Introduction
Multiple myeloma (MM) is an incurable plasma cell
neoplasm; however, patient survival rates have improved in the last
decade due to the introduction of several effective therapies,
including thalidomide and bortezomib (1). These drugs are expensive and the wide
clinical application is limited in China, thus the development of
new drugs is important for the treatment of multiple myeloma. Bone
marrow (BM) angiogenesis exhibits an important role in the
pathogenesis and progression of MM (2). Inducers of angiogenesis in the BM
microenvironment include insulin-like growth factor-1 (IGF-1),
vascular endothelial growth factor (VEGF), and hypoxia-inducible
transcription factor-1 (HIF-1) (3). Thus far, angiogenesis is the
best-documented biological consequence of aberrant HIF expression
in MM. Studies have shown that there is a positive correlation
between HIF-1α expression and the levels of BM angiogenesis, and
expression of VEGF and VEGF receptor in biopsy specimens of
patients with MM (4).
Overexpression of HIF-1 in MM cells significantly enhanced
MM-induced angiogenesis in an in vivo xenograft model
(5). Small interfering
RNA-mediated knockdown of HIF-1α expression in RPMI-8226 cells and
CD138-positive MM cells significantly reduced MM-induced
angiogenesis in vitro (6).
HIF-1 activation promotes the aberrant production of VEGF by MM and
angiogenesis, and is associated with a poor prognosis in patients
with MM (7). IGF-1 has been shown
to activate VEGF expression in MM (8). IGF-1 is a cytokine that exhibits a
role in MM development and promotes angiogenesis (9). The serum levels of IGF-1 in patients
with newly diagnosed MM are positively correlated with markers of
angiogenesis, including VEGF (10).
Several studies have provided evidence that the
levels of nuclear translocation of HIF-1α are increased following
stimulation with IGF-1 (11–13).
IGF1 has been shown to promote VEGF secretion in the 5T33MM model
via the mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase (ERK) signaling pathway, independent of
phosphatidylinositol 3-kinase (PI3K) (14). IGF-1 has been shown to upregulate
the levels of VEGF production via HIF-1α in an AKT-dependent manner
(15). A study has reported that
the regulatory mechanism of HIF-1 activation is closely associated
with ERK (16). HIF-1α is
phosphorylated in hypoxia via an ERK-dependent signaling pathway
(17). HIF-1 is activated by
increased levels of VEGF production and transactivation via the
PI3K/AKT and mitogen-activated protein kinase kinase/ERK signaling
pathways in breast cancer (18).
A previous study confirmed that rosiglitazone (RGZ),
a thiazolidinedione ligand of the peroxisome proliferator-activated
receptor-γ, can inhibit myeloma cell proliferation, cell cycle
arrest, apoptosis and differentiation (19). The expression levels of IGF1-mRNA
have been found to be reduced following RGZ treatment and the
levels of IGF-1 secretion were suppressed (20). Treatment with RGZ can attenuate the
activation and expression of HIF-1 (21). The present study demonstrated that
RGZ reduces the expression of HIF-1α and IGF1 mRNA in RPMI-8226 and
primary myeloma cells from patients. In addition, the molecular
mechanisms underlying its anti-angiogenic effects was
investigated.
Materials and methods
Cell lines and reagents
The RPMI-8226 myeloma cell line was provided by
Professor Xueguang Zhang (Institute of Biological Technology,
Soochow University, China). Cells were maintained in RPMI-1640
supplemented with 10% fetal calf serum, 2 mM glutamine and 1%
penicillin/streptomycin (Gibco-BRL, Grand Island, NY, USA). Cells
were cultured at 37°C in a humidified 5% CO2 atmosphere
and passaged every 2–3 days. RGZ was purchased and dissolved in
dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA).
Anti-mouse phospho-AKT monoclonal antibody and anti-mouse
phospho-ERK ½ monoclonal antibody were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA) and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from
Abcam (Cambridge, UK).
Cell viability assay
The viability of the cells was assessed by an MTT
assay. Cells (2x104 cells/well) were seeded in a 96-well
plate and treated with the vehicle control (<0.1% DMSO) or RGZ
at various concentrations (10, 20 or 40 μM). For the time course
experiment, cells were incubated for 24, 48 or 72 h. A solution of
20 μl/well (MTT, 5 mg/ml; Sigma, St. Louis, MO, USA) was added to
each well for the last 4 h of incubation. After 4 h, the plate was
centrifuged at 1,000 rpm for 10 min the media removed and 150 μl
DMSO was added to each well to dissolve the precipitate. The plate
was then read at 570 nm in an ELISA microplate reader (ELX800;
Bio-Rad, Hercules, CA, USA). Five replicate wells were used for
each analysis. A percentage of the viability of the controls was
presented in culture conditions.
Isolation of BM cells from the
patients
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Soochow University and informed
consent was obtained from all patients in accordance with the
Declaration of Helsinki protocol. Mononuclear cells were freshly
isolated from the BM of five patients with MM and five healthy
patients by Ficoll-Hypaque density gradient centrifugation (Sigma).
