Introduction
Gastric cancer is one of the most frequent
malignancies worldwide, particularly in Eastern Asia, according to
estimations published in 2011 (1).
The common treatment of gastric cancer is radical resection of the
neoplastic tumor tissue with lymphadenectomy (2). Adjuvant radiotherapy and chemotherapy
to eliminate dissemination or minimal residual tumor load have
further improved prognosis. However, the five-year survival rate
for advanced gastric cancer has remained poor (1,3).
Thus, it is of great importance to further investigate the onset
and progression of gastric cancer and novel therapeutic targets. It
has been widely accepted that the pathogenesis of gastric cancer is
a complicated process (4–6) involving dysregulation of multiple
genes (7–9), including oncogenes, tumor suppressors
and DNA repair genes. However, the underlying molecular mechanism
in gastric cancer initiation and progression requires further
investigation.
The 14-3-3 proteins are a family of 28–33 kDa acidic
polypeptides widely expressed in all eukaryotic organisms (10). There are seven mammalian isotypes
denoted by β, γ, ɛ, σ, ζ, τ and η, and every subtype is encoded by
a distinct gene. Due to specific phospho-serine and
phospho-threonine binding activity, 14-3-3 proteins are able to
interact with numerous different proteins and regulate diverse
cellular biological activities, including cytoskeleton
configuration, signal transduction, metabolism, differentiation,
transcription and tumorigenesis (11). The 14-3-3ɛ isoform is the most
conserved member of the 14-3-3 family, with conserved sequences
from plants, yeast and mammals (12). This isoform has been proposed as a
candidate tumor promoter in lung cancer, breast cancer, hepatic
cellular cancer, vulvar squamous cell carcinoma, follicular and
papillary thyroid tumors and meningioma (12–17).
However, the function and possible mechanism of 14-3-3ɛ in gastric
cancer remain unknown.
RNA interference (RNAi) is a general mechanism of
eukaryotic gene regulation and a potent strategy for specific gene
silencing. The vector-based approach of short hairpin (sh)RNA
interference, either as selectable plasmids or as retroviruses, has
previously been demonstrated to achieve specific and persistently
effective suppression of gene expression in mammalian cells
(18). shRNA interference is able
to continuously transcribe and generate small interfering RNA and
degrade complementary messenger RNA persistently inside the cells,
which have been screened by resistance selection, and is
functionally similar to the process of post-transcriptional gene
silencing (19–21). Preliminary experiments performed by
our group indicated a negative correlation between 14-3-3ɛ
expression levels and gastric cancer tissue differentiation and a
positive correlation between 14-3-3ɛ expression levels and tumor
infiltration, lymph node metastasis and tumor, nodes and metastasis
staging. Furthermore, by introducing 14-3-3ɛ and Raf-1 kinase
inhibitor protein (RKIP) genes into SGC7901 cells, 14-3-3ɛ and RKIP
were demonstrated to exert an opposite effect on SGC7901 human
gastric cancer cells by regulating the mitogen-activated protein
kinase signaling pathway (22). In
the present study, the functional role of 14-3-3ɛ in human gastric
cancer was examined using RNAi.
Materials and methods
Chemicals and reagents
shRNA directed against 14-3-3ɛ, control shRNA
encoding a scrambled shRNA sequence that does not result in
specific degradation of any cellular message RNA, monoclonal mouse
anti-14-3-3ɛ, polyclonal rabbit anti-cyclin E and anti-p27
antibody, shRNA Plasmid Transfection Reagent and puromycin were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Goat anti-mouse secondary antibody and goat anti-rabbit secondary
antibody were purchased from Sigma (St. Louis, MO, USA). The
enhanced chemiluminescence (ECL) system for western blot analysis
was provided by Amersham Pharmacia Biotech (Piscataway, NJ, USA).
MTT and Giesma stain were purchased from Amresco Chemical Co., Ltd.
(Solon, OH, USA). All other reagents were of molecular biology
grade and purchased from Sigma (St. Louis, MO, USA) or Ameresco
Chemical Co. Ltd.
Cell lines and animals
The SGC7901 gastric cancer cell line was obtained
from the Key Laboratory of Cancer Proteomics of the Chinese
Ministry of Health, Xiangya Hospital, Central South University,
China. The cells were incubated in complete RPMI-1640 (Hyclone
Laboratories, Inc., Logan, UT, USA) and supplemented with 10% fetal
bovine serum (FBS; Gibco-BRL, Carlsbad, CA, USA), 100 U/ml
penicillin and 100 U/ml streptomycin in a humidified atmosphere at
37°C and 5% CO2.
Female nude athymic mice, aged 4–5 weeks and each
weighing ~20 g, were purchased from the Department of Experimental
Zoology, Central South University (Changsha, China). The
experiments were performed with the approval of the Animal Ethics
Committee of Xiangya Hospital, Central South University (Changsha,
Hunan, China).
Stable transfection
The cells were plated at 3×105 cells per
well in six-well plates. Transfections were conducted using the
shRNA Plasmid Transfection Reagent according to the manufacturer’s
instructions. To establish stable cell lines expressing lower
levels of 14-3-3ɛ, a 1:10 passage of the transfected SGC7901 cells
was performed after 48 h, followed by puromycin selection (1.5
μg/ml). Following ~4 weeks of incubation, the resistant colonies
were selected and transferred to 24-well plates. These colonies
were maintained in selective culture medium with 0.75 μg/ml
puromycin. Another set of SGC7901 cells were transfected with
control shRNA as an experimental control.
