Introduction
Liver cancer is the fifth most common type of
malignancy worldwide and the third most common cause of
cancer-related mortality. As an aggressive disease with a poor
outcome, liver cancer results in >600,000 fatalities per year
(1,2). In total, ~90% of all primary liver
cancer diagnoses are classified as hepatocellular carcinoma (HCC).
Therefore, for the majority of the time, the terms liver cancer and
HCC may be used interchangeably (3). Studies have found that 70–90% HCC
cases are caused by liver cirrhosis; the predominant risk factor
for HCC worldwide is chronic hepatitis B virus infection (4). HCC development occurs in multiple
stages, which include genetic and epigenetic changes, the
activation of oncogenes or the inactivation of tumor suppressor
genes in cancer cells.
MicroRNAs (miRNAs) are a class of small, non-coding
RNAs that regulate gene expression through targeted inhibition of
transcription and translation. Recent studies have suggested that
miRNAs are important in tumor cell biological processes, including
cell proliferation, cell cycle, apoptosis, migration and invasion
(5–7). Furthermore, miRNAs have emerged as
potential therapeutic agents for tumorigenesis due to the ability
to downregulate multiple targets, not only in tumor progression and
metastasis, but also in therapeutic resistance and tumor recurrence
(8). In HCC carcinogenesis and
progression, certain miRNAs have been reported to be dysregulated,
for example, miR-135, miR-21 and miR-17–92 are upregulated, and
miR-122, miR-125, miR-375, miR-101 and miR-150 are downregulated
(6,9–14).
Recently, miR-195 has been reported to exert vital functions in a
number of types of cancer, including bladder, adrenocortical,
breast and gastric cancer (15–18);
thus, miR-195 may be critical in cancer development. The aim of
this study was to investigate the effect of miR-195 on the
biological function of HepG2 cells and to identify the correlation
between miR-195 and Wnt3a in HCC.
Materials and methods
Human tissue samples and cell lines
Surgically removed HCC tumor tissues and matched
adjacent non-cancerous tissues used for reverse transcription
polymerase chain reaction (RT-PCR) and western blotting were
obtained from 28 HCC patients at the First Affiliated Hospital of
Xi’an Jiaotong University College of Medicine (Xi’an, China). No
local or systemic treatment had been conducted prior to surgery.
The SMMC-7721, HepG2, Hep3B and Bel-7402 liver cancer cell lines
and the HL-7702 normal liver cell line were obtained from the Key
Laboratory of Environmentally and Genetically Associated Diseases
at Xi’an Jiaotong University, Ministry of Education (Xi’an,
Shaanxi, China). The cells were maintained in Dulbecco’s modified
Eagle’s medium (PAA Laboratories, Pasching, Austria) supplemented
with 10% fetal bovine serum. All cells were incubated at 37°C in 5%
CO2. The study was approved by the Medical Ethical
Committee of the College of Medicine, Xi’an Jiaotong University,
Xi’an, China and all patients provided written informed
consent.
RNA extraction and RT-PCR
Total RNA, including miRNA from tissue samples and
cells was isolated using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). The concentration of RNA was
measured using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA). The mRNA was reverse
transcribed using a reverse transcription kit (Takara Biotechnology
Co., Ltd., Dalian, China), according to the manufacturer’s
instructions. The relative levels of miR-195 were examined using
altered stem-loop RT-PCR with specific RT and PCR primers; U6
served as a control. The reverse transcription primers of miR-195
and U6 are listed in Table I.
Relative quantification of mRNA expression levels was performed
according to the manufacturer’s instructions (Bio-Rad, Hercules,
CA, USA).
 | Table ImiR-195 and U6 forward and reverse
primers for reverse transcription polymerase chain reaction. |
Table I
miR-195 and U6 forward and reverse
primers for reverse transcription polymerase chain reaction.