Myeloma cells were purified with the CD138 positive selection
method using CD138 immunomagnetic beads and a magnetic cell sorter
(AutoMACS; Miltenyi Biotec Ltd., Surrey, UK), according to the
manufacturer’s instructions. The primary CD138-positive myeloma
cells were viable (95–97%) in vitro. The cell density was
maintained at 5×105 cells/ml and cells were treated with
RGZ at various concentrations (10, 20 or 40 μM) for 48 h.
RNA extraction and reverse
transcription-polymerase chain reaction
Total RNA was obtained from the cultured cells using
TRIzol (Takara Bio, Inc., Shiga, Japan). Total RNA (1 μg) was used
for reverse transcription, which was performed with reverse
transcriptase from Invitrogen Life Technologies (Carlsbad, CA, USA)
at 65°C for 5 min, 42°C for 60 min and 70°C for 15 min.
Amplification started with a 5 min denaturation step at 95°C,
followed by 35 cycles of denaturation at 95°C for 15 sec and
annealing at 60°C for 45 sec for HIF1α, or 35 cycles of
denaturation at 95°C for 30 sec and annealing at 55°C for 45 sec
for IGF1 and GAPDH. The sequences of the oligonucleotides used as
specific primers for each gene were as follows: Forward:
5′-ACAAGTCACAGGACAG3′ and reverse: 5′-AGGGAGAAAATCAAGTCG3′ for
HIF1α; forward: 5′-AGCAGTCTTCCAACCCAATTA3′ and reverse:
5′-CACGGACAGAGCGAGCTG3′ for IGF1; and forward:
5′-GTGGTCTCCTCTGACTTCAAC-3′ and reverse:
5′-TCTCTTCCTCTTGTGCTCTTG-3′ for GAPDH.
Western blot analysis
RPMI-8226 cells (1×106) were seeded in
six-well plates containing RPMI-1640 medium with 10% FBS and 1%
antibiotics, and were then harvested 48 h after RGZ treatment.
Following removal of the medium, the RPMI-8226 cells were washed
twice with cold phosphate-buffered saline and lysed for 30 min in
100 μl ice-cold cell-lysis buffer containing proteinase inhibitors
(1% cocktail and 1 mM phenylmethylsulfonyl fluoride). The protein
concentration was determined using a bicinchoninic acid assay.
Protein samples (50 μg) were denatured in 5× sodium dodecyl
sulfate-polyacrylamide gel electrophoresis sample buffer and were
subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis on 10% Tris-glycine gels. The separated proteins
were transferred onto polyvinylidene fluoride membranes for 1 h at
