changes.
Blood sample preparation
Whole blood (5 ml) was drawn from the femoral veins
of each rabbit prior to execution using a disposable syringe. Blood
was rested in tubes for 30 min, and centrifuged for 10 min at a
speed of 5,000 × g to separate the serum and collect the
supernatant. Supernatants were stored in a freezer at −20°C for
subsequent use.
Perfusion
The rabbits were sacrificed by intravenous injection
with pentobarbital sodium. Subsequently, the chest skin of
sacrificed rabbits in the different time point groups was prepared,
and the pleura and pericardium were exposed with an incision 1.5 cm
from the subcostal area between the two sides of the breastbone.
When the heart and aortic root were exposed completely, an infusion
needle was inserted at the root of the ascending aorta and fixed
with a vascular clamp. The right atrium was cut open to drain
blood. The inferior vena cava was closed using a clamp in order to
exclude backflow of blood. Perfusion was conducted using 0.1 mol/l
phosphate-buffered saline in a 50-ml syringe. When clear liquid
effused from the right atrium, the intracranial artery and brain
tissue were collected by opening the skull, and then fixed with
fixative solution, including 4% paraformaldehyde and phosphate
buffer.
Hematoxylin and eosin (HE) staining
The rabbits were subjected to routine pathological
examinations. When slicing, cross sections and neighboring brain
tissues of the superior segment of the basilar artery (BA) were
selected, in addition to vascular tissues and neighboring brain
tissues of the posterior communicating artery (PCoA). Sections were
prepared by paraffin wax embedding, and routine HE staining was
performed. Vessel diameters and the thickness of vessel walls of
the BA and PCoA were measured under HE staining and ×100