| miRNA | Primer |
|---|
| miR-195 | RT:
GTCGTATCCAGTGCGTGTCGTGGAGTC GGCAATTGCACTGGATACGACGCCAATA
Forward: ATCCAGTGCGTGTCGTG
Reverse: TGCTTAGCAGCACAGAAA |
| U6 | RT:
CGCTTCACGAATTTGCGTGTCAT
Forward: GCTTCGGCAGCACATATACTAAAAT
Reverse: CGCTTCACGAATTTGCGTGTCAT |
Plasmid constructs
The precursors of the hsa-miR-195 sequence were
synthesized with the following EcoRI and HindIII top
oligo:
5′-AATTCAGCTTCCCTGGCTCTAGCAGCACAGAAATATTGGCACAGGGAAGCGAGTCTGCCAATATTGGCTGTGCTGCTCCAGGCAGGGTGGTGA-3′;
and the following bottom oligo:
3′-GTCGAAGGGACCGAGATCGTCGTGTCTTTATAACCGTGTCCCTTCGCTCAGACG
GTTATAACCGAACACGACGAGGTCCGTCCCACCACTT CGA-5′. Then the miR-15b was
inserted into the EcoRI and HindIII sites of the
pcDNA™ 6.2-GW/EmGFP-miR vector (National Center for Biotechnology
Information; http://www.ncbi.nlm.nih.gov/).
MTT cell proliferation and colony forming
assays
A total of 5×103 HepG2 cells were seeded
in 96-well culture dishes, and incubated for 24, 48 or 72 h at 37°C
in a humidified incubator with 5% CO2. Subsequently, 20
μl MTT was added to the cells and the cells were incubated for 4 h.
The supernatant was removed and replaced with 150 μl dimethyl
sulfoxide and the cells were then incubated at 37°C in a humidified
incubator with 5% CO2. The absorbance was measured at
490 nm using the FLUOstar OPTIMA (BMG Labtech, Ortenberg, Germany).
To assess cell colony formation ability, 5×102 HepG2
cells transfected with either miR-195, miR-ctrl, anti-miR-195 or
anti-ctrl were seeded in 6-well culture dishes. After 14 days
incubation at 37°C in a humidified incubator with 5%
CO2, the cells were washed twice with phosphate-buffered
saline (PBS) and stained with crystal violet solution. Images were
obtained using Quantity One computer software (Bio-Rad). All assays
were repeated three times.
Luciferase activity assay
A fragment of the 3′ untranslated region (UTR) of
Wnt3a was synthesized with SacI and XhoI restriction
enzymes using the following primers: Wnt3a,
5′-CGCGAGTCTGCCAATATTGGCTGTGCTGC TCCAGGCAGGGTGGTGC-3′; and Wnt3a
mutation, 5′-CGCGAGTCTGCCAATATTGGCTGAGGTGATCCAGG CAGGGTGGTGC-3′.
HEK293 cells were obtained from the Key Laboratory of
Environmentally and Genetically Associated Diseases at Xi’an
Jiaotong University, Ministry of Education (Xi’an, China). A total
of 1×104 HEK293 cells were seeded into each well of a
96-well plate, subsequent to pmirGLO-Wnt3a-3′UTR-wt or
pmirGLO-Wnt3a-3′UTR-mut vector co-transfection with miR-195 or
miR-ctrl for 24 h using Lipofectamine 2000 (Invitrogen Life
Technologies). Firefly luciferase activity was measured at 24 h
post-transfection using a Dual-Luciferase Reporter Assay system
(Promega Corporation, Madison, WI, USA). The results were
normalized with Renilla luciferase. Each reporter plasmid was
transfected at least three times and each sample was assayed in
triplicate.
Cell cycle analysis
HepG2 cells were transfected with miR-195, miR-ctrl,
anti-miR-195 or anti-ctrl when the cells had grown to 70–90%
confluence. The cells were then incubated at 37°C for 48 h.
Subsequently, the treated cells were collected and fixed in 70%
ethanol at 4°C overnight. The cells were then washed in PBS and
incubated with 1 ml staining solution (20 μg/ml propidium iodide
and 10 U/ml RNase A) for 30 min at room temperature.
The cell-cycle distributions were assayed by
fluorescence-activated cell sorting using a flow cytometer
(FACSort; Becton-Dickinson, Franklin Lakes, NJ, USA).
Apoptosis analysis
HepG2 cells were transfected with miR-195, miR-ctrl,
anti-miR-195 or anti-ctrl when the cells had grown to 70–90%
confluence. The cells were cultured at 37°C for 48 h then harvested
by trypsinization and centrifugation, and washed twice with PBS.
