Introduction
Prostate cancer is the most common type of cancer in
males and second most common cause of cancer-related mortality with
an increasing incidence worldwide (1). Despite the fact that Slovakia has a
higher incidence of prostate cancer than other countries in the
Central and Eastern Europe, it is classified as a country with an
intermediate incidence of prostate cancer. In the year 2003, 1,130
cases of prostate cancer were diagnosed in Slovakia, which
represents a raw incidence of 43.3/100,000 males and an
age-standardized rate (ASR) of 33.9/100,000 (2,3). The
incidence of prostate cancer in Slovakia increased with an ASR of
14.6, 14.5 and 33.9/100,000 in the years 1968, 1980 and 2003,
respectively.
The most well-recognized risk factors for prostate
cancer development are age, geographic origin/ethnicity and family
history of the disease (1). The
known modulating susceptibility factors of prostate cancer are diet
and lifestyle (4,5). Other predisposing risk factors for
prostate cancer may be considered, including heredity (genetics),
hormonal influences and environmental factors (6–8).
Androgens, testosterone and dihydrotestosterone
(DHT) are actively involved in the growth and development of the
prostate, and appear to be essential for carcinogenesis in the
prostate gland (9). In total,
80–90% of prostate cancers are dependent on androgens at initial
diagnosis, and endocrine therapy of prostate cancer is directed
towards the reduction of serum androgens and inhibition of the
androgen receptor (10,11). It has been hypothesized that
variation in the genes involved in androgen biosynthesis and
metabolism may be risk factors for prostate cancer (12). One of these genes is
steroid-5-α-reductase, which irreversibly catalyzes the conversion
of testosterone to its more active and most potent androgen DHT
(13,14).
A total of two steroid-5-α-reductases expressed in
the human prostate have been identified and steroid-5-α-reductase,
type II is the predominant isoenzyme that is encoded by the
SRD5A2 gene. Subsequent to catalysis of this enzyme, the
binding affinity of DHT to an androgen receptor is five times
higher compared with that of testosterone (13). It has been demonstrated that young
Japanese males have a lower steroid-5-α-reductase activity compared
with young Caucasian-American and African-American males (15), and the DHT:testosterone ratio was
highest in African-Americans, intermediate in Caucasians and lowest
in Asian-Americans, corresponding to the respective risk of
developing prostate cancer in these groups (16,17).
Montgomery et al (18)
demonstrated the downregulation of SRD5A2 mRNA in
castration-resistant metastatic prostate cancer tissue. This
finding is consistent with the hypothesis that testosterone may be
equally as important as DHT in promoting the aggressive behavior of
advanced prostate cancer cells.
The SRD5A2 gene is located on the short (p)
arm of chromosome 2 at position 23. It spans over 40 Kbp of genomic
DNA and contains 5 exons and 4 introns (19). Several polymorphisms within the
SRD5A2 gene have been identified (20). One of the most highly polymorphic,
contested and investigated is a single nucleotide polymorphism that
replaces the more frequently encountered valine (V) with a leucine
(L) residue at amino acid position 89 (V89L, rs523349) (21,22).
Experiments with cloned and expressed human SRD5A2 isoforms
reported significant differences in their KM and
Vmax values (23). The
LL genotype tends to have decreased enzyme activity (21). In certain studies, this genotype
was associated with lower androgen levels (22,24);
however, in others this was not observed (25). Controversies remain regarding the
association of this polymorphism with the risk of prostate cancer.
Studies have reported a decreased risk of prostate cancer
associated with the LL genotype (26,27),
an increased risk with the LL genotype (28,29)
or no association with risk and this genotype (17,30).
Given the importance of the SRD5A2 gene in
androgen production and the conflicting epidemiological data
regarding the SRD5A2 polymorphism as a risk factor for
prostate cancer, a case-control study of the association between
the SRD5A2 V89L polymorphism and the risk of prostate cancer
was conducted. The hypothesis of the association between this
polymorphism and tumor grade and disease onset within the study
population was also investigated.
Patients and methods
Study population
A case-control study was conducted that included 260
patients with histologically verified prostate cancer and 196
age-matched control patients who visited the Department of Urology
in the University Hospital Martin (Martin, Slovakia) between May
2005 and June 2010. The mean age of the patients was 63.6 years for
the prostate cancer cases and 62.3 years for the controls. All the
cases and controls were Caucasians. In the prostate cancer group
(n=260), 83 patients (31.9%) had a Gleason score <7
(histological grading system referring to the cancer aggressiveness
in clinical setting) (31) and 159
patients (61.2%) had a Gleason score ≥7. In the remaining 18 (6.9%)
cases the Gleason score was not recorded and these patients were
excluded from the prostate cancer subgroup analysis. Subjects were
recruited to participate in the study if they met the inclusion and
exclusion criteria.