magnification fields. Vessel diameters were represented as the mean
of the vessel diameters of axis of bank and lateral axis within the
lumen, and vessel thickness was measured by Ilker G method
(1,2).
PDGF-β measurement
PDGF-β levels in the sera were measured using ELISA
with the PDGF-β kit. The concentration of PDGF-β was calculated
according to the optical density values of the serum samples and
the constructed standard curve.
IHC
To examine the expression of PDGF-β in vascular
smooth muscle cells in the present study, the streptavidin-biotin
immunoperoxidase method and IHC (PDGF-β IHC kit) were employed
according to the manufacturer’s instructions, and were performed as
the methods elsewhere (5,6). Semi-quantitative analysis was
conducted according to the ratio of the positive cells and the
color of the staining. The Q-test was used to compare the
groups.
Diagnosis criteria for CVS
In order to define post-SAH CVS in the current
study, a narrowing of the lumen diameter of the large vessels was
regarded as the necessary criterion for diagnosis.
Statistical analysis
The data are presented as the mean ± standard
deviation. The statistical significance of the data was determined
by one-way analysis of variance and the F-test in SPSS version 13.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference. P<0.01 was
considered to indicate an highly statistically significant
difference.
Results
Construction of SAH models
SAH is incurred by bleeding into the subarachnoid
space of the brain, and CVS is one of the most common complications
experienced by patients following SAH. To elucidate the mechanism
of SAH and provide useful information for clinical practice, SAH
rabbit models were created. A total of 35 SAH rabbit models were
successfully created out of all 66 rabbits, with a success rate of