The cells were then stained using an Annexin V/fluorescein
isothiocyanate Apoptosis Detection kit (Invitrogen Life
Technologies) according to the manufacturer’s instructions. Cell
apoptosis were examined using a flow cytometer
(Becton-Dickinson).
Western blot analysis
Either miR-195 or anti-miR-195 was transfected into
HepG2 cells using Lipofectamine 2000, with miR-ctrl and anti-ctrl
serving as respective controls. Total proteins were isolated from
the cells by radioimmunoprecipitation assay 48 h post-transfection.
Protein concentrations were measured using a micro bicinchoninic
acid protein assay kit (Pierce Biotechnology, Inc, Rockford, IL,
USA). The proteins were resolved using a 10% SDS-PAGE gel,
transferred onto the nitrocellulose membrane, blocked in 5% non-fat
dry milk in Tris-buffered saline (pH 7.4) containing 0.05% Tween 20
(TBST) and incubated with rabbit polyclonal antibody against Wnt3a
overnight at 4°C (1:200; Beijing Biosynthesis Biotechnology Co.,
Ltd., Beijing, China). After washing three times with TBST, the
membrane was incubated with a goat anti-rabbit IgG (1:5,000; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) antibody for 2 h,
then washed three times with TBST. GAPDH served as a control.
Relative protein expression was measured on the Quantity One
imaging software. All western blot analyses were performed three
times.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Student’s t-test was used for statistical analysis.
P<0.05 and P<0.01 were considered to indicate statistically
significant differences. All analyses were performed using SPSS
13.0 software (SPSS, Inc., Chicago, IL, USA).
Results
miR-195 exhibits low-level expression in
HCC tissues and cell lines
The RT-PCR detection results revealed that the
expression levels of miR-195 were significantly downregulated in
the 28 pairs of HCC tissues in comparison with matched non-tumor
tissues from patients (P<0.05; Fig.
1A). Furthermore, miR-195 was found to be significantly
downregulated in the Bel-7402, SMMC-7721, HepG2 and Hep3B HCC cells
compared with the HL-7702 normal hepatocyte cells (P<0.01;
Fig. 1B). These results reveal
that miR-195 is downregulated in HCC tissues and cell lines. The
reduced expression levels of miR-195 in HCC suggest that miR-195 is