Western blot analysis
The cultured cells were lysed in a lysis buffer [20
mmol/l Tris (pH 7.5), 0.1% Triton X, 0.5% deoxycholate, 1 mmol/l
phenylmethylsulfony fluoride, 10 μg/ml aprotinin and 10 μg/ml
leupeptin] for 60 min on ice. The samples were boiled in 2X SDS
sample buffer. A total of 40 μg lysate per lane was separated by
10% SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Amersham Pharmacia Biotech). The membranes were blocked with 5%
non-fat dry milk in Tris-buffered saline and Tween-20 buffer for 1
h at room temperature and then incubated with the appropriate
primary antibodies for 2 h at room temperature, followed by
incubation with the respective peroxidase-conjugated secondary
antibodies for 45 min at room temperature. Immunoreactive proteins
were visualized using the ECL detection system. β-actin was
detected simultaneously as a loading control. The protein bands
were quantified with Image J 1.48 (National Institutes of Health,
Bethesda, MA, USA) analysis. The expression of each protein was
calculated using the ratio of the intensity of each protein band to
that of β-actin. All experiments were performed in triplicate.
Cell proliferation analysis
Cell proliferation was assessed using the MTT assay.
SGC7901 cells (transfected and untreated) were seeded in 96-well
microplates at a density of 2,000 cells per well in RPMI-1640
medium containing 10% FBS. Cells were incubated for 0, 24, 48 and
72 h. A total of 20 μl MTT substate (5 mg/ml in RPMI-1640) was
added to each well and the plates were incubated for another 4 h at
37°C. The reaction was terminated by adding 150 μl dimethyl
sulfoxide. The optical density (OD) was detected using the Stat
Fax® 2100 multi-well plate reader (Awareness Technology,
Inc., Palm City, FL, USA) by measuring absorbance at 570 nm with
background subtraction of 690 nm. Each assay was performed in
triplicate and repeated three times. Growth curves were constructed
with OD570 on the vertical axis and culture time on the
horizontal axis.
Colony formation assay
Anchorage-dependent cell growth was determined by
analyzing the formation of colonies on the plates. Briefly, 100
cells were plated on six-well plates and incubated for two weeks to
allow for colony formation. The colonies were then fixed with 70%
ethanol, stained with Giemsa solution and counted. The assay was
performed in triplicate.
Flow cytometric analysis of the cell
cycle
For flow cytometric analysis of the cell cycle, the
cells were seeded and maintained on 6 cm-diameter plates in
RPMI-1640 containing 10% FBS overnight. The cells were synchronized
in a serum-free medium for 24 h and then cultured again in
RPMI-1640 containing 10% FBS. After 24 h of incubation, a total of
1×107 cells was harvested and fixed in 70% cold ethanol
for 45 min. Following repeated washings, the cells were stained