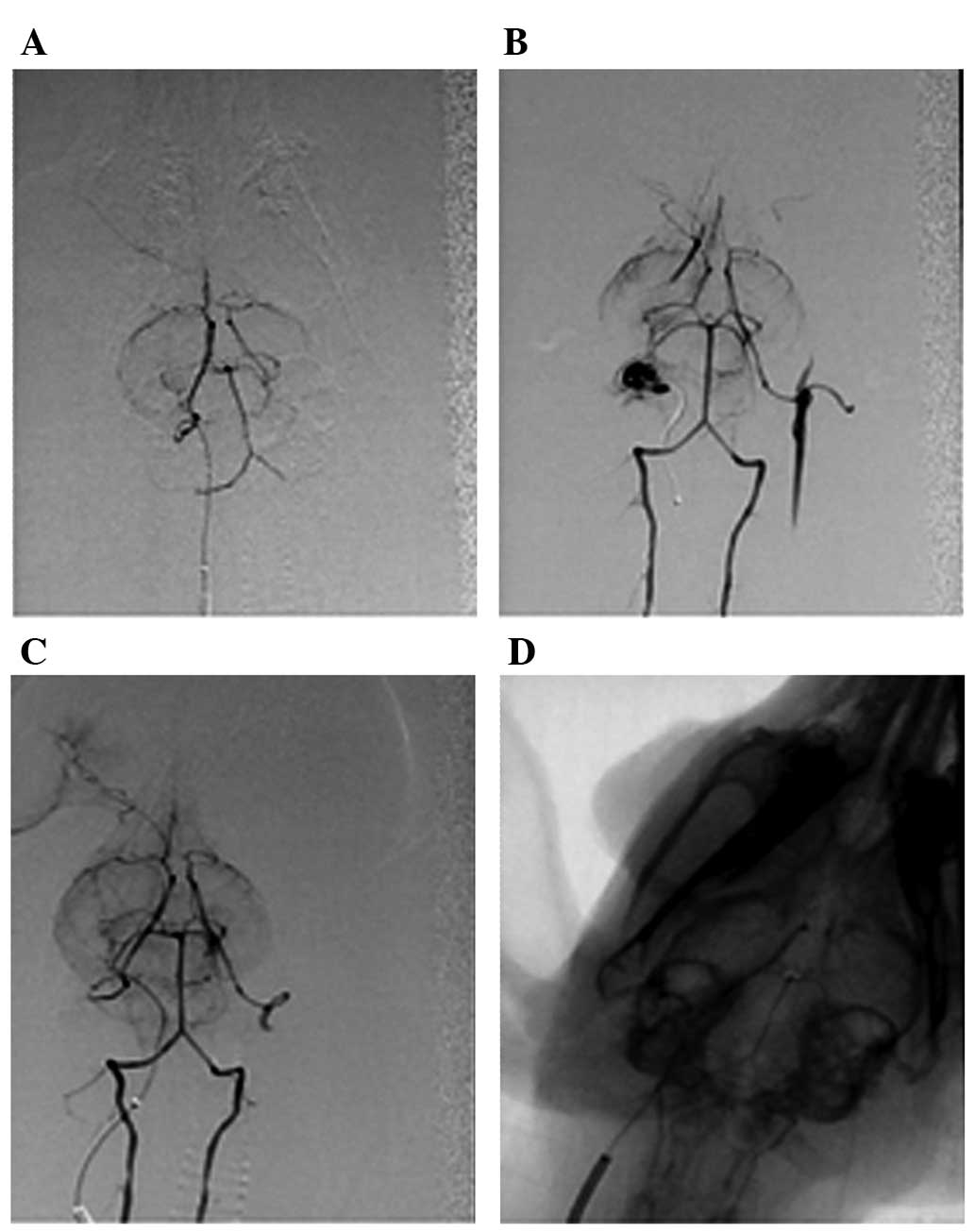
62.5%. The internal carotid artery angiography results from various
groups are represented in Fig.
1.
CT scanning results
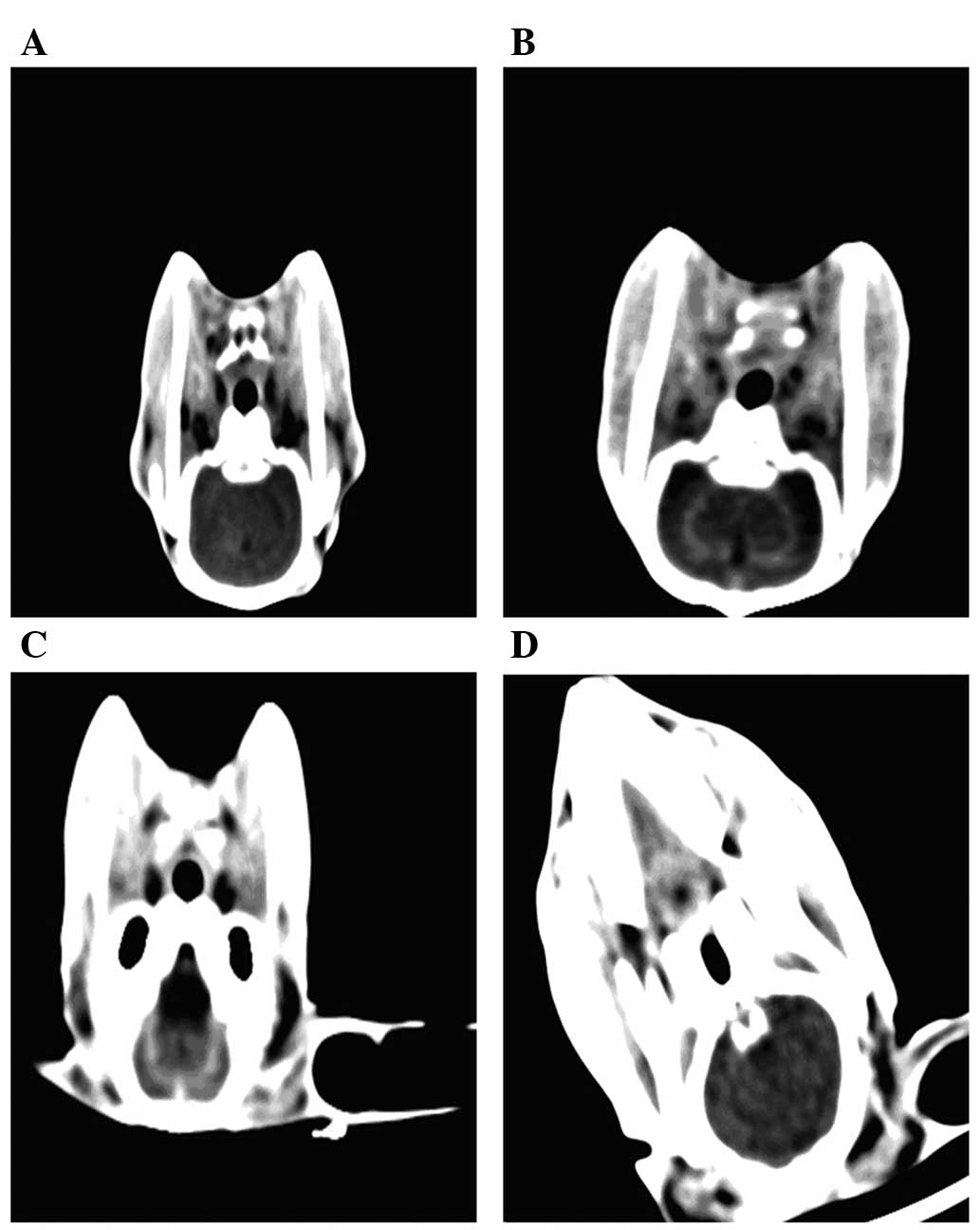
The subarachnoid space was clearly visible in CT
scans of the rabbits prior to surgery (Fig. 2). Examination of the skulls of the
35 successfully constructed SAH rabbit models, and CT scans of the
heads following surgery demonstrated that there were 8 cases of
class I, 16 cases of class II and 11 cases of class III SAH. There
was no evidence of subdural or intracranial hematoma, or changes to
pneumocephalus for all the normal control, sham surgery and model
rabbits. Pre-sacrifice CT scanning of rabbit heads at different
time points demonstrated that there was less rabbit subarachnoid
blood at the 3 h and 2 day time points than that in the
pre-operative rabbits. There were clear subarachnoid spaces in 4
cases in the groups between 3 and 14 days. There were changes to
pneumocephalus and intracranial hematoma with varying amounts of
bleeding, in the rabbits with unintentional mortality. The rabbits
that accidently died were scanned immediately. The animals were
checked every 1–2 days.
Animal ethology
The SAH rabbit groups exhibited numerous
characteristics including somnolence, weakness and anorexia in the
12–16 h following surgery. There were 2 rabbits that slept for 3
days, and 23 rabbits with a significant improvement in mental
status evaluated by the Short Portable Mental Status Questionnaire
by 1 day post-SAH compared with that immediately after SAH. The 14
day SAH group completely recovered, and there was 1 rabbit in the 3
day group with a significant improvement in mental status just 3 h
post-SAH, comparable to the class II SAH, but it died accidently
the following day.