a potential antioncogenic miRNA in HCC and miR-195 downregulation
may be involved in human HCC development.
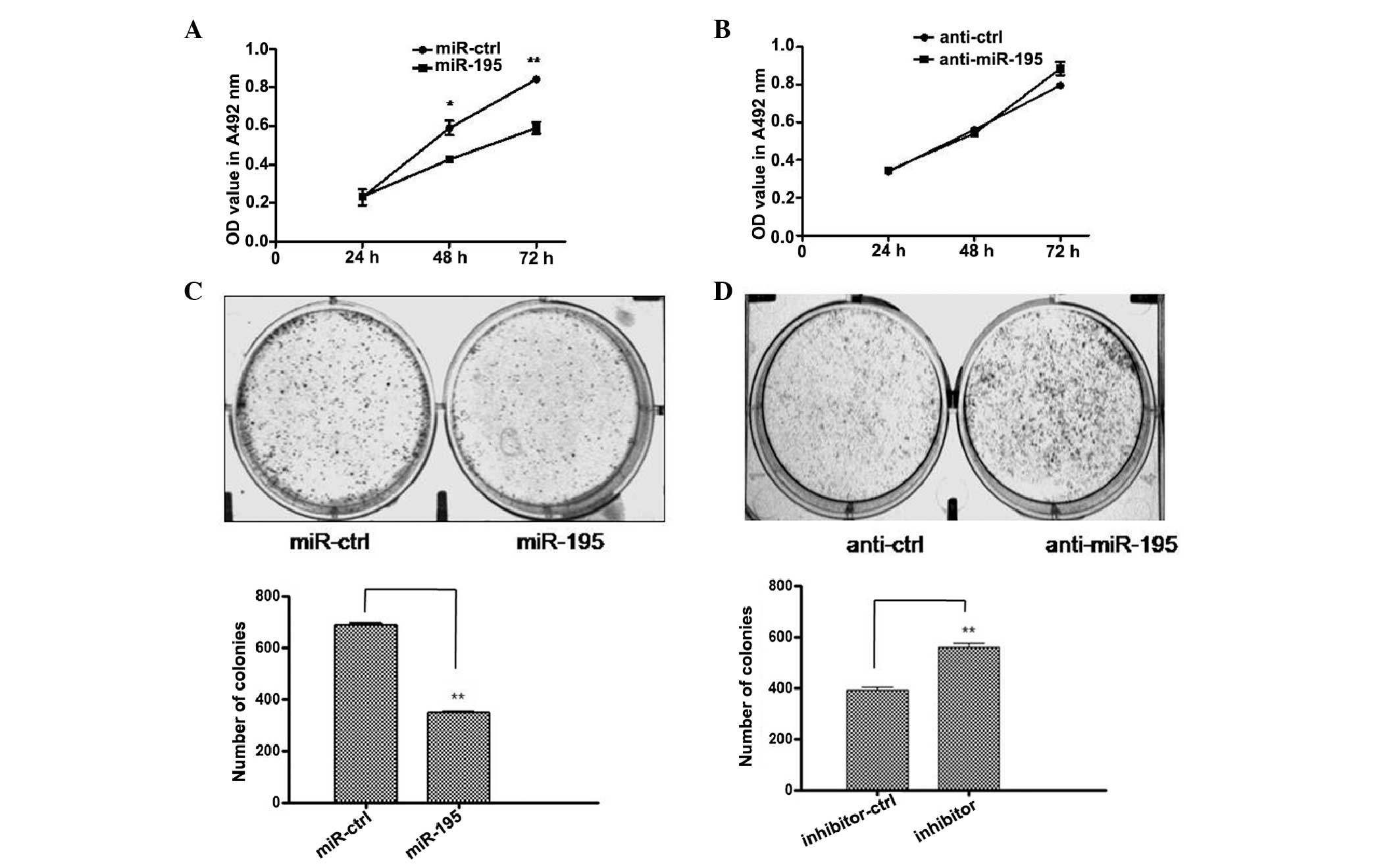
miRNA-195 regulates the proliferation and
colony formation of liver cancer cells
To analyse the function of miR-195 in cell growth,
miR-195-transfected HepG2 cells were compared with cells
transfected with miR-ctrl, anti-miR-195 and anti-ctrl. As
demonstrated by MTT growth assays, overexpression of miR-195
significantly reduced cell proliferation compared with the miR-ctrl
group. Conversely, anti-miR-195 upregulated cell proliferation
compared with the anti-ctrl group (Fig. 2A and B). In addition,
miR-195-transfected cells exhibited lower colony formation ability
compared with miR-ctrl-transfected cells. By contrast,
anti-miR-195-transfected cells exhibited higher colony formation
ability compared with anti-ctrl-transfected cells (Fig. 2C and D). These results indicate
that miR-195 inhibited HepG2 cell proliferation and further
suggests a tumor suppressive effect of miR-195 in HCC.
miR-195 induces cell cycle arrest and
apoptosis in HCC
In order to further demonstrate the significance of
the function of miR-195 in HCC cells, cells transfected with
miR-195 were compared with cells transfected with miR-ctrl, and
cells transfected with anti-miR-195 were compared with anti-ctrl to
detect whether miR-195 exerted apoptotic effects in HCC. miR-195
overexpression resulted in a significant increase in the number of
cells in the G1 phase of the cell cycle compared with miR-ctrl
overexpression (P<0.05; Fig.
3A) and the effect of miR-195 inhibitor transfection was to
significantly reduce the number of HepG2 cells in the G1 phase of
the cell cycle compared with control inhibitor transfection
(P<0.05; Fig. 3B). Furthermore,
the results of cell apoptosis analysis demonstrate that miR-195
transfection significantly increased the number of cells in the
early apoptotic stages compared with control transfection
(P<0.05; Fig. 3C); conversely,
anti-miR-195 transfection significantly reduced the number of cells
in early apoptosis compared with anti-control transfection
(P<0.05; Fig. 3D). Thus,
miR-195 transfection induced G1 phase cell cycle arrest and
promoted HCC cell apoptosis. These results indicate that miR-195
may exert a tumor suppressor function in HCC.
