Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most
common cause of chronic liver disease, which includes a spectrum of
liver diseases from hepatic steatosis to nonalcoholic
steatohepatitis (1–3), where the latter is known to increase
the risk of liver cirrhosis and hepatocellular carcinoma (4). NAFLD is associated with metabolic
syndrome, consisting of obesity, diabetes, hyperlipidemia and
insulin resistance (5). As a form
of NAFLD, hepatic steatosis results from the accumulation of fat in
the liver, primarily through excessive transport of free fatty
acids from visceral adipose tissue into the liver and from an
imbalance in de novo lipid synthesis and catabolism in
hepatocytes (6–8). The prolonged energy imbalance
increases the amount of triglyceride (TG) content in adipose tissue
and these TGs are stored as lipid droplets in the liver. Thus,
hepatic steatosis is generally characterized by excess hepatic
lipid accumulation as esterified TG in the liver. Cellular toxicity
mediated by this excess hepatic lipid accumulation known as
lipotoxicity, has been implicated in the pathophysiology of insulin
resistance, type 2 diabetes and metabolic syndrome (9).
Several agents are known to improve NAFLD
histologically or biochemically in animals and humans (10), although, numerous side effects have
been reported (11).
Rubus coreanus is one of the 100 genera in
the family Rosaceae, subfamily Rosoideae, and there
are currently 250 species of Rubus established around the
world. The dried unripe fruit of Rubus coreanus Miq. Rubi
Fructus (RF) has been used as a traditional oriental medicine
in Asia, including Korea and China (12–14).
RF has been used for the management of impotence and spermatorrhea,
and as a stomachic and tonic in Korea (15–17),
and it has been identified that RF exhibits a hepatoprotective
effect in animal models (18,19).
Ellagic acid from RF protects hepatocytes from damage by inhibiting
mitohondrial production of reactive oxygen species (20), while the same flavonoid inhibits
host immune tolerance induced by the hepatitis B virus-e antigen
(21). Ellagic acid has also been
reported to affect cellular lipid metabolism during benzoyl
peroxide-induced toxicity and effectively reduced the elevations of
plasma cholesterol in hyperlipidemic rabbits (22).
The present study examined the effects of the
Rubi Fructus extract (RFex) containing ellagic acid on the
development and progression of hepatic steatosis and liver
lipotoxicity in mice fed with a high-fat diet (HFD) by assessing
hepatic lipid accumulation, the mRNA expression of lipogenic genes
and enzyme activities.
Materials and methods
Plant materials and the preparation of
extracts
The unripened fruit of RF was collected during June
from Buan (Chunra, Korea). The plant samples were kept in the
herbarium of the Korea Research Institute of Bioscience and
Biotechnology (KRIBB). The RFex containing ellagic acid was
prepared by Lee’s Biotech Co., Ltd. (Yuseonggu, Daejeon,
Korea).
Animal treatment
The male C57BL/6 mice were obtained from Koatech
Technology Corporation (Seoul, Korea). The animals were allowed
free access to rodent food (Purina Co., Seoul, Korea) and tap water
and maintained in a controlled environment at 22°C and 50±10%
relative humidity with a 12-h dark/light cycle, and acclimatized
for at least one week prior to use. The mice were randomly divided
into three groups: the normal diet (ND), high-fat diet (HFD) and
HFD + RFex (100 mg/kg) fed group. The HFD was based on the diet
from Open Source Diet (Central Animal Lab Inc., Seoul, Korea)
containing 60% kcal fat, while the ND contained 10% kcal fat. The
diets were administered for 10 weeks and the weight gain was
measured once a week. Food intake was measured for three
consecutive days per week by subtraction of food jar pre-and
post-weights for 10 weeks. At necropsy, the sides of the
epididymal, retroperitoneal and perirenal adipose tissues were
removed and weighed, and the relative adipose tissue weight to body
weight ratio was calculated.
All animal experiments were approved by the
Institutional Animal Care and Use Committee and Ethics Committee,
which were performed in accordance with the institutional
guidelines at the KRIBB (Daejeon, Korea).
Plasma, hepatic lipid concentrations and
liver histology
Following 10 weeks of RFex administration, blood
samples were collected by a heart puncture method to determine the
levels of plasma enzymes [alanine aminotransferase (ALT) and
aspartate aminotransferase (AST)], plasma TG and total cholesterol
(TC). The plasma was prepared by the centrifugation of blood
(10,000 × g for 5 min at 4°C) and stored at −70°C until analysis.
Plasma ALT and AST levels were measured using an automatic
biochemical analyzer in the Animal Experiment Laboratory at the
KRIBB. Plasma TG and TC levels were measured directly with a BCS
analysis kit (Bioclinical System Co., Anyang, Korea). Plasma leptin
was determined by the sandwich ELISA method using a commercially
available rat leptin kit according to the manufacturer’s
instructions (Bioclinical System Co., Anyang, Korea). Immediately
following sacrification of the mice, half of the liver was removed,
quickly frozen in liquid nitrogen and stored at −70°C. Hepatic
lipids were extracted using the modified procedure of the Folch
method (23). Briefly, frozen
liver tissue was homogenized in 0.9% NaCl solution and chloroform -
methanol, 1:2 v/v was added to the homogenate. Then the mixture was
vortexed and centrifuged (2,000 × g for 20 min), and the upper
phase was aspirated and filtered. The collected chloroform phase
was used for analysis. Hepatic TG and TC concentrations were
analyzed using an enzymatic analysis kit (BCS analysis kit; Asan
Pharmaceutical Co. Ltd., Seoul, Korea).
The remaining half of the liver was removed and
immediately fixed in a buffer solution of 10% formalin for
pathological analysis. Fixed tissues were processed routinely for
paraffin embedding and 5 μm sections were prepared and stained with
haematoxylin and eosin. Stained areas were viewed using an optical
microscope.