80 V using a Mini Trans-Blot Electrophoretic Transfer Cell
(Bio-Rad). The membranes were blocked with 5% non-fat milk at room
temperature for 1 h. Anti-phospho-AKT (Cell Signaling Technology,
Inc.), anti-phospho-ERK 1/2 (Cell Signaling Technology, Inc.) and
GAPDH (Abcam; dilution ratio 1:1,000) were used to probe the
protein levels of the different desired molecules at 4°C overnight.
Further incubation with goat anti-mouse IgG peroxidase-conjugated
secondary antibodies (Abcam) was conducted at room temperature for
2 h. Protein bands were detected using an Enhanced
Chemiluminescence kit (Amersham Biosciences, Little Chalfont,
UK).
Statistical analysis
Statistical analysis was performed with the
Statistical Program for Social Sciences software, version 19.0
(IBM, Armonk, NY, USA). All data are expressed as the mean ±
standard deviation. Analysis of variance was applied for comparison
of the means of two or multiple groups, in which Student’s t-test
was further used for the comparison of two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
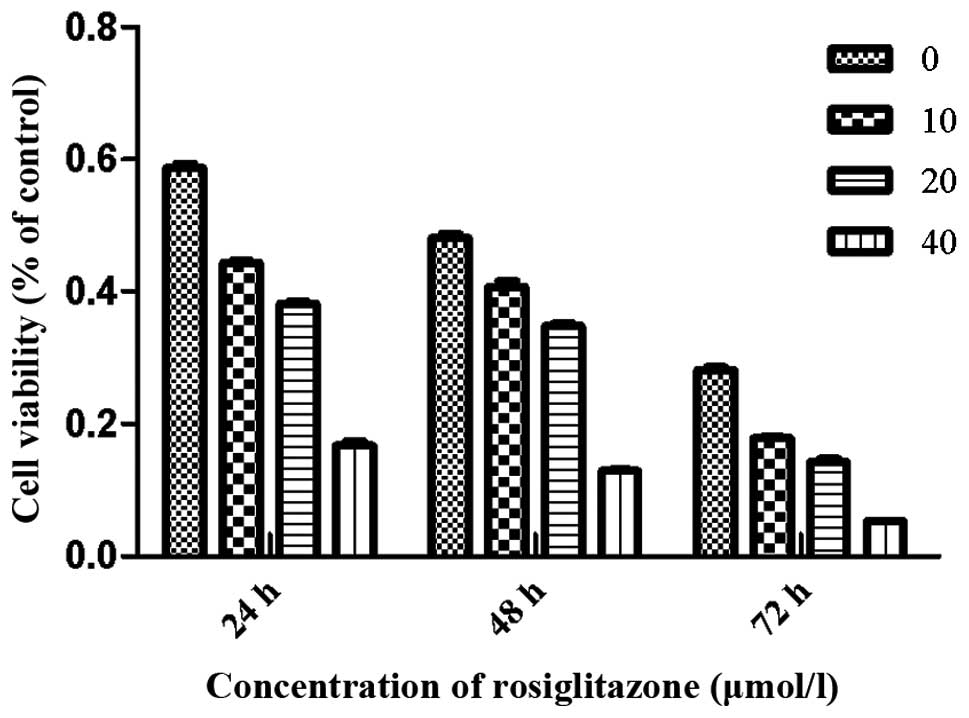
RGZ inhibits RPMI-8226 cell growth
To examine the effects of RGZ on myeloma cell
growth, concentration- and time-response experiments were
conducted. Cells were treated with RGZ at various concentrations
dissolved in DMSO. After treatment with 10, 20 and 40 μmol/l RGZ
for 48 h, the viability of RPMI-8226 cells was 40.8±1.5, 33.4±2.9
and 11.9±1.1%, respectively. After 24, 48 and 72 h treatment with
20 μM RGZ, the cell viability was 36.3±2.7, 33.4±2.9 and 14.3±
2.4%, respectively (Fig. 1).
Higher levels of cell growth inhibition were observed at a RGZ
concentration of 40 μM after 24, 48 and 72 h compared with those of
the cells treated with 10 or 20 μM RGZ.
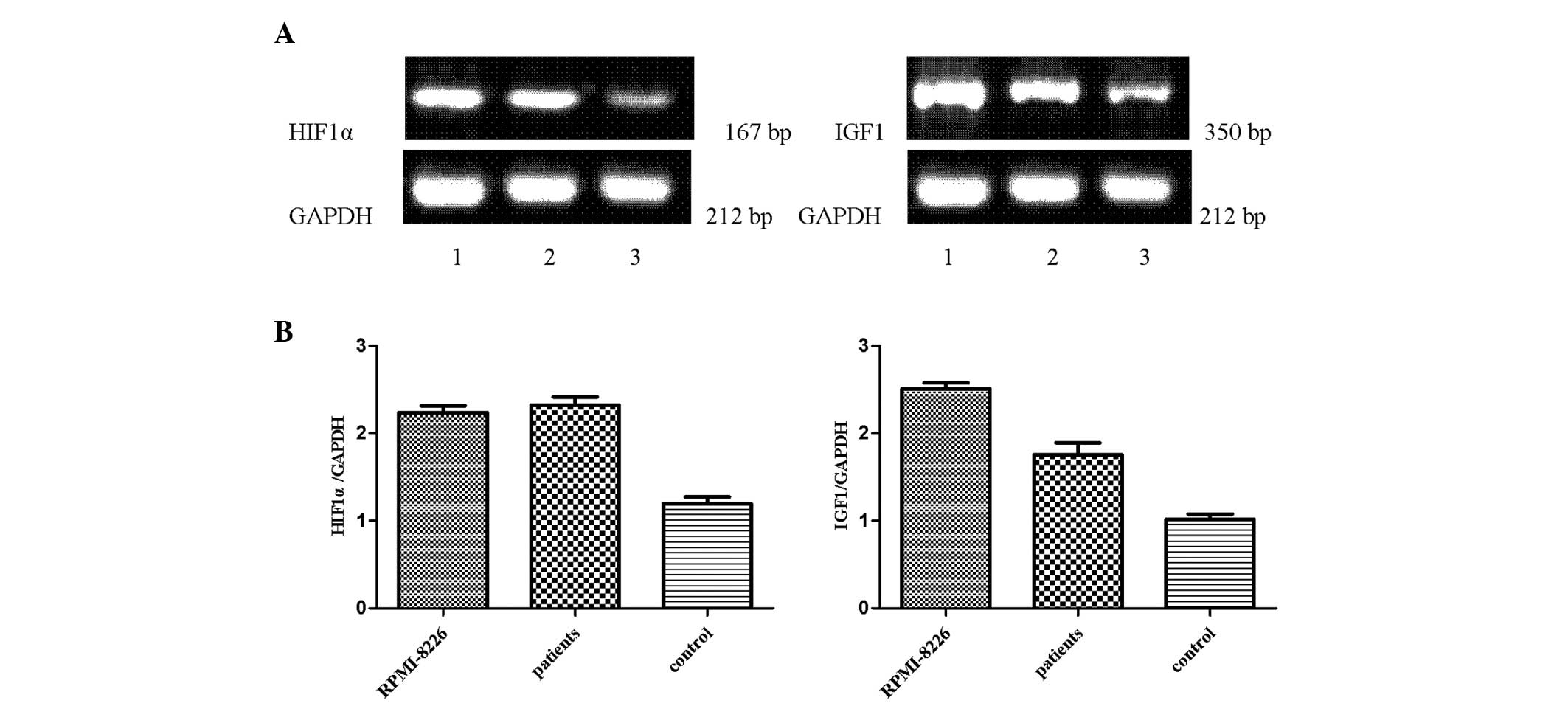
HIF1α and IGF1 mRNA are expressed in
primary myeloma, RPMI-8226 and healthy donor cells
Mononuclear cells were freshly isolated from the BM
of healthy patients, RPMI-8226 cells and primary myeloma cells by
Ficoll-Hypaque density gradient centrifugation. The results
demonstrated that a higher expression of HIF1α and IGF1 was
detected in RPMI-8226 cells and primary myeloma cells compared with
healthy donor cells (Fig. 2).