with a solution containing 40 μg/ml propidium iodide and 100 μg/ml
RNase A. The samples were immediately analyzed by a FAC-Scan flow
cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA). The
distribution of the cell cycle was determined using CellQuest™ Pro
software (BD Biosciences, Franklin Lakes, NJ, USA) and ModFit
LT™ software (Verity Software House, Topsham, ME, USA).
Three independent experiments were performed.
Tumorigenicity in nude mice
A total of 5×106 logarithmically growing
cells were resuspended in 0.2 ml RPMI-1640 medium and injected into
the right oxter of the 4–5 week old female Balb/c-nu/nu mice. Each
experimental group consisted of four mice. Tumor growth was
measured every three days after injection using calipers. All mice
were sacrificed on day 21 after injection. The sizes of the tumors
were calculated according to the following formula: Length ×
width2/2, where length was the longest diameter and
width was the shortest diameter of each tumor.
Statistical analyses
The data were presented as the mean ± standard
deviation or the mean ± standard error of the mean. The statistical
analyses were performed using SPSS software, version 19.0 (IBM,
Armonk, NY, USA). Comparisons among all groups were analyzed by
one-way analysis of variance and P<0.05 was considered to
indicate a statistically significant difference.
Results
Stable downregulation of 14-3-3ɛ in the
SGC7901 cell line
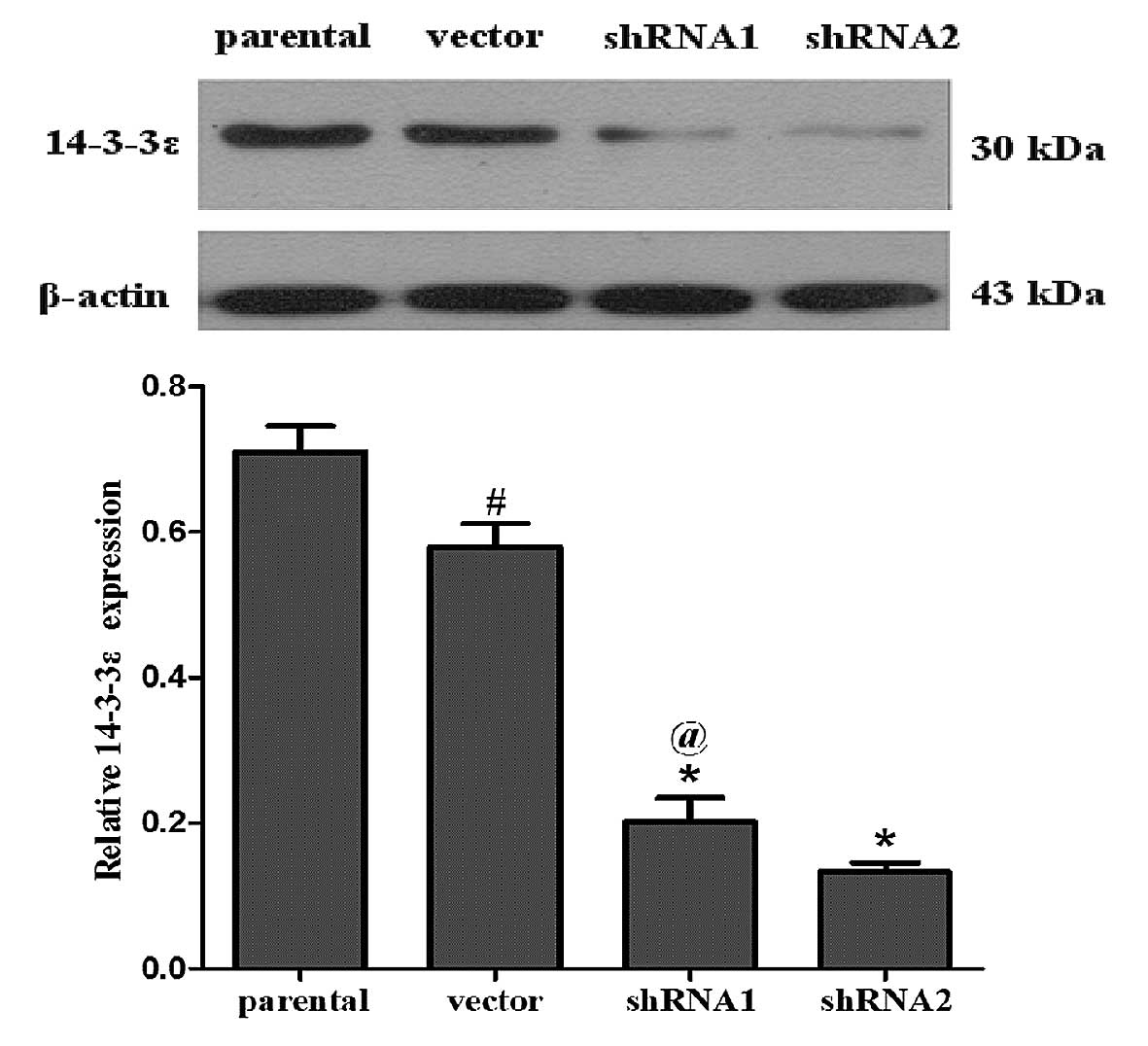
SGC7901 human gastric cancer cells were transfected
with 14-3-3ɛ shRNA and control shRNA, respectively. Following
puromycin selection, western blot analysis was performed to
determine the knockdown efficiency of 14-3-3ɛ. As shown in Fig. 1, the expression levels of 14-3-3ɛ
protein were significantly reduced in the shRNA1 and shRNA2 groups
in comparison with the parental SGC7901 and vector groups (cells
transfected with control shRNA). The experiment was repeated three
times. The three independent experiments confirmed that 14-3-3ɛ
protein expression was successfully downregulated in the shRNA1 and
shRNA2 groups, but no significant differences in 14-3-3ɛ expression
levels were detected between the cells transfected with the control
vector and the parental group.
14-3-3ɛ downregulation inhibits cell
proliferation and colony formation in vitro
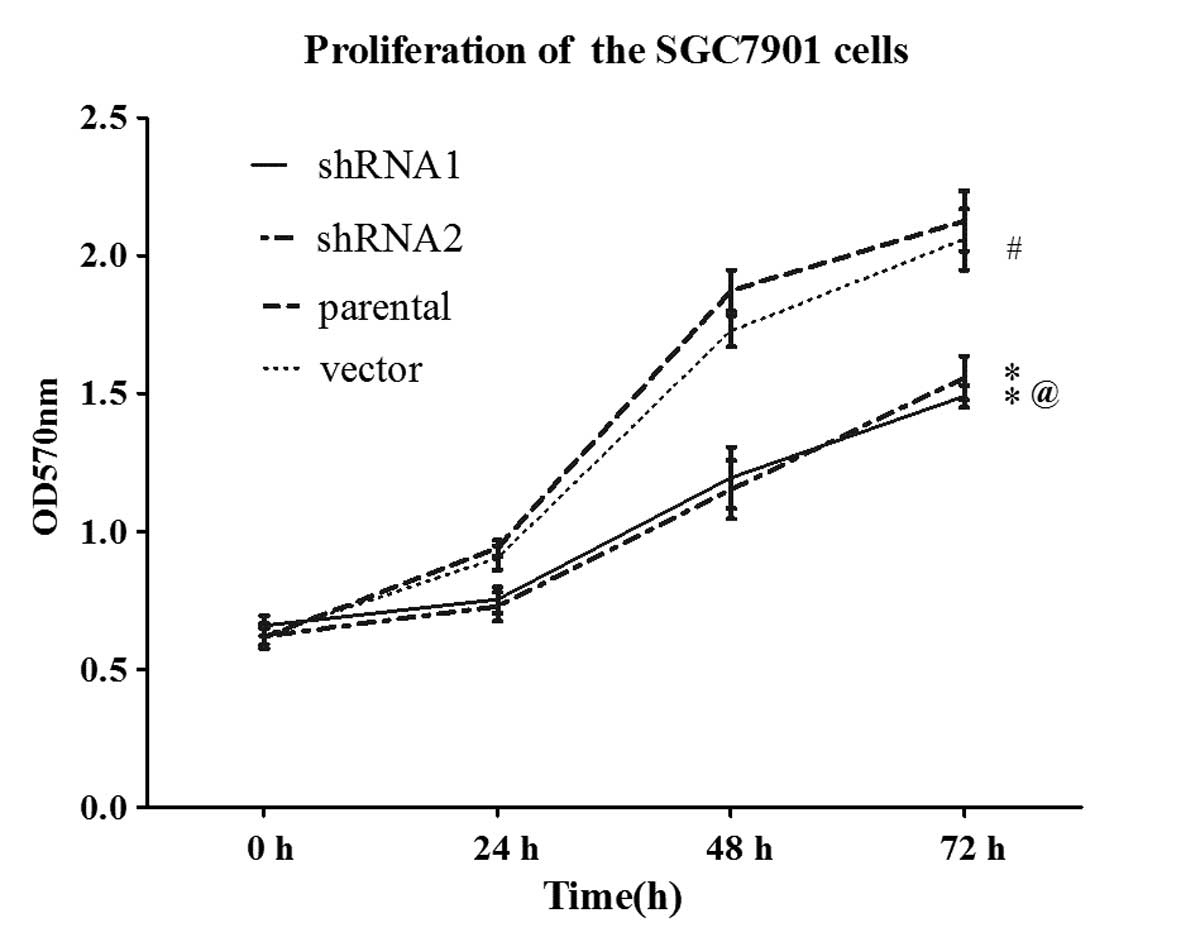
To determine the impact of 14-3-3ɛ on gastric cancer
cell proliferation, parental SGC7901 cells and the cells from the
variant groups were seeded onto 96-well culture plates and growth
was analyzed every day by MTT assay. As revealed by the growth
curves in Fig. 2, the vector cells
showed a similar growth rate to parental SGC7901 cells, whereas the
shRNA1 and shRNA2 cells exhibited a significantly lower growth rate
compared with the parental SGC7901 cells (P<0.05).