Quantitative polymerase chain reaction
(qPCR) analysis for hepatic lipogenic gene expression
Total RNA from the liver was isolated and the
samples were reverse-transcribed using the TOPscript™ cDNA
synthesis kit (Enzynomics, Seoul, Korea). The resulting cDNA was
amplified using a PCR system (Astec, Fukuoka, Japan) and the Premix
Taq (Bioneer, Daejeon, Korea) according to the manufacturer’s
instructions. The sequences of oligonucleotides used as primers
were designed using Primer Express 3.0 (Applied Biosystems,
Carlsbad, CA, USA). The sequences are listed in Table I. All primer sets produced
amplicons of the expected size and their identity was also verified
by sequencing. The cycling conditions were 95°C for 10 min,
followed by 50 cycles of 95°C for 10 sec and 60°C for 1 min. The
results were normalized against GAPDH and are presented as fold
changes versus the reference gene.
 | Table INucleotide sequences of primers for
qPCR of mRNA. |
Table I
Nucleotide sequences of primers for
qPCR of mRNA.
| Gene description | Primer sequences
(5′–3′) | Annealing temperature
(°C) | PCR product (bp) |
|---|
| SREBP-1c | F:
GCCTGCTTGGCTCTTCTCTT
R: AGGTCAGCTTGTTTGCGATG | 55 | 102 |
| ACC1 | F:
TGATGCAGAGGTACC
R: CGTAGTGGCCGTCT | 55 | 100 |
| LPL | F:
TGCCGCTGTTTTGTTTTACC
R: TCACAGTTTCTGCTCCCAGC | 55 | 172 |
| LXR | F:
ACAGCCAGACGCTACAACCA
R: TGGCGATAAGCAAGGCATAC | 55 | 193 |
| CD36 | F:
TGACGTGGCAAAGAACAGC
R: GAAGGCTCAAAGATGGCTCC | 55 | 160 |
| FAS | F:
CTGGCATTCGTGATGGAGTC
R: TGTTTCCCCTGAGCCATGTA | 55 | 101 |
| GAPDH | F:
GGAGCCAAAAGGGTCATCAT
R: GTGATGGCATGGACTGTGGT | 60 | 321 |
Measurement of hepatic lipid-regulating
enzyme activity
Hepatic enzymes were prepared according to the
method of Hulcher and Oleson (24)
with a slight modification. A homogenate was prepared in a buffer
containing 0.1 mol/l of triethanolamine, 0.02 mol/l of EDTA and 2
mmol/l of dithiothreitol (pH 7.0). The homogenate was centrifuged
(600 × g for 10 min) to remove any cell debris and the supernatant
was again centrifuged (10,000 × g, followed by 12,000 × g for 20
min at 4°C) to remove the mitochondrial pellet. The supernatant was
then ultracentrifuged twice (100,000 × g for 60 min at 4°C) to
remove the cytosolic supernatant. The mitochondrial and microsomal
pellets were then redissolved in 800 μl of homogenization buffer
and the protein content determined using the Bradford method using
bovine serum albumin as the standard. Fatty acid synthase (FAS)
activity was measured in the cytosolic fraction according to the
method by Nepokroeff et al (7) by monitoring the malonyl-coenzyme
A-dependent oxidation of nicotinamide adenine dinucleotide
phosphate (NADPH) at 340 nm, where the activity was represented by
the oxidized NADPH nmol/min/mg protein. Carnitine
palmitoyltrasferase-1 (CPT) activity was determined in the
mitochondrial fraction according to the method by Markwell et
al (25) and the results are
expressed as nmol/min/mg protein. The activity of
3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase was
measured in microsomes based on a modification of the method of
Hulcher and Oleson (24). The
results are expressed as released CoA-SH nmol/min/mg protein.
Statistical analysis
All values from in vivo (n=6) and in
vitro (n=3) experiments are expressed as the mean ± standard
deviation. One-way analysis of variance and Duncan’s test were used
for multiple comparisons (SPSS, version 10.0; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of RFex on body weight, total
liver, adipose tissue weight and hepatic enzyme activities in HFD
fed mice
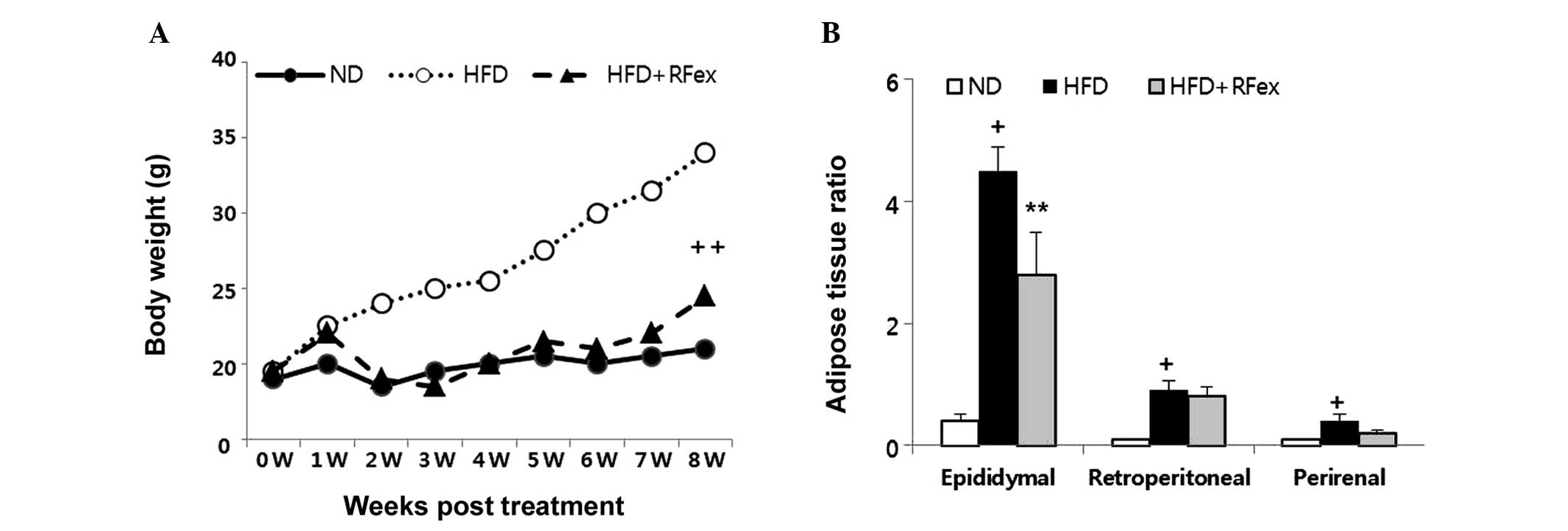
The body weight increased with time in HFD groups,
however, it decreased significantly in the HFD + RFex group
(P<0.01; Fig. 1A). The adipose
tissue ratio also decreased significantly in HFD + RFex fed mice
(Fig. 1B). A significant decrease
in epididymal (P<0.01), retroperitoneal and perirenal weight to
body weight was identified in the RFex fed group.