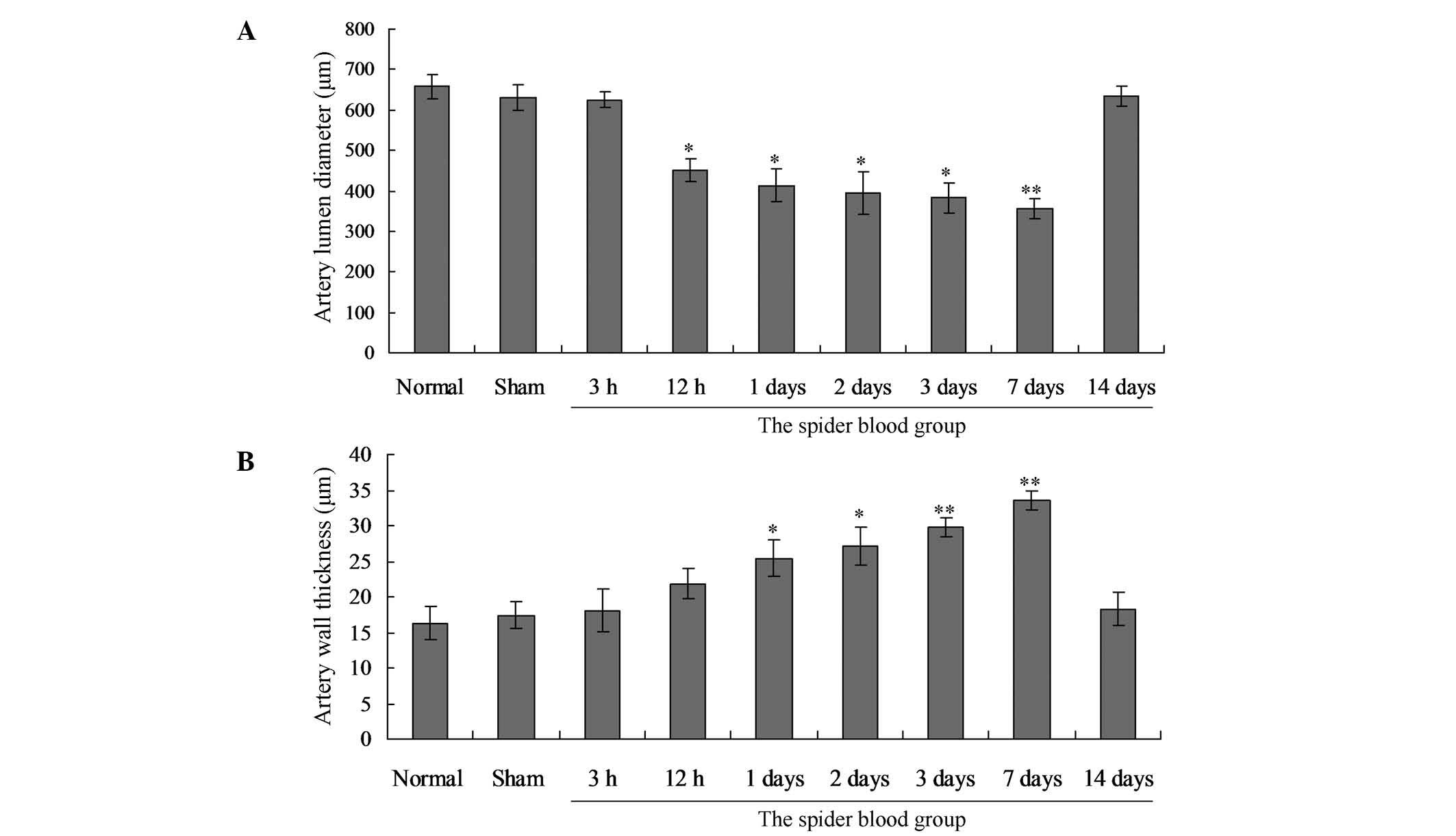
Vasospasm morphology
The narrowing of the PCoA occurred throughout the
1–7 day subgroups, and there was a 31.2% reduction in the 7 day
subgroup (this was the greatest decrease compared with the 1 day
subgroup). There were significant differences between the narrowing
of lumens in the SAH groups and in the normal control or sham
surgery groups (P<0.01). Statistical differences in the 3 h
group were observed. However, there was no difference in the groups
of 12 h, 1 day, 2 days, 3 days, 7 days and 14 days (Fig. 3A).
The thickness of the PCoA wall increased markedly in
the 7 day group compared with that of the normal control group.
Statistically significant differences in the vessel wall thickness
between the 3 and 12 h, and 1, 2 and 3 day SAH groups and the
normal control or sham surgery group were observed. Specifically,
there was a 58.1% increase in vessel wall thickness compared with
that of the control and the sham surgery groups. No statistically
significant difference was identified between other groups
(Fig. 3B).
Similar to the PCoA, the diameter of the BA lumen
narrowed significantly (decrease of 52.3%) in the 7 day group
compared with that in the normal control group. The thickness of
the BA wall increased in the 7 day group (48.8% increase) compared
with that of the control group and the sham surgery group (data not
shown).
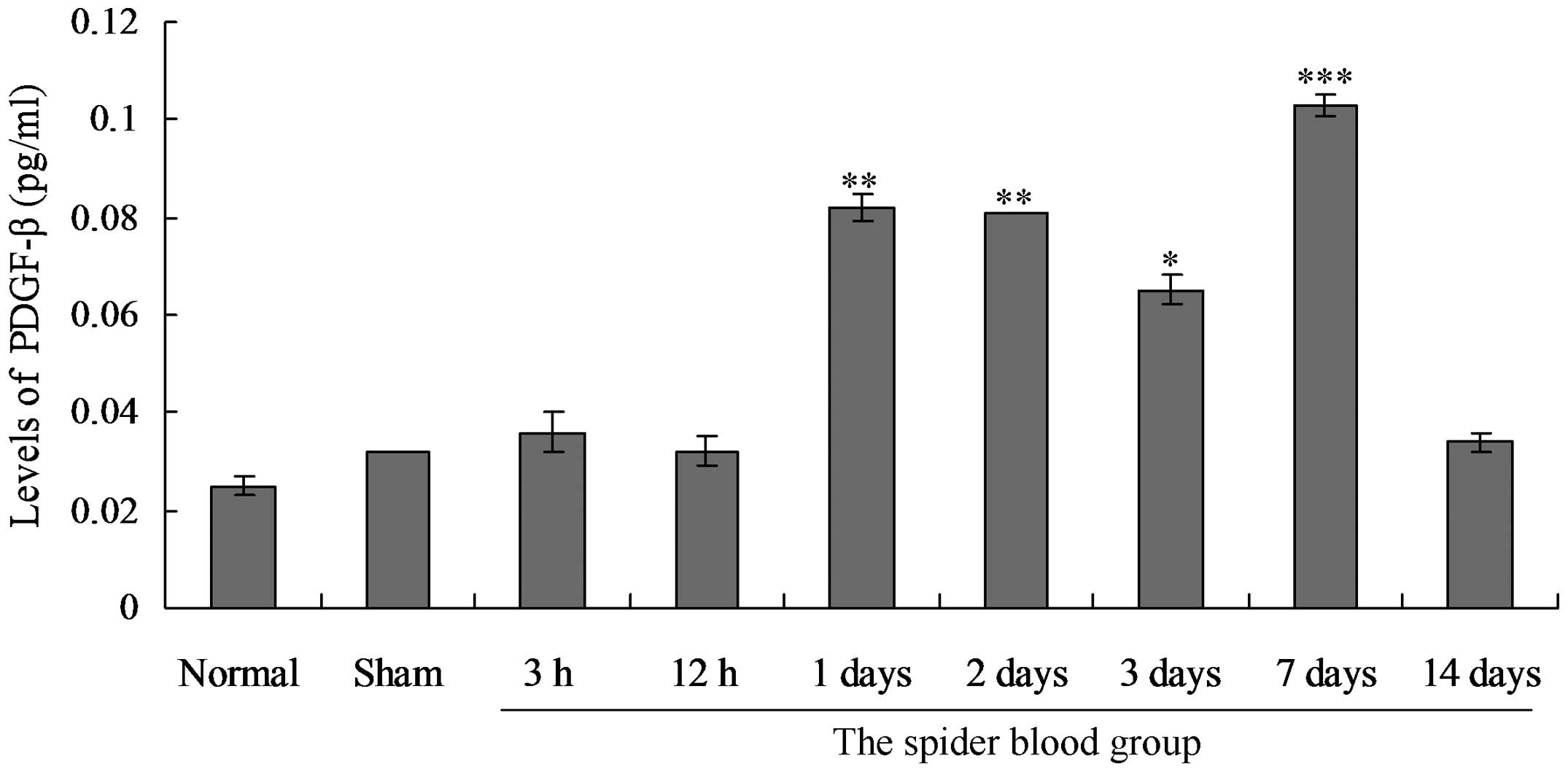
PDGF-β expression in serum of SAH rabbit
models
The expression of PDGF-β in serum was detected in
all groups. Serum PDGF-β expression levels were not significantly
different between the normal control group and the sham surgery
group (P>0.05) (Fig. 4). In the
SAH groups, expression of PDGF-β was first detected in the 3 h
group at low levels. The PDGF-β expression levels were markedly