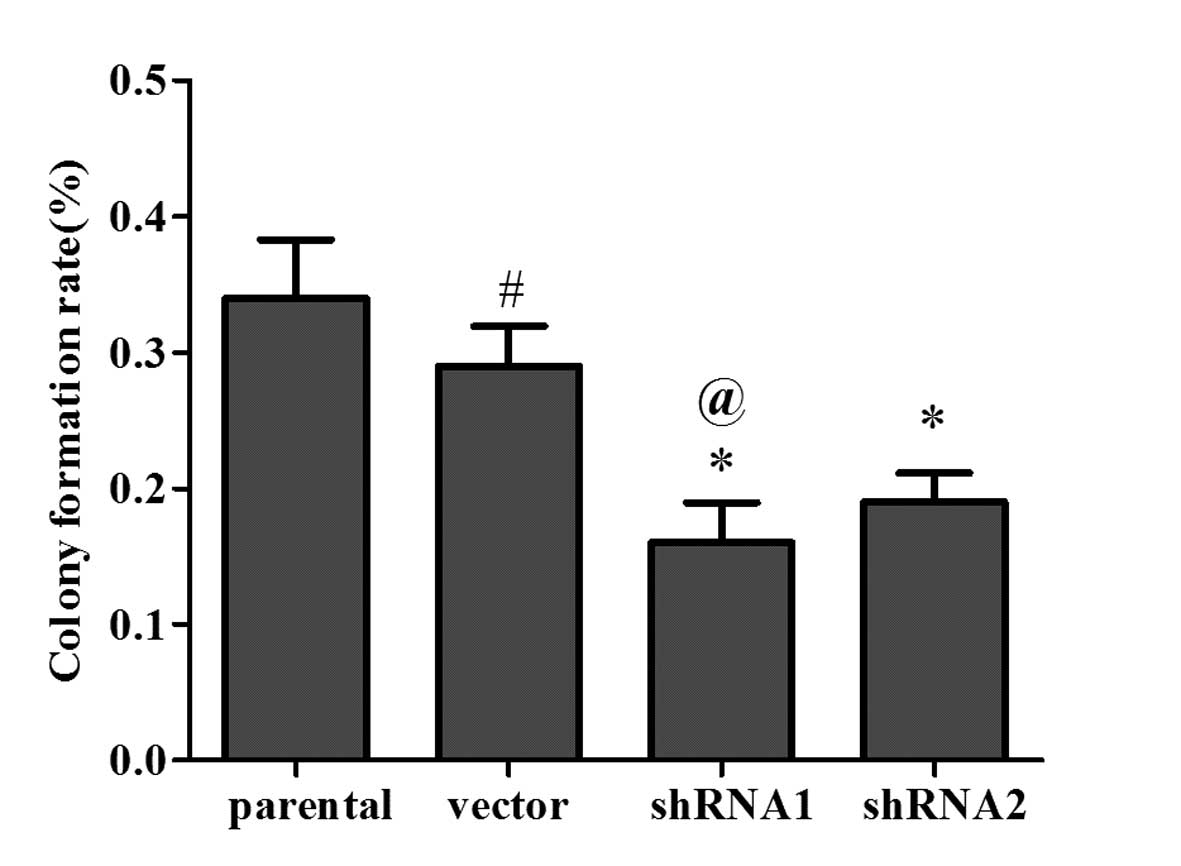
To investigate whether the levels of 14-3-3ɛ
expression affect the colony formation ability in vitro, a
colony formation assay was conducted to detect cell growth
viability in shRNA1-, shRNA2- and vector-transfected as well as
parental SGC7901 cells. The colony formation capacity was estimated
at 14 days after transduction. As shown in Fig. 3, the colony formation rates of
shRNA1 cells and shRNA2 cells were significantly reduced in
comparison with those of the parental SGC7901 cells (P<0.05).
These results confirm the anti-proliferative role of 14-3-3ɛ in
gastric cancer cells.