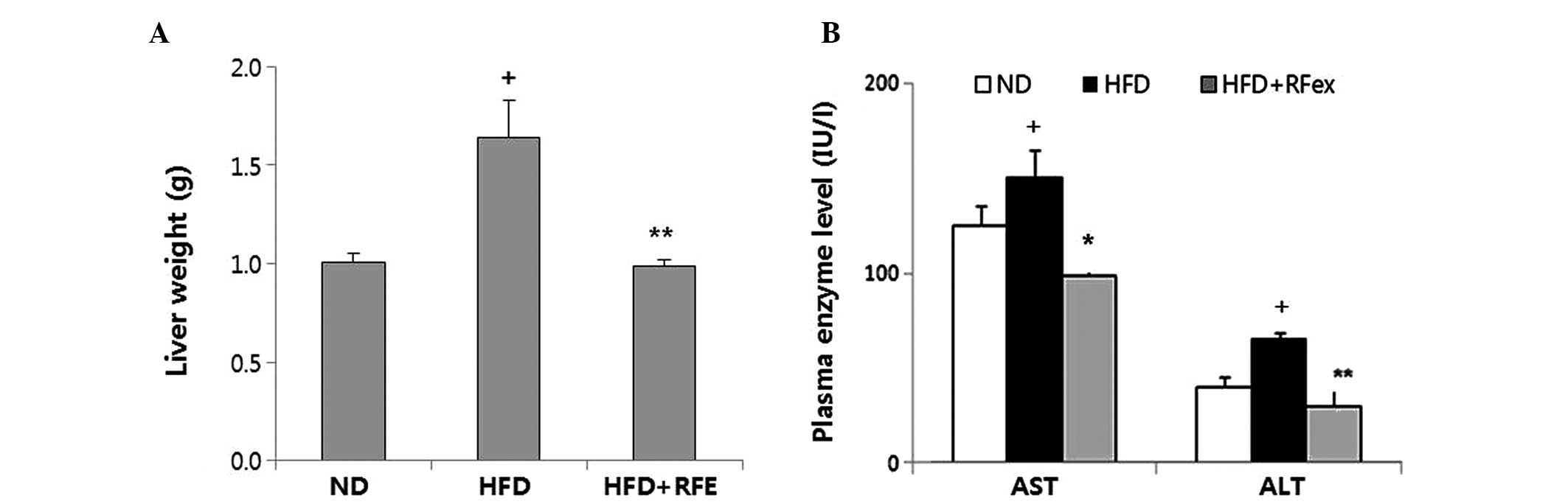
The total liver weight decreased significantly in
RFex fed HFD mice compared with the HFD mice (P<0.01; Fig. 2A). The HFD fed group had elevated
ALT and AST levels compared with the ND group (P<0.05; Fig. 2B), while RFex treatment
significantly attenuated HFD-induced ALT and AST levels (P<0.01
and P<0.05, respectively). RFex reversed the increase in ALT and
AST, suggesting that it has the potential to prevent liver
injury.
Effects of RFex on lipid profiles, free
fatty acid levels and fatty droplet accumulations in the livers of
HFD fed mice
To analyze the possible role of RFex in lipid
metabolism, which is the key factor relative to fatty liver
formation, plasma lipid profiles in experimental mice were
investigated and are presented in (Table II).
 | Table IIPlasma lipid profiles from mice
exposed to different experimental diets. |
Table II
Plasma lipid profiles from mice
exposed to different experimental diets.
| Group | Total cholesterol
(mg/dl) | Triglycerides
(mg/dl) | HDL-cholesterol
(mg/dl) | LDL-cholesterol
(mg/dl) |
|---|
| ND | 112.4±3.3 | 69.4±3.4 | 97.4±4.0 | 78.6±5.8 |
| HFD |
128.6±3.2a |
110.8±19.4b | 101.0±2.1 |
96.6±7.1a |
| HFD + RFex |
109.5±2.1c |
82.8±5.6c | 97.7±3.0 | 74.3±4.5 |
The mice exposed to HFD exhibited increased TC (by
14%), TG (by 59%) and low-density lipoprotein-cholesterol (LDL-C;
by 22.9%) levels and decreased high-density lipoprotein-cholesterol
(HDL-C; by 11%) levels in the respective animal groups compared
with the ND fed mice.