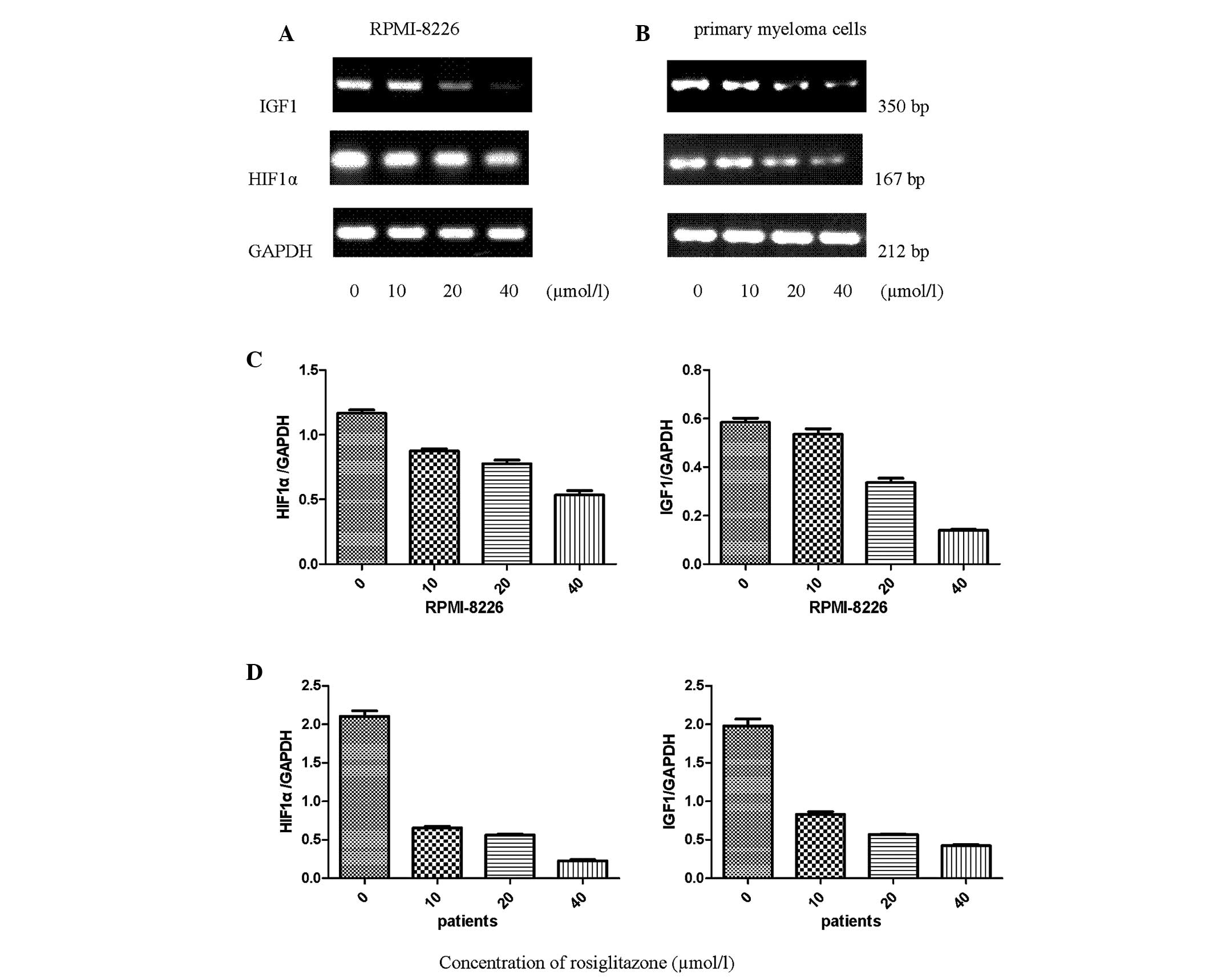
RGZ downregulates the expression of HIF1α
and IGF1 mRNA in RPMI-8226 and primary myeloma cells
To determine whether HIF1α and IGF1 expression is
affected in myeloma cells isolated from patients with MM and
RPMI-8226 cells after 48 h incubation with RGZ, the expression
levels of the corresponding mRNA were measured using reverse
transcription-polymerase chain reaction amplification. The HIF1α
and IGF1 gene expression levels were detected in the RPMI-8226 and
primary myeloma cells. Following treatment with RGZ, the mRNA
expression levels of HIF1α and IGF1 were concentration-dependently
downregulated compared with those in the untreated cells (Fig. 3). GAPDH mRNA was used as the
control.