higher in the 1 day group, and greatest in the 7 day group.
Significant differences in the expression levels of PDGF-β between
the SAH groups and controls were observed. Furthermore, PDGF-β was
highly expressed in the majority of SAH groups throughout the
experiment (P<0.05), and the levels of PDGF-β in the 14 day
group were higher than those of the control and sham surgery
groups.
Histopathology results
In the control and sham surgery groups, the
structure of the vessel walls was normal, and the tunica intima
consisted of endothelial cells with normal structures (Fig. 5). The tunica media consisted of
smooth muscle cells and their surrounding extracellular matrices.
The tunica adventitia was composed of fibroblasts and loose
connective tissues. In comparison, all vessel endothelial cells
were disrupted in all SAH groups; intercellular spaces of smooth
muscle cells increased in size, with changes to the cytoplasm,
vacuoles and nuclear membrane; and a condensed nucleus were present
in a number of cells. One day following SAH, the nuclei of the
endothelial cells changed (with the condensed nucleus) and this was
most evident in the 2 and 3 day groups. There were also aggregated
platelets in the endothelial cells, which was most clear in the 7
day group, and elastic membranes were twisted.
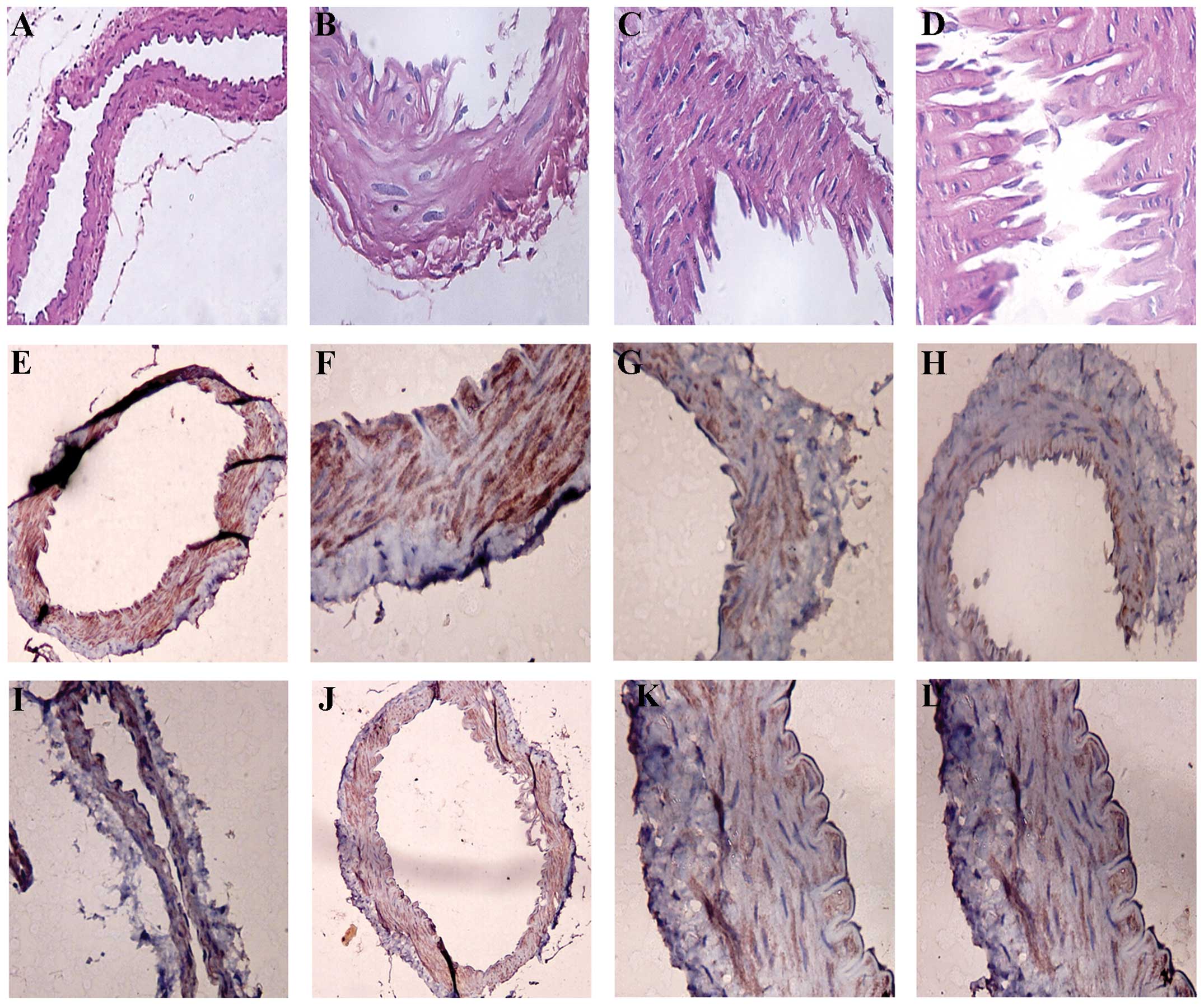 | Figure 5Results of hematoxylin and eosin (HE)
staining in various groups. (A) Normal basilar artery (staining,
HE; magnification, ×100). (B and C) Basilar artery wall is slightly
thickened in the 3 day subarachnoid hemorrhage (SAH) group
(staining, HE; magnification, ×400). (D) Posterior communicating
artery stenosis wall thickness in the 7 day SAH group (staining HE;
magnification, ×400). (E) The normal traffic artery, smooth muscle