However, RFex feeding attenuated the serum lipid
profile, which manifested in a reduced TC, TG and LDL-C
concentration and a increased HDL-C levels as compared with the HFD
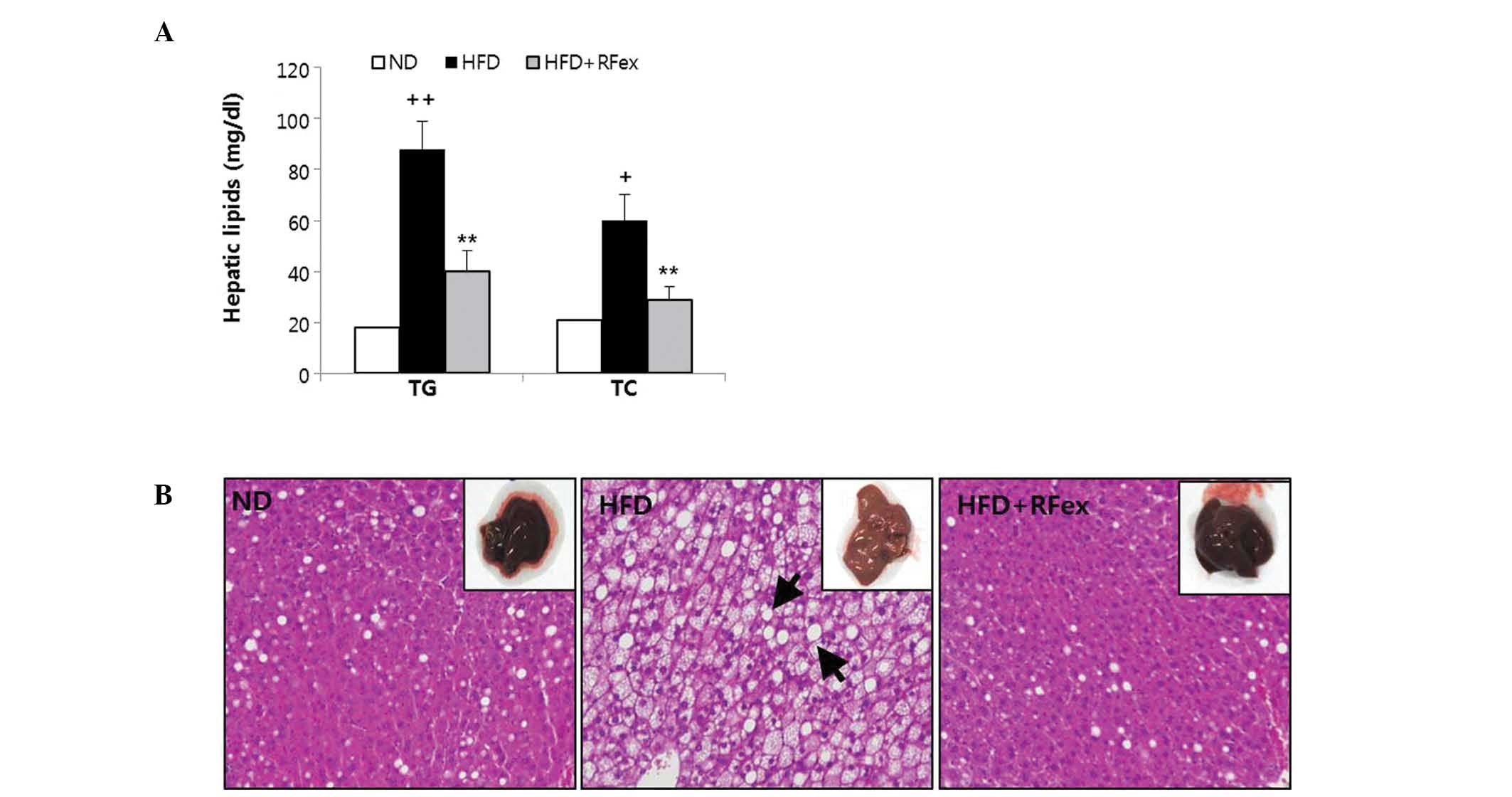
group (P<0.05). As shown in Fig.
3A, the increase of liver TG and TC in the HFD group (P<0.01
and P<0.05) was significantly suppressed by RFex
(P<0.05).
Representative photomicrographs of liver histology
are shown in Fig. 3B. HFD fed
control mice demonstrated a high accumulation of
microvesicular-type fat in the cytoplasm of the hepatocytes and
demonstrated focal hepatitis characterized by scattered
inflammatory cells and/or inflammatory foci. Treatment with RFex
significantly improved the microvesicular hepatic steatosis
(Fig. 3B; HFD + RFex) and liver
histology results were almost the same as those of the ND (Fig. 3B; ND). These data clearly suggest
that RFex is able to prevent hepatosteatosis via downregulating the
accumulation of lipids in liver cells.