14-3-3ɛ downregulation triggers G0/G1
arrest in the SGC7901 cell line
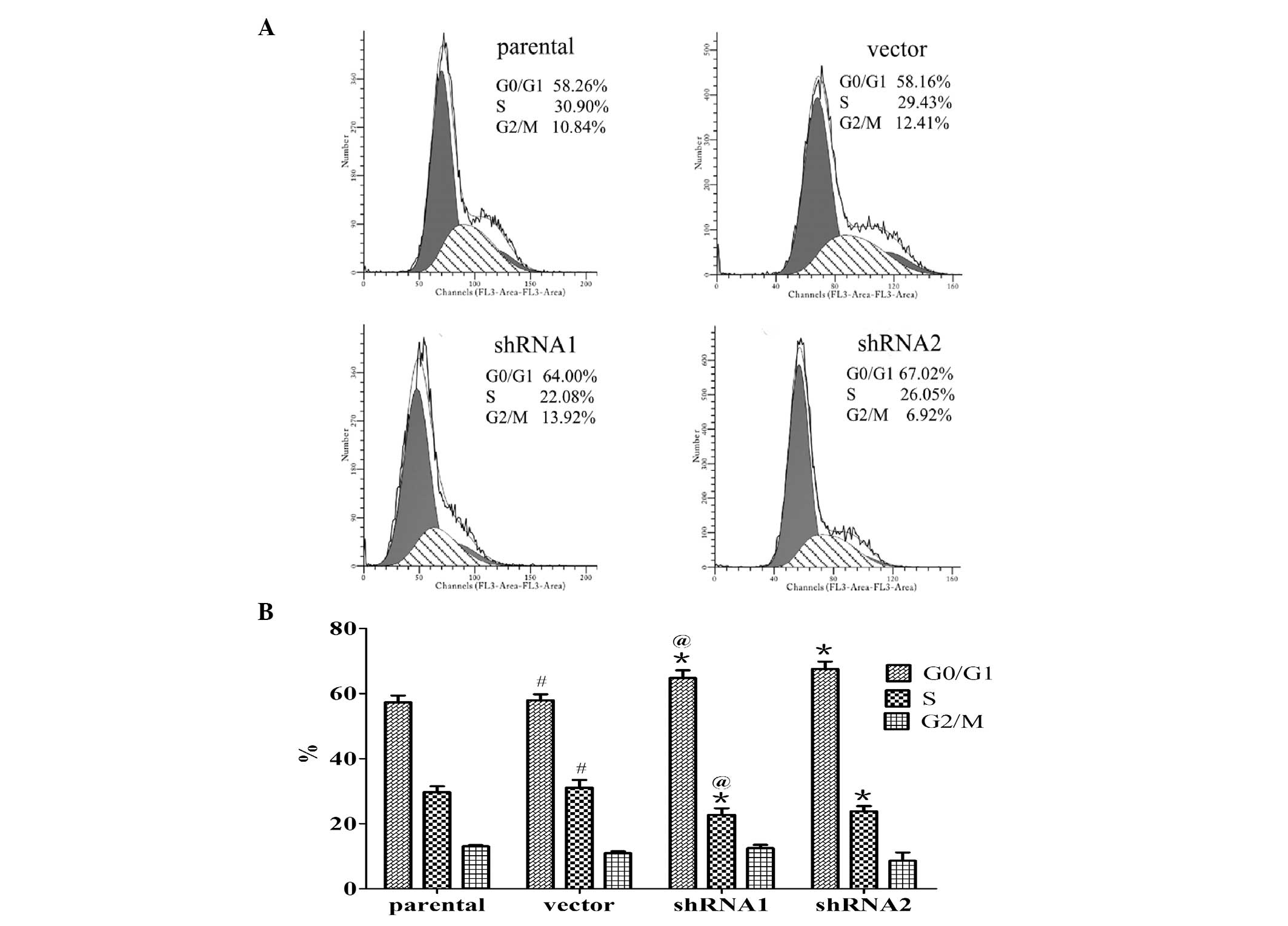
To uncover the mechanism underlying the growth
suppression effect of 14-3-3ɛ on SGC7901 cells, the cell cycle
distribution was analyzed by fluorescence-activated cell sorting
analysis. As shown in Fig. 4,
14-3-3ɛ knockdown induced cell cycle arrest in G0/G1 phase in the
SGC7901 cell line. As compared with the parental SGC7901 cells, the
percentage of cells in G0/G1 phase in the shRNA1 and shRNA2 groups
was significantly increased by 13.06 and 17.81% (P<0.05), and
the percentage of cells in S phase was significantly reduced by
6.94 and 5.85% (P<0.05), respectively. However, no significant
differences in cell cycle distribution were observed between the
parental SGC7901 cells and the cells transfected with control
shRNA. This suggests that 14-3-3ɛ downregulation induced G0/G1
arrest in SGC7901 cells.
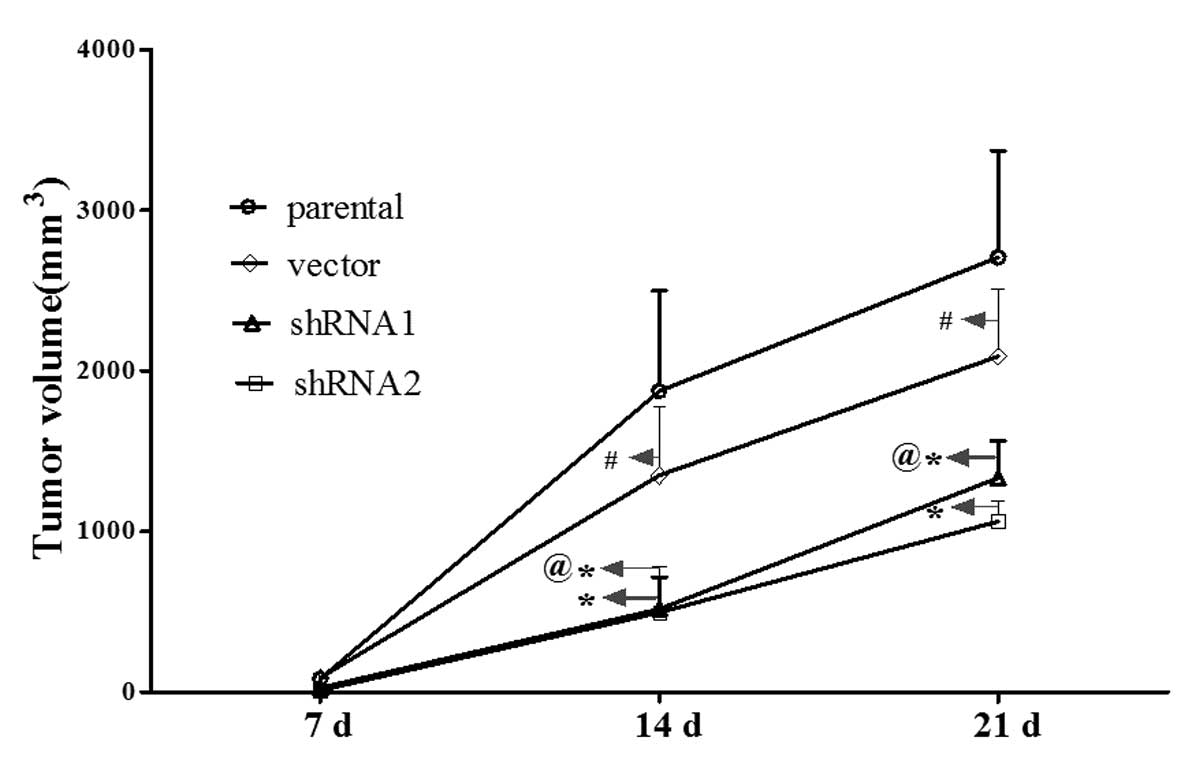
14-3-3ɛ downregulation inhibits tumor
growth of gastric cancer cells in vivo
To further confirm the effects of 14-3-3ɛ on the
tumor growth of gastric cancer, shRNA1-, shRNA2- and
vector-transfected as well as parental SGC7901 cells were
inoculated into female nude mice. Three weeks after injection, the
mice were sacrificed and the tumors were excised. As shown in
Fig. 5, the velocities of tumor
growth in the shRNA1 and shRNA2 groups were significantly slower
than those of the parental SGC7901 or vector groups (P<0.05).