cells of platelet-derived growth factor β (PDGF-β) (staining, SP;
magnification, ×100). (F) Normal basilar artery, smooth muscle
cells of PDGF-β (staining, SP; magnification, ×100). (G) PDGF-β in
the 3 day SAH group (staining, SP; magnification, ×400). (H) PDGF-β
in the 2 day SAH group (staining, SP; magnification, ×400). (I)
PDGF-β in the 1 day SAH group (staining, SP; magnification, ×100).
(J) PDGF-β in the 7 day SAH group (staining, SP; magnification,
×100). (K) PDGF-β in the 1 day SAH group (staining, SP;
magnification, ×400). (L) PDGF-β in the 7 day SAH group (staining,
SP; magnification, ×400). SP, streptavidin peroxidase. |
Histopathology of PDGF-β
In the control group and the sham surgery group, the
nuclei of smooth muscle cells were blue and there was no expression
of PDGF-β (Fig. 5). In
PDGF-β-positive cells, the cytoplasm and cytomembranes were pale
brown, and the nuclei were not stained (Fig. 5). A small number of PDGF-β-positive
cells from the PCoA were present in the 3 and 12 h SAH groups.
There were PDGF-β-positive smooth muscle cells in the 1 day group,
which were observed in the 2 and 3 day groups and peaked in 7 days
(Table I). By contrast, the 14 day
group had markedly lower numbers of positive cells in comparison to
the days 1–7 groups, which were still slightly higher than the
number in the normal control and the sham surgery groups. With
respect to PDGF-β expression levels, statistically significant
differences between the 1, 2 and 3 day groups and the normal
control and sham surgery groups were observed (P<0.05). There
were statistically significant differences between the 1 and 2 day
groups, and the 12 h and 1 day groups with respect to PDGF-β
expression levels (P<0.05).
 | Table IPDGF-β-positive cells and the gray
intensity scan. |
Table I
PDGF-β-positive cells and the gray
intensity scan.