RGZ downregulates the functions of
PI3K/AKT and ERK in RPMI-8226 cells
Based on the results shown in Fig. 4, RGZ was clearly able to inhibit
pAKT and pERK expression after 48 h of treatment. When RPMI-8226
cells were cultured with various concentrations of RGZ for 48 h,
the expression levels of pAKT and pERK in the RPMI-8226 cells
gradually decreased in a concentration-dependent manner. In the
RPMI-8226 cells treated for 48 h with 10 μM RGZ incubation, the
protein expression levels of pAKT and pERK were relatively lower
compared with those of the control. When the RGZ concentration was
increased to 20 μM, the protein expression levels of pAKT and pERK
were further reduced. At the RGZ concentration of 40 μM, the pAKT
and pERK protein expression levels were significantly lower
compared with those of the control.
Discussion
A previous study confirmed that RGZ is able to
induce cell cycle arrest, cell differentiation and apoptosis of MM
cells (19). Numerous studies have
shown that RGZ inhibits angiogenesis in various types of tumor,
including human endometrial carcinoma (22), human ovarian cancer (23), lung cancer (24) and pancreatic carcinoma (25), and in other tissues, including in
human umbilical vein endothelial cells (26). The present study assessed the
inhibitory effects and molecular mechanisms of RGZ using RPMI-8226
myeloma cells and primary myeloma cells from patients. The MTT
assay showed that RGZ inhibited the growth of RPMI-8226 cells in a
time- and concentration-dependent manner. However, the optimal
therapeutic strategy of targeting angiogenesis in MM with RGZ has
not yet been identified. To the best of our knowledge, the present
study reports for the first time, that RGZ has a potential
antiangiogenic effect and may inhibit the PI3K/AKT and ERK
signaling pathways in MM.
BM angiogenesis is important in the pathogenesis and
progression of MM. The role of angiogenesis in growth, progression
and metastatic spread of solid tumors has already been broadly
confirmed (27). The progression
of several cancers of hematopoietic lineages suggests a positive
correlation between angiogenesis and progression, including that of
non-Hodgkin’s lymphoma, lymphoblastic leukemia, B-cell chronic
lymphocytic leukemia, acute myeloid leukemia and MM (28). A study has also shown that BM
angiogenesis is a hallmark of MM progression (29). Tumor angiogenesis mainly depends on
growth factors that are released by neoplastic cells and are able
to stimulate the growth of the blood vessels of the host,
particularly those of endothelial cells (30). An increasing number of studies have
found that HIF1α and IGF1 exhibit a significant role in tumor
angiogenesis. HIF1α promotes the formation of blood vessels in MM
(7,4) and in other types of solid tumor,
including bladder cancer (31),
colon cancer (32) oral squamous
cell carcinoma (33) and cervical
carcinoma (34). IGF1 promotes
angiogenesis in hepatocellular carcinoma (35) lung cancer (36) pancreatic ductal adenocarcinoma
(37) and breast cancer (38).
In the present study, HIF1α and IGF1 mRNA expression
levels were significantly increased in RPMI-8226 and primary
myeloma cells compared with those in healthy donor cells. The
results have confirmed the data of previous studies (3–5,10,39),
which have suggested that high levels of HIF1α and IGF1 contribute
to angiogenesis and promote MM disease progression.
Several studies have shown that RGZ-induced
activation of peroxisome proliferator-activated receptor-γ inhibits
angiogenesis in various types of tumor (22–25).
Furthermore, it has been demonstrated that RGZ can suppress the
levels of IGF-1 or HIF1α expression in vitro and in
vivo (20,21). In the present study, when RPMI-8226
cells were incubated for 48 h with different concentrations of RGZ,
the mRNA expression levels of HIF1α and IGF1 were downregulated in
a concentration-dependent manner. In particular, when the
concentration of RGZ increased to 40 μM, the mRNA expression levels
of HIF1α and IGF1 were significantly reduced. Similar results were
observed in CD138-positive myeloma cells from patients. These
results suggest that when treated with RGZ, the expression levels
of HIF1α and IGF1 decreased significantly in a
concentration-dependent manner. These results indicated that RGZ
may inhibit angiogenesis in a concentration-dependent manner in
RPMI-8226 and primary myeloma cells through downregulation of the
expression levels of HIF1α and IGF1.
A number of studies have suggested that the PI3K/AKT
and ERK signaling pathways have an important role in angiogenesis
(40–42). In a previous study, IGF-1 increased
the expression levels of VEGF through the PI3K/AKT and ERK
signaling pathways (14,43). Other studies have suggested that
HIF1α promotes vascularization, which is mediated by the PI3K/AKT
and MEK/ERK signaling pathways (42,44).