These results indicated that downregulation of 14-3-3ɛ expression
resulted in inhibited tumor growth in vivo.
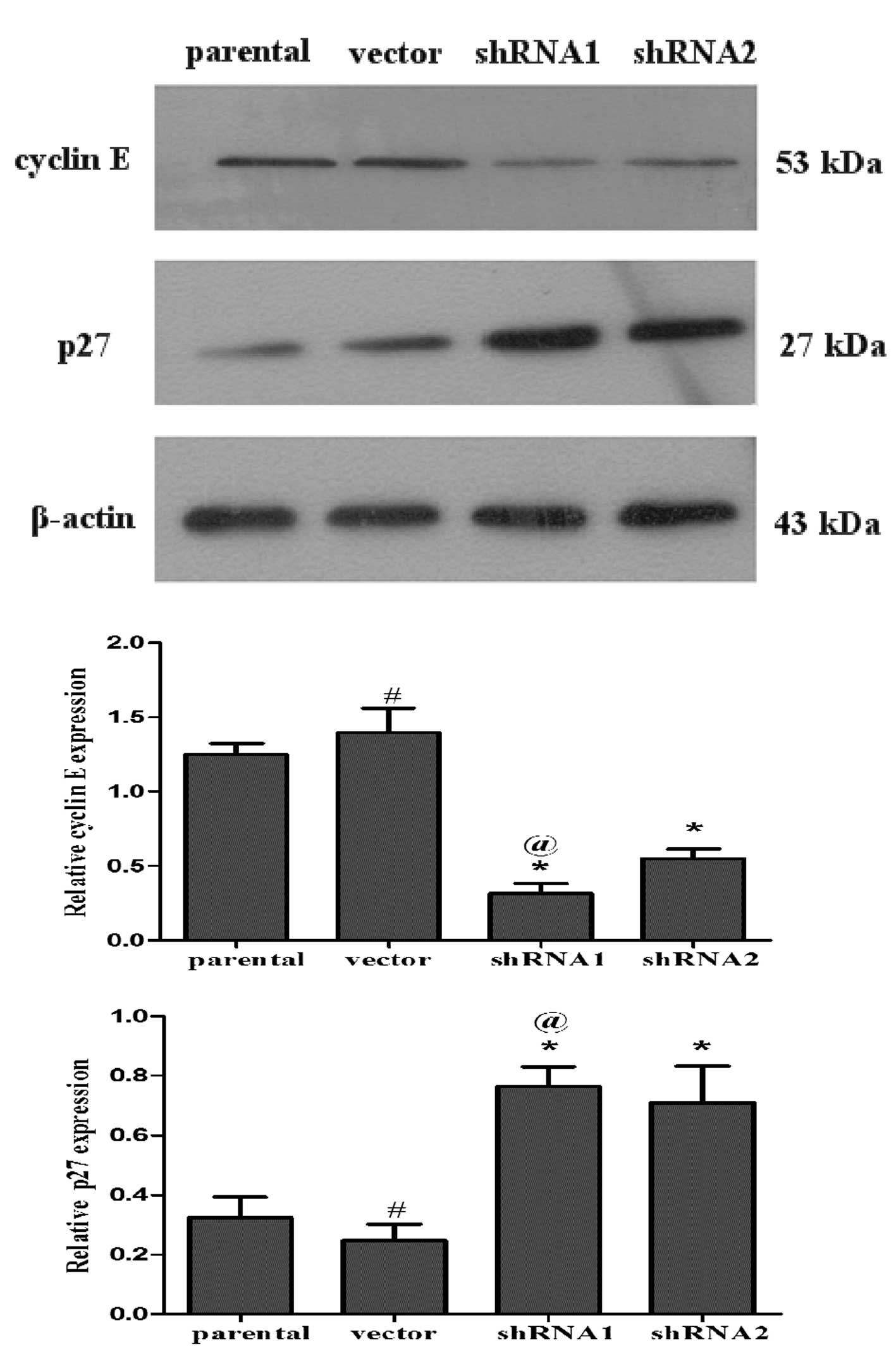
Inhibition of 14-3-3ɛ suppresses cyclin E
expression and induces p27kip1 expression in SGC7901
cells
Cyclin E is an important cell cycle regulator, which
forms a complex with cyclin-dependent kinase (CDK) 2 and
facilitates cell cycle progression from G1 to S phase.
P27kip1, known as a CDK inhibitor, mainly inhibits the
activity of cell cycle complexes, including cyclin E-CDK2, which
results in G1 phase arrest (23).