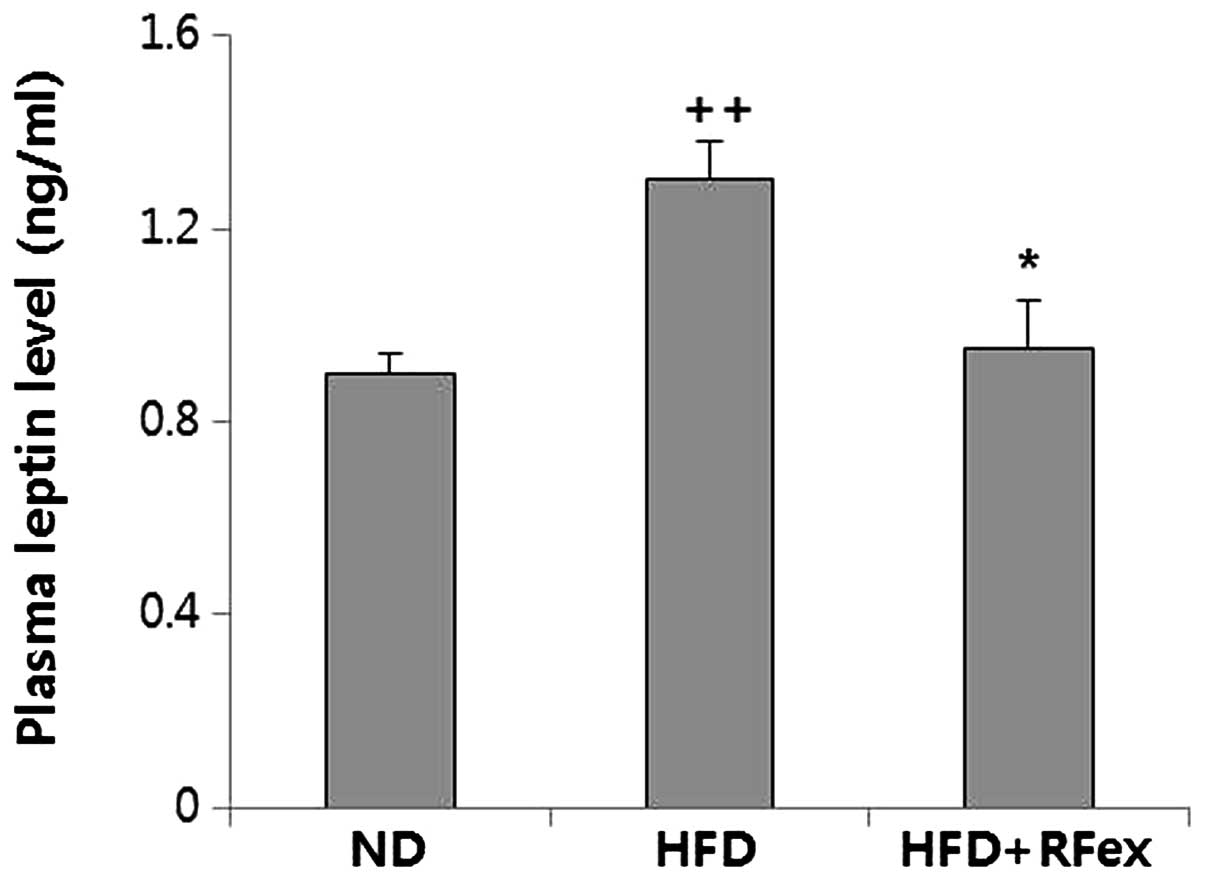
Effects of RFex on plasma leptin levels
in mice
Leptin levels in the plasma were examined to assess
the role of RFex in the prevention of obesity when the mice were
fed a HFD (Fig. 4). There was a
significant elevation in the levels of plasma leptin in the HFD (by
44%) group compared with that of the ND group (P<0.05). In
addition, the results implied that obesity in mice is accompanied
with leptin resistance. However, the leptin concentration was
significantly reduced in the RFex groups following 10 weeks of
exposure to a HFD.
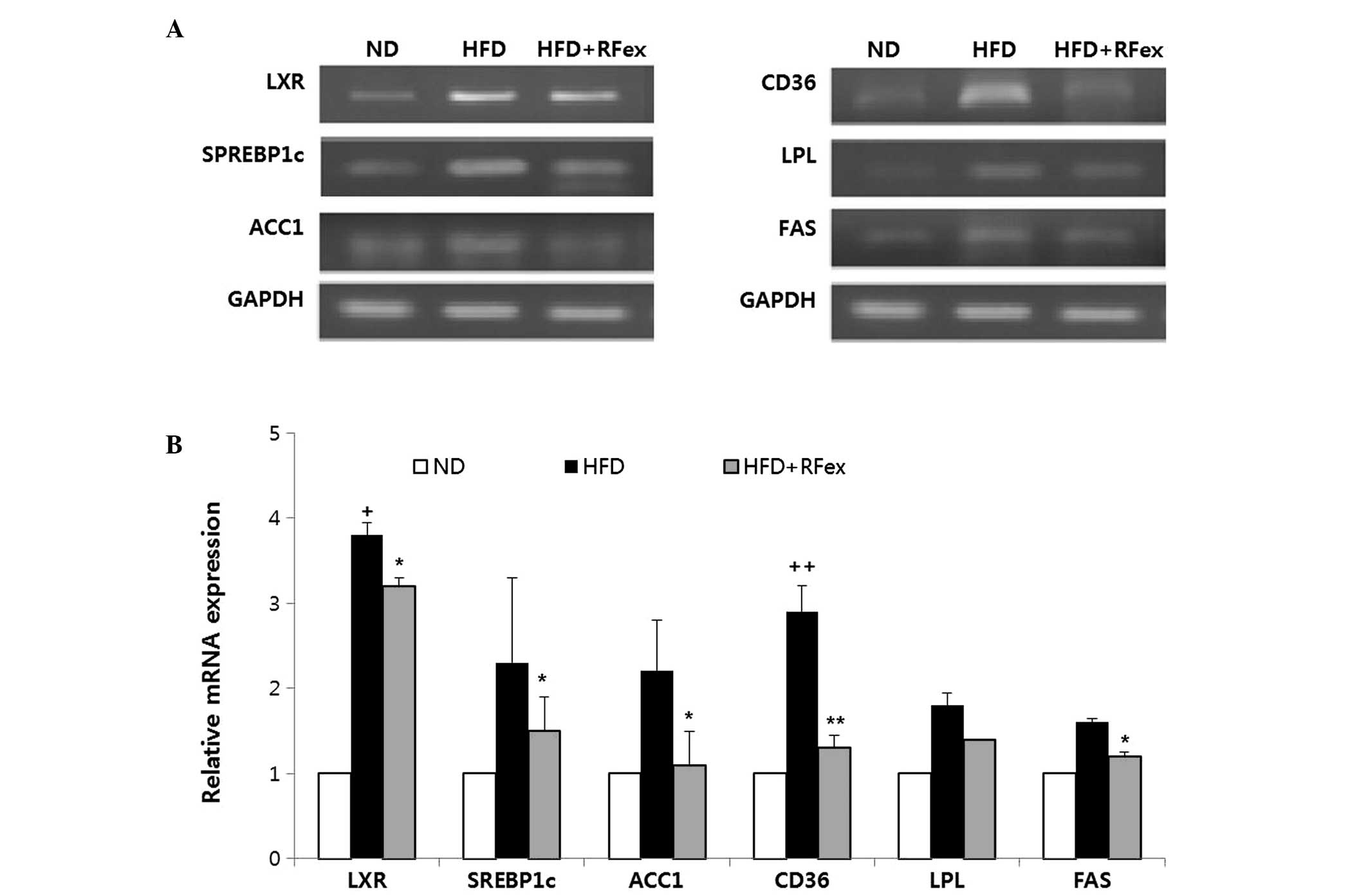
Effects of RFex on hepatic lipid
regulatory gene expression in mice
To investigate the underlying mechanism responsible
for the inhibitory effect of RFex on HFD-induced hepatic steatosis,
hepatic expression levels of lipogenesis-regulating genes were
determined, including liver X receptor (LXR), sterol regulatory
element binding protein-1c (SREBP-1c), acetyl-CoA carboxylase 1
(ACC1), cluster of differentiation 36 (CD36), lipoprotein lipase
(LPL) and FAS. GAPDH was used as a control (Fig. 5). Compared with the HFD group, mice
treated with RFex demonstrated significantly reduced mRNA
expression of the tested genes (P<0.05, P<0.01 compared with
the HFD-fed mice; Fig. 5A). The
relative levels of specific mRNA levels are shown (Fig. 5B).