RGZ has been shown to exert an inhibitory effect on cell
proliferation by downregulation of the PI3K/AKT and ERK1/2
signaling pathways (45,46). Our previous study showed that RGZ
can suppress IGF-1 or HIF1α expression in RPMI-8226 and primary
myeloma cells. Therefore, it was hypothesized that RGZ
downregulates IGF-1 or HIF1α expression levels through the PI3K/AKT
and ERK signaling pathways. In the present study, when RPMI-8226
cells were cultured with various concentrations of RGZ for 48 h,
the expression levels of pAKT and pERK gradually decreased
concentration-dependently. At an RGZ concentration of 40 μmol/l,
pAKT and pERK protein expression levels were significantly reduced
compared with those in the untreated cells. Therefore, RGZ may
inhibit IGF-1 or HIF1α expression in a concentration-dependent
manner through the PI3K/AKT and ERK signaling pathways.
In conclusion, a previous study has demonstrated
that treatment with RGZ can induce growth inhibition in MM cells
through cell cycle arrest, cell differentiation and apoptosis. The
current study extends the data of previous studies to demonstrate
that HIF1α and IGF1 are highly expressed in primary myeloma cells
and RPMI-8226 cells, and provides novel evidence that RGZ may
inhibit angiogenesis concentration-dependently in RPMI-8226 cells
and primary myeloma cells through downregulation of the expression
levels of HIF1α and IGF1. Furthermore, the findings of the present
study provide additional evidence that RGZ may inhibit IGF-1 or
HIF1α expression concentration-dependently through the PI3K/AKT and
ERK signaling pathways. Therefore, these findings suggest that the
peroxisome proliferator-activated receptor-γ ligand RGZ can be
regarded as an angiogenesis inhibitor for the clinical treatment of
myeloma and that it constitutes a promising therapeutic approach
for patients with MM.
Acknowledgements
This study was supported by grants from the project
of the Jiangsu Natural Science Foundation (no. BK2012610), The
Colleges and universities Natural Science Fund of Jiangsu Province
(no. SZ12306612) and The Social Development Fund of Suzhou City
(no. SYS201134). The authors would like to thank all of the
patients who provided samples for this study.
References
|
1
|
Rajkumar SV: Treatment of multiple
myeloma. Nat Rev Clin Oncol. 8:479–491. 2011. View Article : Google Scholar
|
|
2
|
Ribatti D, Vacca A, Dammacco F and English
D: Angiogenesis and anti-angiogenesis in hematological
malignancies. J Hematother Stem Cell Res. 12:11–22. 2003.
View Article : Google Scholar
|
|
3
|
Ribatti D, Nico B and Vacca A: Importance
of the bone marrow microenvironment in inducing the angiogenic
response in multiple myeloma. Oncogene. 25:4257–4266. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giatromanolaki A, Bai M, Margaritis D, et
al: Hypoxia and activated VEGF/receptor pathway in multiple
myeloma. Anticancer Res. 30:2831–2836. 2010.PubMed/NCBI
|
|
5
|
Martin SK, Diamond P, Williams SA, et al:
Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12
expression in multiple myeloma plasma cells. Haematologica.
95:776–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Colla S, Storti P, Donofrio G, et al: Low
bone marrow oxygen tension and hypoxia-inducible factor-1α
overexpression characterize patients with multiple myeloma: role on
the transcriptional and proangiogenic profiles of CD138(+) cells.
Leukemia. 24:1967–1970. 2010.
|
|
7
|
Zhang J, Sattler M, Tonon G, et al:
Targeting angiogenesis via a c-Myc/hypoxia-inducible
factor-1alpha-dependent pathway in multiple myeloma. Cancer Res.
69:5082–5090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Menu E, Jernberg-Wiklund H, De Raeve H, et
al: Targeting the IGF-1R using picropodophyllin in the
therapeutical 5T2MM mouse model of multiple myeloma: beneficial
effects on tumor growth, angiogenesis, bone disease and survival.
Int J Cancer. 121:1857–1861. 2007. View Article : Google Scholar
|
|
9
|
Menu E, van Valckenborgh E, van Camp B and
Vanderkerken K: The role of the insulin-like growth factor 1
receptor axis in multiple myeloma. Arch Physiol Biochem. 115:49–57.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pappa CA, Tsirakis G, Psarakis FE, Kolovou
A, Tsigaridaki M, Stafylaki D, Sfiridaki K and Alexandrakis MG:
Lack of correlation between angiogenic cytokines and serum
insulin-like growth factor-1 in patients with multiple myeloma. Med
Oncol. Mar;30:3632013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stoeltzing O, Liu W, Reinmuth N, et al:
Regulation of hypoxia-inducible factor-1alpha, vascular endothelial
growth factor, and angiogenesis by an insulin-like growth factor-I
receptor autocrine loop in human pancreatic cancer. Am J Pathol.