Wnt3a is a direct target of miR-195
To identify the molecular targets of miR-195, as
miRNAs function mainly through posttranscriptional inhibition of
target mRNAs via binding to the 3′UTR, an online miRNA target
database TargetScan (http://www.targetscan.org) was used to predict the
target genes. Wnt3a has been indicated to be a potential target of
miR-195 with a complementary 3′UTR binding site for the seed
sequence of miR-195 (Fig. 4A).
Co-transfection of miR-195 along with Wnt3a wild-type 3′UTR
resulted in a significant reduction in luciferase activity compared
with that of the control (P<0.05). However, co-transfection with
miR-195 along with the Wnt3a-mut did not reduce luciferase activity
(Fig. 4B). These results suggest
that miR-195 directly targets Wnt3a. Additionally, western blot
analysis was performed to measure the expression levels of Wnt3a
protein in miR-195-transfected HCC cells and miR-195 transfection
was found to reduce Wnt3a protein expression levels compared with
miR-ctrl transfection. The expression levels of Wnt3a protein
following transfection with anti-miR-195 or anti-ctrl were also
determined (Fig. 4C). The
expression levels of Wnt3a protein were significantly increased in
the cells transfected with anti-miR-195 compared with the cells
treated with anti-miRNA-control after 48 h. The results revealed
that the endogenously expressed Wnt3a was regulated by miR-195.
These data demonstrate that Wnt3a is a direct target of
miR-195.
 | Figure 4Wnt3a was a direct target of miR-195.
(A) The miR-195-binding sequence in the 3′UTR of Wnt3a mRNA. A
mutation was generated in the Wnt3a 3′UTR sequence in the
complementary site for the seed region of miR-195, as shown. (B)
HEK293 cells seeded in 96-well dishes were co-transfected with
either the wild-type or mutant Wnt3a-3′UTR, together with miR-195
or pcDNA6.2-control. After 48 h, the relative luciferase values
were measured and normalized by Renilla luciferase activity. The
expression of Wnt3a was directly downregulated by miR-195. Data
indicate the mean ± standard deviation (n=3). Similar results were
obtained from three independent experiments. *P<0.05.
(C) HepG2 cells were transfected with miR-195, miR-ctrl,
anti-miR-195 or anti-ctrl. After 48 h, the expression levels of
Wnt3a were detected by western blot analysis. GAPDH served as an
internal control. The result revealed that miR-195 transfection
repressed the translation of Wnt3a protein in HepG2 cells, compared
with miR-ctrl transfection, and that transfection with anti-miR-195
promoted the expression of Wnt3a, compared with anti-ctrl
transfection. miR, microRNA; UTR, untranslated region; CMV,
cytomegalovirus; WT, wild-type; ctrl, control. |
In conclusion, these results suggest that
overexpression of miR-195 inhibited HCC proliferation by repressing
Wnt3a expression and that downregulation of miR-195 is important in
the progression of HCC.
Discussion
Recent studies have shown that miRNAs exert wide
effects through regulating the expression of a variety of genes in
the majority of biological processes. Therefore, it is no surprise
that miRNAs regulate the development of cancer. Investigating the
role of individual miRNAs in cancer has generated great interest
(20); the effects of differently
expressed miRNAs may contribute to human carcinogenesis by
regulating multivarious types of target gene expression (21). Recently, studies have demonstrated
that miR-195 suppresses human glioma cell proliferation by directly
targeting the 3′UTRs of cyclin D1 and cyclin E1 (22). miR-195 also exhibited an
anti-apoptotic function in human colorectal cancer cells by
targeting Bcl-2 (23).
In recent years, a number of signaling pathways have
been found to be associated with HCC (24), including the AKT/mammalian target
of rapamycin (25)
mitogen-activated protein kinase kinase/extracellular
signal-regulated kinase (26),
c-Jun N-terminal kinase (27),
nuclear factor-kappa B (28) and
Wnt/β-catenin signaling pathways (29). The Wnt/β-catenin signaling pathway
is vital in tumorigenesis and the progression of numerous types of
tumor (30,31). The Wnt signaling pathway was first
associated with cancer when Wnt1a was identified as an oncogene in
mouse breast cancer in 1982 (32).