Western blot analysis was performed to detect the protein
expression levels of these cell cycle regulators. As indicated in
Fig. 6, the expression of
p27kip1 was upregulated and the expression of cyclin E
was downregulated in the shRNA1 and shRNA2 groups (P<0.05). The
results demonstrated that 14-3-3ɛ may have been involved in
regulating the expression of cyclins binding to CDKs and the
expression of CDK inhibitors in the SGC7901 gastric cancer cell
line.
Discussion
Increasing evidence suggests that 14-3-3ɛ is
critical in carcinogenesis and cancer progression. Altered 14-3-3ɛ
expression levels have been implicated in the initiation and
development of different types of tumors. Previous studies have
shown that the role of 14-3-3ɛ in the progression of specific
tumors is controversial. However, it has been demonstrated that
14-3-3ɛ expression is upregulated and functions as an oncogene in a
number of tumors, including breast and hepatic cellular cancer,
vulvar squamous cell carcinoma, follicular and papillary thyroid
tumors and meningioma (13–17).
For example, Ko et al (17)
demonstrated that overexpression of 14-3-3ɛ in primary hepatic
cellular cancer tissues conveys a high risk of extrahepatic
metastasis and shortened survival rate. In addition, 14-3-3ɛ has
been shown to act as an antioncogene in certain instances. Che
et al (24) revealed that
reduced expression levels of 14-3-3ɛ contributed to laryngeal
squamous cell carcinoma (LSCC) development and that 14-3-3ɛ was
able to promote apoptosis and inhibit the invasiveness of LSCC.
This discrepancy in response to 14-3-3ɛ is suggestive of a
specificity for different cell types. To date, little is known
regarding the role of 14-3-3ɛ in gastric cancer.
Preliminary results performed by our group using
immunohistochemical staining revealed that the protein expression
levels of 14-3-3ɛ in gastric cancer tissues were significantly
higher than those of paired tumor tissues. The present study
attempted to further demonstrate the involvement of 14-3-3ɛ in cell
growth by using RNAi. MTT and colony formation assays showed that
the proliferation of gastric cancer cells was significantly
inhibited in cells in which 14-3-3ɛ was downregulated. Furthermore,
tumorigenicity in xenografts was reduced upon 14-3-3ɛ
downregulation. The above results indicated that 14-3-3ɛ may act as
an oncogene in gastric cancer. However, downregulation of 14-3-3ɛ
reduced but did not completely halt cell proliferation, indicating
that, although 14-3-3ɛ was clearly involved in gastric cancer cell
proliferation, other proteins were also involved in this process.
In addition, a component of the tumor-promotion activity of 14-3-3ɛ
was demonstrated to proceed via the inhibition of cell cycle
progression as indicated by increased accumulation of cancer cells
in the G1 phase and a corresponding reduction of cells in the S
phase of the cell cycle in the SGC7901 gastric cancer cell
line.
The exact molecular mechanism of how 14-3-3ɛ
downregulation stimulates G1-S arrest remains to be elucidated. One
notable study suggested that 14-3-3ɛ regulates compact ventricular
myocardium growth by modulating the cardiomyocyte cell cycle via
cyclin E and p27kip1 (25). In the present study, a possible
correlation between 14-3-3ɛ and cyclin E/p27kip1 protein
expression was investigated in vitro. The study revealed
that the expression levels of cyclin E were reduced, whereas the
expression levels of p27kip1 were increased upon 14-3-3ɛ
knockdown in SGC7901 cells. It is known that p27kip1 is
a CDK inhibitor acting on cyclin E-CDK2, which promotes G1/S phase
transition (26,27). Therefore, changes in the expression
levels of cyclin E and p27kip1 may contribute to the
effect of 14-3-3ɛ on gastric cancer cell proliferation and G1/S
checkpoint control. Of note, 14-3-3ɛ in association with DP-3 has
also been reported to upregulate the transcription of cyclin E
(28). 14-3-3ɛ was originally
considered to regulate signal transduction by modulating
protein-protein interactions in the cytoplasm (29). A mechanism by which 14-3-3ɛ may
promote the progression of the cell cycle may be through
facilitating the localization of p27kip1 to the
cytoplasm (30,31), where phosphorylated
p27kip1 is degraded by proteases following
ubiquitination (32). In future
studies, the analysis of 14-3-3ɛ interactions with protein-binding
partners may reveal the mechanisms by which 14-3-3ɛ modulates the
expression levels of different cell cycle-associated proteins.
The findings of the present study are contradictory
to those of Leal et al (33), who demonstrated that 14-3-3ɛ
expression levels are reduced in gastric cancer, comparing 14-3-3ɛ
protein expression levels in 20 pairs of gastric cancer samples
with corresponding non-neoplastic gastric tissues using western
blot analysis. The insufficient sample size and the lack of further
investigation in vitro and in vivo may be responsible
for this difference. In addition, the role of 14-3-3ɛ in other
gastric cancer cell lines and the manner in which cyclin E and
p27kip1 are regulated require further investigation.
In conclusion, the present study demonstrated that
knockdown of 14-3-3ɛ inhibited the proliferation of gastric cancer
cells, partially through downregulation of cyclin E and
upregulation of p27kip1. This provides novel insight
into the function of 14-3-3ɛ and suggests that downregulation of
14-3-3ɛ by RNAi may be another possible approach in the management
of human gastric cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81072038).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Orditura M, Galizia G, Sforza V, et al:
Treatment of gastric cancer. World J Gastroenterol. 20:1635–1649.
2014. View Article : Google Scholar
|
|
3
|
Yasui W, Oue N, Aung PP, Matsumura S,
Shutoh M and Nakayama H: Molecular-pathological prognostic factors
of gastric cancer: a review. Gastric Cancer. 8:86–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.
|
|
5
|
Uemura N, Okamoto S, Yamamoto S, et al:
Helicobacter pylori infection and the development of gastric
cancer. N Engl J Med. 345:784–789. 2001. View Article : Google Scholar
|
|
6
|
Houghton J, Stoicov C, Nomura S, et al:
Gastric cancer originating from bone marrow-derived cells. Science.