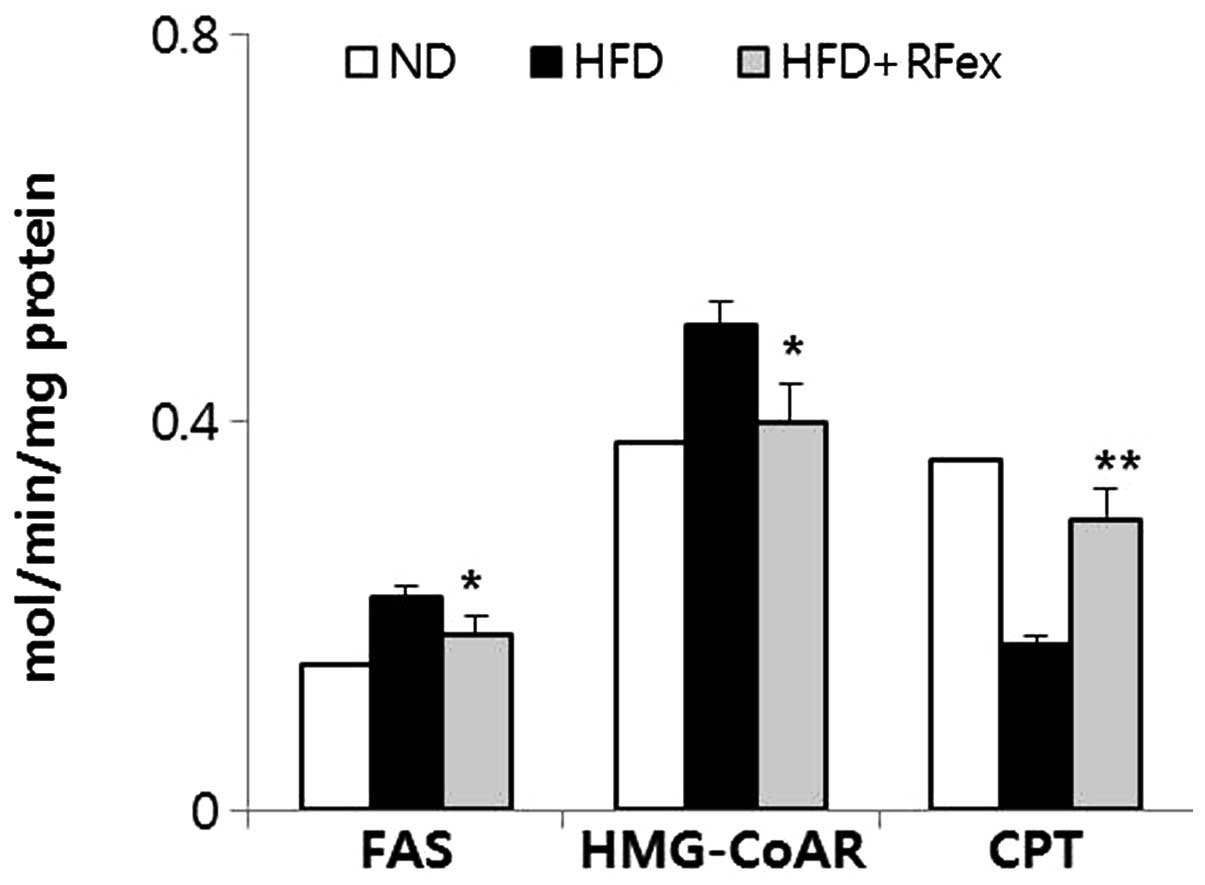
Effects of RFex on hepatic
lipid-regulating enzyme activities in mice
As representative enzymes, mediating lipid
biogenesis and lipid degradation, the activities of FAS, HMG-CoA
reductase and CPT were assessed to investigate whether RFex is able
to affect the enzyme activities of lipid metabolism in the liver
(Fig. 6). Hepatic FAS and HMG-CoA
reductase activity was significantly decreased (P<0.05 each),
whereas CPT activity was increased in the RFex-fed group compared
with the HFD-fed group (P<0.01).
Discussion
Hepatic steatosis is emerging as the most important
cause of chronic liver disease associated with the increasing
incidence of obesity (9).
It is able to progress to nonalcoholic
steatohepatitis in 10–20% of patients (26) and even to advanced cirrhosis and
hepatocellular carcinoma (27).