163:1001–1011. 2003. View Article : Google Scholar
|
|
12
|
Fukuda R, Hirota K, Fan F, et al:
Insulin-like growth factor 1 induces hypoxia-inducible
factor1-mediated vascular endothelial growth factor expression,
which is dependent on MAP kinase and phosphatidylinositol 3-kinase
signaling in colon cancer cells. J Biol Chem. 277:38205–38211.
2002. View Article : Google Scholar
|
|
13
|
Treins C, Giorgetti-Peraldi S, Murdaca J,
et al: Regulation of hypoxia-inducible factor (HIF)-1 activity and
expression of HIF hydroxylases in response to insulin-like growth
factor I. Mol Endocrinol. 19:1304–1317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Menu E, Kooijman R, Van Valckenborgh E, et
al: Specific roles for the PI3K and the MEK-ERK pathway in
IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of
multiple myeloma cells: study in the 5T33MM model. Br J Cancer.
90:1076–1083. 2004. View Article : Google Scholar
|
|
15
|
Poulaki V, Mitsiades CS, McMullan C, et
al: Regulation of vascular endothelial growth factor expression by
insulin-like growth factor I in thyroid carcinomas. J Clin
Endocrinol Metab. 88:5392–5398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sang N, Stiehl DP, Bohensky J, Leshchinsky
I, Srinivas V and Caro J: MAPK signaling up-regulates the activity
of hypoxia-inducible factors by its effects on p300. J Biol Chem.
278:14013–14019. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minet E, Arnould T, Michel G, Roland I,
Mottet D, Raes M, Remacle J and Michiels C: ERK activation upon
hypoxia: involvement in HIF-1 activation. FEBS Lett. 468:53–58.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi YH, Wang YX, Bingle L, Gong LH, Heng
WJ, Li Y and Fang WG: In vitro study of HIF-1 activation and VEGF
release by bFGF in the T47D breast cancer cell line under normoxic
conditions: involvement of PI-3K/Akt and MEK1/ERK pathways. J
Pathol. 205:530–536. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang H, Wu D, Fu J, Chen G, Chang W, Chow
HC, Leung AY and Liang R: All-trans retinoic acid can intensify the
growth inhibition and differentiation induction effect of
rosiglitazone on multiple myeloma cells. Eur J Haematol.
83:191–202. 2009. View Article : Google Scholar
|
|
20
|
Lecka-Czernik B, Ackert-Bicknell C, Adamo
ML, et al: Activation of peroxisome proliferator-activated receptor
gamma (PPARgamma) by rosiglitazone suppresses components of the
insulin-like growth factor regulatory system in vitro and in vivo.
Endocrinology. 148:903–911. 2007. View Article : Google Scholar
|
|
21
|
Kang BY, Kleinhenz JM, Murphy TC and Hart
CM: The PPARγ ligand rosiglitazone attenuates hypoxia-induced
endothelin signaling in vitro and in vivo. Am J Physiol Lung Cell
Mol Physiol. 301:L881–L891. 2011.
|
|
22
|
Nickkho-Amiry M, McVey R and Holland C:
Peroxisome proliferator activated receptors modulate proliferation
and angiogenesis in human endometrial carcinoma. Mol Cancer Res.
10:441–453. 2012. View Article : Google Scholar
|
|
23
|
Yokoyama Y, Xin B, Shigeto T and Mizunuma
H: Combination of ciglitazone, a peroxisome proliferator activated
receptor gamma ligand, and cisplatin enhances the inhibition of
growth of human ovarian cancers. J Cancer Res Clin Oncol.
137:1219–1228. 2011. View Article : Google Scholar
|
|
24
|
Reka AK, Goswami MT, Krishnapuram R,
Standiford TJ and Keshamouni VG: Molecular cross-regulation between
PPAR-γ and other signaling pathways: implications for lung cancer
therapy. Lung Cancer. 72:154–159. 2011.
|
|
25
|
Dong YW, Wang XP and Wu K: Suppression of
pancreatic carcinoma growth by activating peroxisome
proliferator-activated receptor gamma involves angiogenesis
inhibition. World J Gastroenterol. 15:441–448. 2009. View Article : Google Scholar
|
|
26
|
Kim KY, Ahn JH and Cheon HG:
Anti-angiogenic action of PPARγ ligand in human umbilical vein
endothelial cells is mediated by PTEN upregulation and VEGFR-2
downregulation. Mol Cell Biochem. 358:375–385. 2011.
|
|
27
|
Weidner N, Folkman J, Pozza F, Bevilacqua
P, Allred EN, Moore DH, Meli S and Gasparini G: Tumor angiogenesis:
a new significant and independent prognostic indicator in
early-stage breast carcinoma. J Natl Cancer Inst. 84:1875–1887.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bertolini F, Mancuso P, Gobbi A and
Pruneri G: The thin red line: angiogenesis in normal and malignant
hematopoiesis. Exp Hematol. 28:993–1000. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bhaskar A, Gupta R, Vishnubhatla S, Kumar
L, Sharma A, Sharma MC, Das P and Thakur SC: Angiopoietins as
biomarker of disease activity and response to therapy in multiple
myeloma. Leuk Lymphoma. 54:1473–1478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ribatti D, Nico B, Crivellato E, Roccaro
AM and Vacca A: The history of the angiogenic switch concept.