306:1568–1571. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiyama E, Yokoyama T, Tatsumoto N, et al:
Telomerase activity in gastric cancer. Cancer Res. 55:3258–3262.
1995.PubMed/NCBI
|
|
8
|
Zheng L, Wang L, Ajani J and Xie K:
Molecular basis of gastric cancer development and progression.
Gastric Cancer. 7:61–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tahara E: Genetic pathways of two types of
gastric cancer. IARC Sci Publ. 157:327–349. 2004.PubMed/NCBI
|
|
10
|
Aitken A: 14-3-3 proteins: a historic
overview. Semin Cancer Biol. 16:162–172. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mackintosh C: Dynamic interactions between
14-3-3 proteins and phosphoproteins regulate diverse cellular
processes. Biochem J. 381:329–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qi W, Liu X, Qiao D and Martinez JD:
Isoform-specific expression of 14-3-3 proteins in human lung cancer
tissues. Int J Cancer. 113:359–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li DQ, Wang L, Fei F, et al:
Identification of breast cancer metastasis-associated proteins in
an isogenic tumor metastasis model using two-dimensional gel
electrophoresis and liquid chromatography-ion trap-mass
spectrometry. Proteomics. 6:3352–3368. 2006. View Article : Google Scholar
|
|
14
|
Wang Z, Nesland JM, Suo Z, Trope CG and
Holm R: The prognostic value of 14-3-3 isoforms in vulvar squamous
cell carcinoma cases: 14-3-3beta and epsilon are independent
prognostic factors for these tumors. PLoS One. 6:e248432011.
View Article : Google Scholar
|
|
15
|
Sofiadis A, Becker S, Hellman U, et al:
Proteomic profiling of follicular and papillary thyroid tumors. Eur
J Endocrinol. 166:657–667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Tian RF, Li YM, et al: The
expression of seven 14-3-3 isoforms in human meningioma. Brain Res.
1336:98–102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ko BS, Chang TC, Hsu C, et al:
Overexpression of 14-3-3epsilon predicts tumour metastasis and poor
survival in hepatocellular carcinoma. Histopathology. 58:705–711.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gradilone SA, Radtke BN, Bogert PS, Huang
BQ, Gajdos GB and LaRusso NF: HDAC6 inhibition restores ciliary
expression and decreases tumor growth. Cancer Res. 73:2259–2270.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vázquez-Vega S, Contreras-Paredes A,
Lizano-Soberón M, Amador-Molina A, García-Carrancá A,
Sánchez-Suárez LP and Benítez-Bribiesca L: RNA interference (RNAi)
and its therapeutic potential in cancer. Rev Invest Clin. 62:81–90.
2010.(In Spanish).
|
|
20
|
Sen GL and Blau HM: A brief history of
RNAi: the silence of the genes. FASEB J. 20:1293–1299. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian J, Liu H, Chen W, Wen K, Lu W, Huang
C and Fu Z: Knockdown of Slug by RNAi inhibits the proliferation
and invasion of HCT116 colorectal cancer cells. Mol Med Rep.
8:1055–1059. 2013.PubMed/NCBI
|
|
22
|
Yan L, Gu H, Li J, et al: RKIP and
14-3-3epsilon exert an opposite effect on human gastric cancer
cells SGC7901 by regulating the ERK/MAPK pathway differently. Dig
Dis Sci. 58:389–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawauchi S, Yamamoto Y, Uchida K, Chochi
Y, Kondo T, Oga A and Sasaki K: Significance of cyclin E and p27
expression in malignant ovarian germ cell tumors: correlation with
the cell proliferation activity and clinicopathologic features.
Oncol Rep. 16:1029–1033. 2006.PubMed/NCBI
|
|
24
|
Che XH, Chen H, Xu ZM, Shang C, Sun KL and
Fu WN: 14-3-3epsilon contributes to tumour suppression in laryngeal
carcinoma by affecting apoptosis and invasion. BMC Cancer.
10:3062010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kosaka Y, Cieslik KA, Li L, et al:
14-3-3epsilon plays a role in cardiac ventricular compaction by
regulating the cardiomyocyte cell cycle. Mol Cell Biol.
32:5089–5102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mazumder S, Plesca D and Almasan A: A
jekyll and hyde role of cyclin E in the genotoxic stress response:
switching from cell cycle control to apoptosis regulation. Cell
Cycle. 6:1437–1442. 2007. View Article : Google Scholar
|
|
27
|
Mitrea DM, Yoon MK, Ou L and Kriwacki RW:
Disorder-function relationships for the cell cycle regulatory
proteins p21 and p27. Biol Chem. 393:259–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milton AH, Khaire N, Ingram L, O’Donnell
AJ and La Thangue NB: 14-3-3 proteins integrate E2F activity with
the DNA damage response. EMBO J. 25:1046–1057. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mackintosh C: Dynamic interactions between
14-3-3 proteins and phosphoproteins regulate diverse cellular
processes. Biochem J. 381:329–342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fujita N, Sato S, Katayama K and Tsuruo T:
Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3
and cytoplasmic localization. J Biol Chem. 277:28706–28713. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fujita N, Sato S and Tsuruo T:
Phosphorylation of p27Kip1 at threonine 198 by p90 ribosomal
protein S6 kinases promotes its binding to 14-3-3 and cytoplasmic
localization. J Biol Chem. 278:49254–49260. 2003. View Article : Google Scholar
|
|
32
|
Wang W, Ungermannova D, Jin J, Harper JW
and Liu X: Negative regulation of SCFSkp2 ubiquitin ligase by
TGF-beta signaling. Oncogene. 23:1064–1075. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leal M, Calcagno D, Demachki S, Assumpção
P, Chammas R, Burbano R and Smith M: Clinical implication of 14-3-3
epsilon expression in gastric cancer. World J Gastroenterol.
18:1531–1537. 2012. View Article : Google Scholar : PubMed/NCBI
|