The present study demonstrated that the administration of RFex
protected against the development and progression of hepatic
steatosis induced by a HFD in C57BL/6 mice. Furthermore, the
reduction in levels of hepatic lipids (TG and TC) and plasma lipids
(TG, TC and LDL-C) and the elevation of plasma lipid (HDL-C) and
leptin levels were observed in RFex-treated C57BL/6 mice. The
inhibitory effect of RFex on hepatic steatosis appeared to be
associated with the suppression of lipogenesis enzyme activity and
the acceleration of fatty acid oxidation in HFD-fed mice. However,
the other possible mechanisms underlying the effect of RFex on
hepatic steatosis, including intestinal lipid absorption,
pancreatic lipase activity and the modulation of fecal sterol
excretion by RFex treatment requires to be further investigated. A
significant reduction in body weight was observed in the HFD + RFex
groups compared with the HFD-fed group (Fig. 1A), although the amount of food
consumption was similar between the groups (data not shown). These
results suggested that suppressed lipid production and increased
energy expenditure were involved in preventing hepatic steatosis in
RFex-fed mice.
To further investigate the reduced hepatic lipid
production in RFex-fed mice, the mRNA expression levels of
lipogenic and lipid-regulating enzymes, including SREBP-1c, FAS,
LXR, ACC1, LPL and CD36, were examined using qPCR. LXR is a member
of a nuclear receptor superfamily that regulates the expression of
key proteins involved in lipid metabolism and inflammation, serving
as a type of nuclear cholesterol sensor (28).
LXR also increases the expression of SREBP-1c, which
leads to increased hepatic TG synthesis. The present study
demonstrated that LXR expression was decreased in RFex-fed mice.
SREBP-1c is a key transcription factor regulating the expression of
enzymes involved in lipogenesis and fatty acid desaturation as well
as in response to fat and insulin (29). Its expression was significantly
higher in NAFLD, which was almost 5-fold greater than that in the
controls. SREBP-1c is positively regulated by insulin signaling
pathways, including insulin receptor substrates 1 and 2. It is
known that SREBP-1c is negatively regulated by AMP-activated
protein kinase (30). In NAFLD,
insulin signaling via insulin receptor substrate 1 causes the
upregulation of SREBP1-c, leading to an increased synthesis of
fatty acids by the hepatocytes (30).
In the present study, a significant suppression of
SREBP-1c and LXR mRNA by RFex was confirmed, which in turn may be
expected to lead to the downregulation of lipogenic genes,
including FAS and ACC. The present study also confirmed the
downregulation of FAS and ACC, which are involved in fatty acid
biosynthesis, in the RFex-fed group.
The expression levels of LPL and CD36 genes were
also assessed. LPL hydrolyzes TG in lipoproteins (chylomicrons and
very low density lipoproteins) and is also involved in promoting
the cellular uptake of chylomicron remnants, including
cholesterol-rich lipoproteins and very low-density lipoprotein.
CD36, identified on numerous types of cell surfaces, acts as a
fatty acid translocase. The present study demonstrated that LPL and
CD36 mRNA expression levels were decreased in RFex-fed groups.
Three enzyme activities, HMG-CoA reductase, FAS and
CPT, which are involved in hepatic lipogenesis and lipolytic
reactions, were measured. HMG-CoA reductase, an enzyme important in
cholesterol synthesis, catalyzes the reduction of HMG-CoA to CoA
and mevalonate. The experimental results demonstrated a significant
decrease in HMG-CoA reductase and FAS activities in RFex-fed mice.
By contrast, the hepatic activity of CPT, an enzyme involved in
fatty acid β-oxidation was increased in RFex-fed mice.
Bioassay-guided fractionation of the methanol
extract of Geum japonicum Thunb using a FAS inhibition assay
led to the isolation of ellagic acid with six other known compounds
(31). Several studies also
demonstrated that ellagic acid is involved in lipid metabolism
(32). Yu et al (33) have reported that ellagic acid
supplementation effectively reduced the elevation of plasma
cholesterol in hyperlipidemic rabbits. The exact mechanism by which
ellagic acid lowers other lipid levels is not known. However, it is
likely that ellagic acid may have decreased the activity of HMG CoA
reductase or enhanced the rate of lipid degradative processes and
increased the hepatic bile acids and fecal neutral sterol, and thus
decreased the levels of other lipids.
In conclusion, the present study demonstrated that
RFex inhibits hepatic steatosis and has plasma lipid-lowering
effects in HFD-fed C57BL/6 mice. These effects are mediated by the
downregulation of hepatic genes involved in lipogenesis and the
modulation of lipid metabolism-associated enzyme activities.
These results suggested that RFex is useful as a
potential dietary food supplement for intervention in hepatic
steatosis and hyperlipidemia.
Acknowledgements
This study was financially supported by the Ministry
of Trade Industry and Energy (MOTIE) and the Korea institute for
advancement of Technology (KIAT) through the Promoting Regional
Specialized Industry Project.
References
|
1
|
Marchesini G, Bugianesi E, Forlani G,
Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N,
Melchionda N and Rizzetto M: Nonalcoholic fatty liver,
steatohepatitis, and the metabolic syndrome. Hepatology.
37:917–923. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schreuder TC, Verwer BJ, van Nieuwkerk CM
and Mulder CJ: Nonalcoholic fatty liver disease: an overview of
current insights in pathogenesis, diagnosis and treatment. World J
Gastroenterol. 14:2474–2486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kotronen A and Yki-Järvinen H: Fatty
liver: a novel component of the metabolic syndrome. Arterioscler
Thromb Vasc Biol. 28:27–38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edmison J and McCullough AJ: Pathogenesis
of non-alcoholic steatohepatitis: human data. Clin Liver Dis.