Leukemia. 21:44–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen MC, Lee CF, Huang WH and Chou TC:
Magnolol suppresses hypoxia-induced angiogenesis via inhibition of
HIF-1α/VEGF signaling pathway in human bladder cancer cells.
Biochem Pharmacol. 85:1278–1287. 2013.PubMed/NCBI
|
|
32
|
Chen C, Cai S, Wang G, Cao X, Yang X, Luo
X, Feng Y and Hu J: c-Myc enhances colon cancer cell-mediated
angiogenesis through the regulation of HIF-1α. Biochem Biophys Res
Commun. 430:505–511. 2013.PubMed/NCBI
|
|
33
|
Zhou H, Fei W, Bai Y, Zhu S, Luo E, Chen K
and Hu J: RNA interference-mediated downregulation of
hypoxia-inducible factor-1α inhibits angiogenesis and survival of
oral squamous cell carcinoma in vitro and in vivo. Eur J Cancer
Prev. 21:289–299. 2012.
|
|
34
|
Jeong JH, Jeong YJ, Cho HJ, et al:
Ascochlorin inhibits growth factor-induced HIF-1α activation and
tumor-angiogenesis through the suppression of EGFR/ERK/p70S6K
signaling pathway in human cervical carcinoma cells. J Cell
Biochem. 113:1302–1313. 2012.PubMed/NCBI
|
|
35
|
Chen Y, Gou X, Ke X, Cui H and Chen Z:
Human tumor cells induce angiogenesis through positive feedback
between CD147 and insulin-like growth factor-I. PLoS One.
7:e409652012. View Article : Google Scholar
|
|
36
|
Tsai AC, Pan SL, Lai CY, et al: The
inhibition of angiogenesis and tumor growth by denbinobin is
associated with the blocking of insulin-like growth factor-1
receptor signaling. J Nutr Biochem. 22:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakamura K, Sasajima J, Mizukami Y, et al:
Hedgehog promotes neovascularization in pancreatic cancers by
regulating Ang-1 and IGF-1 expression in bone-marrow derived
pro-angiogenic cells. PLoS One. 5:e88242010. View Article : Google Scholar
|
|
38
|
Tang X, Zhang Q, Shi S, Yen Y, Li X, Zhang
Y, Zhou K and Le AD: Bisphosphonates suppress insulin-like growth
factor 1-induced angiogenesis via the HIF-1alpha/VEGF signaling
pathways in human breast cancer cells. Int J Cancer. 126:90–103.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shushanov SS, Mar’ina LG, Kravtsova TA,
Chernykh YB and Kakpakova ES: Coexpression of two mRNA isoforms of
insulin-like growth factor-1 gene and mRNA of YB-1 gene in patients
with multiple myeloma. Bull Exp Biol Med. 154:654–657. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Baek YY, Cho DH, Choe J, et al:
Extracellular taurine induces angiogenesis by activating ERK-,
Akt-, and FAK-dependent signal pathways. Eur J Pharmacol.
674:188–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chung BH, Cho YL, Kim JD, et al: Promotion
of direct angiogenesis in vitro and in vivo by Puerariae
flos extract via activation of MEK/ERK-, PI3K/Akt/eNOS-, and
Src/FAK-dependent pathways. Phytother Res. 24:934–940.
2010.PubMed/NCBI
|
|
42
|
Shen K, Ji L, Gong C, Ma Y, Yang L, Fan Y,
Hou M and Wang Z: Notoginsenoside Ft1 promotes angiogenesis via
HIF-1α mediated VEGF secretion and the regulation ofPI3K/AKT and
Raf/MEK/ERK signaling pathways. Biochem Pharmacol. 84:784–792.
2012.PubMed/NCBI
|
|
43
|
Zhu C, Qi X, Chen Y, Sun B, Dai Y and Gu
Y: PI3K/Akt and MAPK/ERK1/2 signaling pathways are involved in
IGF-1-induced VEGF-C upregulation in breast cancer. J Cancer Res
Clin Oncol. 137:1587–1594. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yang XM, Wang YS, Zhang J, et al: Role of
PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of
HIF-1alpha and VEGF in laser-induced rat choroidal
neovascularization. Invest Ophthalmol Vis Sci. 50:1873–1879. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Choi IJ, Kim SY, Kwon CH and Kim YK:
Rosiglitazone inhibits proliferation of renal proximal tubular
cells via down-regulation of ERK and Akt. Ren Fail. 32:103–111.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cantini G, Lombardi A, Piscitelli E, et
al: Rosiglitazone inhibits adrenocortical cancer cell proliferation
by interfering with the IGF-IR intracellular signaling. PPAR Res.
2008:9040412008. View Article : Google Scholar : PubMed/NCBI
|