Wnt/β-catenin signaling pathway-induced G1 phase progression
through cyclin D1 and c-Myc transcriptional inductions has been
considered to be a mediator between the cell cycle and Wnt
signaling (33–36). Subsequent studies have revealed
that cell proliferation, differentiation, apoptosis and other
biological processes are affected by the Wnt signaling pathway
(31). Multiple factors in the Wnt
signaling pathway, including Wnt, β-catenin, adenomatosis polyposis
coli, Axin and secreted frizzled-related protein 1, have been shown
to be overexpressed in hepatocellular carcinoma cells (37,38).
The β-catenin/T-cell factor heterodimer activates >60 target
genes of the Wnt signaling pathway, such as c-myc, c-jun and cyclin
D1. These unusual activated oncogenes induce rapid proliferation in
liver cells and may result in HCC (36,39,40).
In the present study, miR-195 overexpression was
identified to directly target Wnt3a-induced G1 phase cell cycle
arrest and promote apoptosis. Furthermore, the reduction in cell
proliferation was marked. The data suggest that miR-195 is a
potential diagnostic marker and that Wnt3a may be a key target in
gene treatment of HCC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81171398), the Special Major
Scientific and Technological Research Projects of Shaanxi Province
(grant no. 2010ZDKG-50) and the Program for Changjiang Scholars and
Innovative Research Team in University (grant no. PCSIRT:1171).
References
|
1
|
Roberts LR: Sorafenib in liver cancer -
just the beginning. N Engl J Med. 359:420–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamashita T and Wang XW: Cancer stem cells
in the develop ment of liver cancer. J Clin Invest. 123:1911–1918.
2013. View
Article : Google Scholar
|
|
3
|
Nordenstedt H, White DL and El-Serag HB:
The changing pattern of epidemiology in hepatocellular carcinoma.
Dig Liver Dis. 42(Suppl 3): S206–S214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
McGlynn KA and London WT: The global
epidemiology of hepatocellular carcinoma: present and future. Clin
Liver Dis. 15:223–243. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Long XH, Mao JH, Peng AF, et al: Tumor
suppressive microRNA-424 inhibits osteosarcoma cell migration and
invasion via targeting fatty acid synthase. Exp Ther Med.
5:1048–1052. 2013.PubMed/NCBI
|
|
6
|
Hiyoshi Y, Kamohara H, Karashima R, et al:
MicroRNA-21 regulates the proliferation and invasion in esophageal
squamous cell carcinoma. Clin Cancer Res. 15:1915–1922. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moriyama T, Ohuchida K, Mizumoto K, et al:
MicroRNA-21 modulates biological functions of pancreatic cancer
cells including their proliferation, invasion, and chemoresistance.
Mol Cancer Ther. 8:1067–1074. 2009. View Article : Google Scholar
|
|
8
|
Chen D, Zhang Y, Wang J, et al:
MicroRNA-200c overexpression inhibits tumorigenicity and metastasis
of CD117+CD44+ ovarian cancer stem cells by
regulating epithelial-mesenchymal transition. J Ovarian Res.
6:502013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coulouarn C, Factor VM, Andersen JB,
Durkin ME and Thorgeirsson SS: Loss of miR-122 expression in liver
cancer correlates with suppression of the hepatic phenotype and
gain of metastatic properties. Oncogene. 28:3526–3536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang L, Wong CM, Ying Q, et al:
MicroRNA-125b suppressed human liver cancer cell proliferation and
metastasis by directly targeting oncogene LIN28B2. Hepatology.
52:1731–1740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu AM, Poon RT and Luk JM: MicroRNA-375
targets Hippo-signaling effector YAP in liver cancer and inhibits
tumor properties. Biochem Biophys Res Comm. 394:623–627. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meng F, Henson R, Wehbe-Janek H, et al:
MicroRNA-21 regulates expression of the PTEN tumor suppressor gene
in human hepatocellular cancer. Gastroenterology. 133:647–658.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Su H, Yang JR, Xu T, et al: MicroRNA-101,
down-regulated in hepatocellular carcinoma, promotes apoptosis and
suppresses tumorigenicity. Cancer Res. 69:1135–1142. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Luo N, Luo Y, et al: microRNA-150
inhibits human CD133-positive liver cancer stem cells through
negative regulation of the transcription factor c-Myb. Int J Oncol.
40:747–756. 2012.PubMed/NCBI
|
|
15
|
Ichimi T, Enokida H, Okuno Y, et al:
Identification of novel microRNA targets based on microRNA
signatures in bladder cancer. International Journal of Cancer.