11:75–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marchesini G, Brizi M, Bianchi G,
Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S,
Forlani G and Melchionda N: Nonalcoholic fatty liver disease: a
feature of the metabolic syndrome. Diabetes. 50:1844–1850. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Varela-Rey M, Embade N, Ariz U, Lu SC,
Mato JM and Martinez-Chantar ML: Non-alcoholic steatohepatitis and
animal models: understanding the human disease. Int J Biochem Cell
Biol. 41:969–976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nepokroeff CM, Lakshmanan MR and Porter
JW: Fatty-acid synthase from rat liver. Methods Enzymol. 35:37–44.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Targher G, Bertolini I, Rodella S, Zoppini
G, Lippi G, Day C and Muggeo M: Non-alcoholic fatty liver disease
is independently associated with an increased prevalence of chronic
kidney disease and proliferative/laser-treated retinopathy in type
2 diabetic patients. Diabetologia. 51:444–450. 2008. View Article : Google Scholar
|
|
9
|
Unger RH, Clark GO, Scherer PE and Orci L:
Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim
Biophys Acta. 1801:209–214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lindor KD, Kowdley KV, Heathcote EJ,
Harrison ME, Jorgensen R, Angulo P, Lymp JF, Burgart L and Colin P:
Ursodeoxycholic acid for treatment of nonalcoholic steatosis
steatohepatitis: results of a randomized trial. Hepatology.
39:770–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sgro C and Escousse A: Side effects of
fibrates (except liver and muscle). Therapie. 46:351–354. 1991.(In
French).
|
|
12
|
Cha HW, Park MS and Park KM: Physiological
activities of Rubus coreanus Miquel. Korean J Food Sci Tech.
33:409–415. 2001.
|
|
13
|
Yang HM, Oh SM, Lim SS, Shin HK, Oh YS and
Kim JK: Antiinflammatory activities of Rubus coreanus depend
on the degree of fruit ripening. Phytother Res. 22:102–107.
2008.
|
|
14
|
Oh MS, Yang WM, Chang MS, Park W, Kim do
R, Lee HK, Kim WN and Park SK: Effect of Rubus coreanus on
sperm parameters and cAMP responsive element modulator (CREM)
expression in rat testes. J Ethnopharmacol. 114:463–467. 2007.
|
|
15
|
Lee MW: Phenolic compounds from the leaves
of Rubus coreanus. Yakhak Hoeji. 39:200–204. 1995.
|
|
16
|
Perry LM: Medicinal plants of east and
southeast Asia, attributed properties and uses. MIT press;
Cambridge, MA: pp. 346–356. 1980
|
|
17
|
Sohn DW, Kim HY, Kim SD, Lee EJ, Kim HS,
Kim JK, Hwang SY, Cho YH and Kim SW: Elevation of intracavernous
pressure and NO-cGMP activity by a new herbal formula in penile
tissues of spontaneous hypertensive male rats. J Ethnopharmacol.
120:176–180. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee YI, Choi SK, Yang JY, Cho JS and Kim
TH: Hepatoprotective activities of Rubus coreanus depends on
the degree of ripening. Natural Product Sciences. 15:156–161.
2009.
|
|
19
|
Lee YI, Whang KE, Cho JS, Ahn BM, Lee SB,
Dong MS and Kim TH: Rubus coreanus extract attenuates
acetaminophen induced hepatotoxicity; involvement of cytochrome
P450 3A4. Biomol Ther. 17:455–460. 2009. View Article : Google Scholar
|
|
20
|
Hwang JM, Cho JS, Kim TH and Lee YI:
Ellagic acid protects hepatocytes from damage by inhibiting
mitochondrial production of reactive oxygen species. Biomed
Pharmacother. 64:264–270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang EH, Kown TY, Oh GT, Park WF, Park SI,
Park SK and Lee YI: The flavonoid ellagic acid from a medicinal
herb inhibits host immune tolerance induced by the hepatitis B
virus-e antigen. Antiviral Res. 72:100–106. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaul A and Khanduja KL: Plant polyphenols
inhibit benzoyl peroxide-induced superoxide anion radical
production and diacylglyceride formation in murine peritoneal
macrophages. Nutr Cancer. 35:207–211. 1999. View Article : Google Scholar
|
|
23
|
Folch J, Lees M and Sloane Stanley GH: A
simple method for the isolation and purification of total lipids
from animal tissues. J Biol Chem. 226:497–509. 1957.
|
|
24
|
Hulcher FH and Oleson WH: Simplified
spectrophotometric assay for microsomal 3-hydroxy-3-methylglutaryl
CoA reductase by measurement of coenzyme A. J Lipid Res.
14:625–631. 1973.
|
|
25
|
Markwell MA, McGroarty EJ, Bieber LL and
Tolbert NE: The subcellular distribution of carnitine
acyltransferases in mammalian liver and kidney. J Biol Chem.
248:3426–3432. 1973.PubMed/NCBI
|
|
26
|
Angulo P: Nonalcoholic fatty liver
disease. N Eng J Med. 346:1221–1231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dixon JB, Bhathal PS and O’Brien PE:
Nonalcoholic fatty liver disease: predictors of nonalcoholic
steatohepatitis and liver fibrosis in the severely obese.
Gastroenterology. 121:91–100. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zelcer N and Tontonoz P: Liver X receptors
as integrators of metabolic and inflammatory signaling. J Clin
Invest. 116:607–614. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N,
Hirshman MF, Goodyear LJ and Moller DE: Role of AMP-activated
protein kinase in mechanism of metformin action. J Clin Invest.
108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu H, Li J, Zhao W, Bao L, Song X, Xia Y,
Wang X, Zhang C, Wang X, Yao X and Li M: Fatty acid synthase
inhibitors from Geum japonicum Thunb. var chinense.
Chem Biodivers. 6:402–410. 2009. View Article : Google Scholar
|
|
32
|
Meyer AS, Heinonen M and Frankel EN:
Antioxidant interactions of catechin, cyanidin, caffeic acid,
quercetin and ellagic acid on human LDL oxidation. Food Chem.
61:71–75. 1998. View Article : Google Scholar
|
|
33
|
Yu YM, Chang WC, Wu CH and Chiang SY:
Reduction of oxidative stress and apoptosis in hyperlipidemic
rabbits by ellagic acid. J Nutr Biochem. 16:675–681. 2005.
View Article : Google Scholar : PubMed/NCBI
|