| Groups | Gray intensity of
positive signals | No. of positive
cells |
|---|
| Normal control | 55.55±2.02 | 3.00±4.00 |
| Sham surgery | 56.66±2.22 | 9.00±6.60 |
| 3 h | 49.64±2.51a | 8.20±5.20b |
| 12 h | 51.90±1.88a | 4.20±6.72b |
| 1 day | 42.99±3.27b | 9.00±5.20b |
| 2 day | 50.88±1.88a | 4.60±9.80b |
| 3 day | 46.50±2.20a | 0.80±7.70b |
| 7 day | 40.81±3.34b | 10.60±9.24b |
| 14 day | 53.05±1.97a | 66.40±6.77b |
Discussion
Thus far, the mechanisms of the pathogenesis of CVS
are unknown, so it is necessary to create novel, more reliable
animal models to further investigate these mechanisms. Megyesi
et al (8) reported that 28%
of CVS animal models were created using endovascular puncture or
vascular laceration, and 72% by injecting blood into the
subarachnoid space or inserting a blood clot in vessels to induce
convulsion. However, there are various limitations to these SAH
animal models. For example, there is a high mortality rate in mouse
SAH models, so the models are mainly used in pathological and
physiological analysis of acute CVS. Furthermore, there are
numerous differences in vascular anatomy between mice and humans.
Cerebral ischemia and spasm do not usually appear throughout the
rich collateral circulation in such a short time window (8,9).
Therefore, a novel animal model for SAH is required. Rabbit SAH
models were first reported by Offerhau and Van Gool (7) in 1969. The periods of time prior to
spasm in the models were consistent with those in humans, so they
have been widely employed in SAH and CVS studies. In the present
study, rabbit CVS models were constructed and used. The mortality
rate of the model was ~37.2%, which is consistent with of previous
studies (7,10–12).
The limitations of the rabbit model in the present study included a
high mortality rate and high levels of intracranial bleeding, which
may be alleviated by improvements to surgical methods and
instruments.
Gules et al (13) discovered that the establishment
method of SAH model was not effective. There were also five rabbits
that did not establish the SAH model. Therefore, our result was
consistent with the study by Gules et al (13). The reason may be that the
micro-wire hard-head did not enter intracranial artery due to the
tortuosity of the initial part of the intracranial vessels. As the
effects of bleeding on the pathological and physiological changes
of vessels were difficult to evaluate in the CVS rabbit models
induced by endovascular puncture in the present study, CT scans of
heads were used and changes were evaluated according to Fisher
rating methods. Survival rates were high in class I and II cases,
with clear changes in vessel pathology. Additionally, spider blood
disappeared quickly, mostly in 2–3 days, and there was no spider
blood in the 14 day group based on the CT scan analysis. It was
reported by Woodcock et al (11) that magnetic resonance imaging
fluid-attenuated inversion recovery was better and more sensitive
than CT in diagnosing acute subarachnoid, which is consistent with
the present study.
PDGF is an important mitogenic agent that enhances
cell proliferation of vascular smooth muscle cells, and there is a
positive parallel correlation between expression of PDGF in the
injured vascular smooth muscle cells and cell proliferation
(14). Roles of PDGF in the
occurrence and development of CVS have attracted the interest of
numerous researchers since Borel et al (6) reported that PDGF-β expression levels
were greater in SAH patients compared with those of controls, and
that PDGF was involved in cell proliferation in mouse convulsion
models. Increased expression levels of PDGF were also detected in
ischemic brain injury and brain puncture wounds in a previous
study. Vieweg et al (12)
found that levels of PDGF-β in serum of dog convulsion models
increased in the third day and the seventh day, which was not
correlated with convulsion in radiography analysis. Although PDGF
is expressed in vascular endothelial cells and smooth muscle cells
in human patients and animal models, there has been little study of
the expression of PDGF in vasospasm animal models induced by
vascular rupture hemorrhage similar to that of humans. Therefore,
expression patterns of PDGF-β in post-SAH CVS rabbit models induced
by endovascular puncture method were analyzed in the present study.
Specifically, levels of serum PDGF-β were analyzed using ELISA and
expression levels of PDGF-β in vascular smooth muscle cells were
examined by IHC.
The results demonstrated that PDGF-β was expressed
in the 3 h post-SAH group, but not in the 12 h group. The
expression levels of PDGF-β increased in the 1 day group, and the
expression was clear in the 2 and 3 day groups. In addition, the
levels of PDGF-β peaked in the 7 day group, and expression levels
of PDGF-β decreased in the 14 day group, but were still higher than
those of the normal control group and the sham surgery group. The
majority of PDGF-β-positive cells were smooth muscle cells at the
earlier stages, the number of which increased in the 1 day group
and peaked in 7 day group. There were PDGF-β-positive endothelial
cells in some small vessels. Time-course expression of PDGF-β was
consistent with the activation and proliferation of smooth muscle
cells. There were PDGF-β-positive endothelial cells and vascular
smooth muscle cells, indicating that PDGF-β may participate in
activation and proliferation of smooth muscle cells, and
pathogenesis and development of vascular proliferation. This is
consistent with the findings of Borel et al (6), who also observed that PDGF-β was
highly expressed 7 days subsequent to SAH.