125:345–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soon PSH, Tacon LJ, Gill AJ, et al:
miR-195 and miR-483-5p identified as predictors of poor prognosis
in adrenocortical cancer. Clin Cancer Res. 15:7684–7692. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li D, Zhao Y, Liu C, et al: Analysis of
MiR-195 and MiR-497 expression, regulation and role in breast
cancer. Clin Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo J, Miao Y, Xiao B, et al: Differential
expression of microRNA species in human gastric cancer versus
non-tumorous tissues. J Gastroenterol Hepatol. 24:652–657. 2009.
View Article : Google Scholar
|
|
19
|
Cadigan KM and Nusse R: Wnt signaling: a
common theme in animal development. Genes Dev. 11:3286–3305. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Majid S, Dar AA, Saini S, et al:
MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in
bladder cancer. PloS One. 8:e676862013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Volinia S, Calin GA, Liu CG, et al: A
microRNA expression signature of human solid tumors defines cancer
gene targets. Proc Natl Acad Sci USA. 103:2257–2261. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hui W, Yuntao L, Lun L, et al:
MicroRNA-195 inhibits the proliferation of human glioma cells by
directly targeting cyclin D1 and cyclin E1. PloS One. 8:e549322013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu L, Chen L, Xu Y, Li R and Du X:
microRNA-195 promotes apoptosis and suppresses tumorigenicity of
human colorectal cancer cells. Biochem Biophys Res Commun.
400:236–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fatima S, Lee NP and Luk JM: Dickkopfs and
Wnt/β-catenin signalling in liver cancer. World J Clin Oncol.
2:311–325. 2011.
|
|
25
|
Calvisi DF, Wang C, Ho C, et al: Increased
lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes
development of human hepatocellular carcinoma. Gastroenterology.
140:1071–1083. 2011. View Article : Google Scholar
|
|
26
|
Caja L, Sancho P, Bertran E, et al:
Overactivation of the MEK/ERK pathway in liver tumor cells confers
resistance to TGF-β-induced cell death through impairing
up-regulation of the NADPH oxidase NOX4. Cancer Res. 69:7595–7602.
2009.PubMed/NCBI
|
|
27
|
Chang Q, Zhang Y, Beezhold KJ, et al:
Sustained JNK1 activation is associated with altered histone H3
methylations in human liver cancer. J Hepatol. 50:323–333. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y, Zhang H, Yin J, et al: IκBα gene
promoter polymorphisms are associated with hepatocarcinogenesis in
patients infected with hepatitis B virus genotype C.
Carcinogenesis. 30:1916–1922. 2009.
|
|
29
|
Whittaker S, Marais R and Zhu A: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu D, Zhao Y, Tawatao R, et al: Activation
of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc
Natl Acad Sci USA. 101:3118–3123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ilyas M: Wnt signalling and the
mechanistic basis of tumour development. J Pathol. 205:130–144.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gougelet A and Colnot S: A Complex
Interplay between Wnt/β-Catenin Signalling and the Cell Cycle in
the Adult Liver. Int J Hepatol. 2012:8161252012.
|
|
34
|
He TC, Sparks AB, Rago C, et al:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shtutman M, Zhurinsky J, Simcha I, et al:
The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc
Natl Acad Sci USA. 96:5522–5527. 1999.
|
|
36
|
Tetsu O and McCormick F: β-Catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999.
|
|
37
|
Kaur P, Mani S, Cros MP, et al: Epigenetic
silencing of sFRP1 activates the canonical Wnt pathway and
contributes to increased cell growth and proliferation in
hepatocellular carcinoma. Tumor Biol. 33:325–336. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan CN, Chen XM, Lou HQ, et al: Clinical
Significance of Axin and β-catenin Protein Expression in Primary
Hepatocellular Carcinomas. Asian Pac J Cancer Prev. 13:677–681.
2012.
|
|
39
|
Loeppen S, Koehle C, Buchmann A and
Schwarz M: A β-catenin-dependent pathway regulates expression of
cytochrome P450 isoforms in mouse liver tumors. Carcinogenesis.
26:239–248. 2005.
|
|
40
|
Tien LT, Ito M, Nakao M, et al: Expression
of β-catenin in hepato-cellular carcinoma. World J Gastroenterol.
11:2398–2401. 2005.
|