Human chronic CVS occurs ~3 days following SAH,
peaks at 6–7 days, and then is alleviated by ~14 days (15). In the present study, there was
further narrowing in the 7 day group which was alleviated in the 14
day group, consistent with patterns in humans. Vessel wall
thickness in the BA and PCoA were greatest in the 7 day group than
that at every other time point, which is consistent with the high
expression levels of PDGF-β in the 7 day group. This may be due to
smooth muscle cell spacing and numbers increased, and collencyte
and fibroblast numbers increased, leading to an enhanced thickness
of the vessel wall and narrowed lumen. However, the underlying
mechanism remains to be investigated. Future studies should focus
on time-course expression of PDGF-β in animal models and clinical
samples along with large-sample statistics. Occurrence, development
and the signaling pathways in CVS should also be emphasized.
In conclusion, there was PDGF-β expression in the
CVS rabbit models in the present study, which may aid the
elucidation of the pathogenesis of CVS, and also provide useful
information for diagnosis and treatment of CVS.
Acknowledgements
This study was supported by a grant of a Research
Contact with the Science and Technology Agency of Henan Province
(no. 082102310014).
References
|
1
|
Lee CI, Chou AK, Lin CC, et al: Immune and
inflammatory gene signature in rat cerebrum in subarachnoid
hemorrhage with microarray analysis. Mol Med Rep. 5:118–125.
2012.PubMed/NCBI
|
|
2
|
Beard EF, Robertson JW and Robertson RC:
Spontaneous subarachnoid hemorrhage simulating acute myocardial
infarction. Am Heart J. 58:755–759. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ecker A and Riemenschnieder PA:
Arteriographic demonstration of spasm of the intracranial arteries,
with special reference to saccular arterial aneurysms. J Neurosurg.
8:660–667. 1951. View Article : Google Scholar
|
|
4
|
Ecker A and Riemenschneider PA:
Arteriographic evidence of spasm in cerebral vascular disorders.
Neurology. 3:495–502. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosiak M, Postula M, Kaplon-Cieslicka A,
et al: Lack of effect of common single nucleotide polymorphisms in
leukotriene pathway genes on platelet reactivity in patients with
diabetes. Mol Med Rep. 8:853–860. 2013.PubMed/NCBI
|
|
6
|
Borel CO, McKee A, Parra A, et al:
Possible role for vascular cell proliferation in cerebral vasospasm
after subarachnoid hemorrhage. Stroke. 34:427–433. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gokal R, Alexander S, Ash S, Chen TW,
Danielson A and Holmes C: Peritoneal catheters and exit-site
practices towards optimum peritoneal access: 1998 update. Perit
Dial Int. 18:11–33. 1998.PubMed/NCBI
|
|
8
|
Offerhaus L and van Gool J:
Electrocardiographic changes and tissue catecholamines in
experimental subarachnoid haemorrhage. Cardiovasc Res. 3:433–440.
1969. View Article : Google Scholar
|
|
9
|
Shaw MD, Vermeulen M, Murray GD, et al:
Efficacy and safety of the endothelin, receptor antagonist TAK-044
in treating subarachnoid hemorrhage: a report by the Steering
Committee on behalf of the UK/Netherlands/Eire TAK-044 Subarachnoid
Haemorrhage Study Group. J Neurosurg. 93:992–997. 2000. View Article : Google Scholar
|
|
10
|
Mayberg MR, Okada T and Bark DH: The
significance of morphological changes in cerebral arteries after
subarachnoid hemorrhage. J Neurosurg. 72:626–633. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woodcock RJ Jr, Short J, Do HM, et al:
Imaging of acute subarachnoid hemorrhage with a fluid-attenuated
inversion recovery sequence in an animal model: comparison with
non-contrast-enhanced CT. AJNR Am J Neuroradiol. 22:1698–1703.
2001.
|
|
12
|
Vieweg U, Schramm J and Urbach H:
Platelet-derived growth factor (PDGF-AB) like immune reactivity in
serum and in cerebral spinal fluid following experimental
subarachnoid haemorrhage in dogs. Acta Neurochir (Wien).
141:861–866. 1999. View Article : Google Scholar
|
|
13
|
Gules I, Satoh M, Clower BR, et al:
Comparison of three rat models of cerebral vasospasm. Am J Physiol
Heart Circ Physiol. 283:H2551–H2559. 2002.PubMed/NCBI
|
|
14
|
Ellis EF, Nies AS and Oates JA: Cerebral
arterial smooth muscle contraction by thromboxane A2. Stroke.
8:480–483. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou HW, Li XG, Yan M, et al: Increased
leukocyte Rho-kinase activity in a population with acute coronary
syndrome. Mol Med Rep. 8:250–254. 2013.PubMed/NCBI
|