Patients with histologically proven prostate cancer
(in a biopsy specimen or radical prostatectomy specimen) regardless
of the pathological stage were enrolled in the study. Patients with
the presence of any other type of cancer or major urological
pathology, or patients who had a first-degree relative (brother or
father) with a confirmed diagnosis of prostate cancer were
excluded.
In the control group, age-matched healthy men
without any clinically suspected infection of the prostate, benign
prostate hyperplasia, benign prostate enlargement or prostatitis
were included. The presence of prostate cancer was clinically
excluded by a negative digital rectal examination finding and
prostate-specific antigen (PSA) serum level within the normal age
specific range (32). Controls
that had a first-degree relative (brother or father) with a
confirmed diagnosis of prostate cancer were excluded.
The basic characteristics of the study population
are described in Table I. The
present study was approved by the Ethical Board of Jessenius
Faculty of Medicine, Comenius University (Martin, Slovakia) and
informed written consent was obtained from all participants prior
to the study.
 | Table IBasic characteristics of the study
population. |
Table I
Basic characteristics of the study
population.
| Parameter | Cases, n=260 | Controls,
n=196 | P-valuea |
|---|
| Age, years | | | NS |
| Mean | 63.63 | 62.30 | |
| SD | 7.27 | 6.34 | |
| Median | 63.00 | 62.07 | |
| Min-max | 48–84 | 52–76 | |
| PSA, ng/ml | | | <0.001 |
| Mean | 27.39 | 1.05 | |
| SD | 69.59 | 0.77 | |
| Median | 9.34 | 0.81 | |
| Min-max | 1.87–810.5 | 0.17–3.90 | |
| Gleason score, n
(%) | | | |
| <7 | 83 (31.9) | NA | - |
| ≥7 | 159( 61.2) | NA | - |
| Unknown | 18 (6.9) | NA | - |
DNA extraction
In order to collect 5 ml of blood from all the study
participants a standard venipuncture was used
(ethylenediaminetetraacetic acid was added as an anticoagulant).
Briefly, selected polymorphisms were investigated in isolated DNA
from 5 ml peripheral blood which was collected into heparinized
test tubes using ethylenediaminetetraacetic acid (EDTA) and were
stored at −20°C until analysis. Genomic DNA from peripheral blood
leukocytes was isolated after digestion with proteinase K by
extraction with organic solvents (phenol, chloroform
isoamylalcohol) and precipitation using ethanol. Blood samples were
lysed using lysing solution (0.77 M NH4Cl, 0.046 M
NaHCO3, 0.01 M EDTA) and a sediment was placed into SE
solution (0.075 M NaCl, 0.025 M EDTA) and incubated at 55°C for
three hours (or incubation at 37°C overnight). Isolation continued
with a standard phenol/chloroform methodology (33) was used to extract genomic DNA,
which was stored at −20°C until further genotype analysis.
Genotyping
Restriction fragment length polymorphism analysis
polymerase chain reaction (RFLP-PCR) was used to detect the
SRD5A2 polymorphism on codon 89 according to the study by
Cicek et al (34). The PCR
reaction mixture (25 μl) included 100 ng genomic DNA, 200 μmol/l
deoxynucleoside triphosphates, 1 unit of Taq polymerase in
10X PCR buffer [composed of 16.6 mmol/l
(NH4)2SO4 and 20.0 mmol/l MgCl2;
pH 8.8] and 25 pmol of each of the SRD5A2 primers
(SRD5A2-F, 5′-TGG CCT TGT ACG TCG CGA AG-3′ and
SRD5A2-R, 5′-AGC AGG GCA GTG CGC TGC ACT-3′). For
amplification, PCR was used with the following steps: Initial
denaturation for 5 min at 94°C, 35 cycles at 94°C for 45 sec, 62°C
for 1 min and 72°C for 1 min. The final elongation step was
performed at 72°C for 10 min. Each PCR product (261 bp) was
digested at 37°C for 16 h by the restriction enzyme RsaI (5
units, Fermentas, Denmark) in order to determine the presence of
the V89L variant (9), separated by
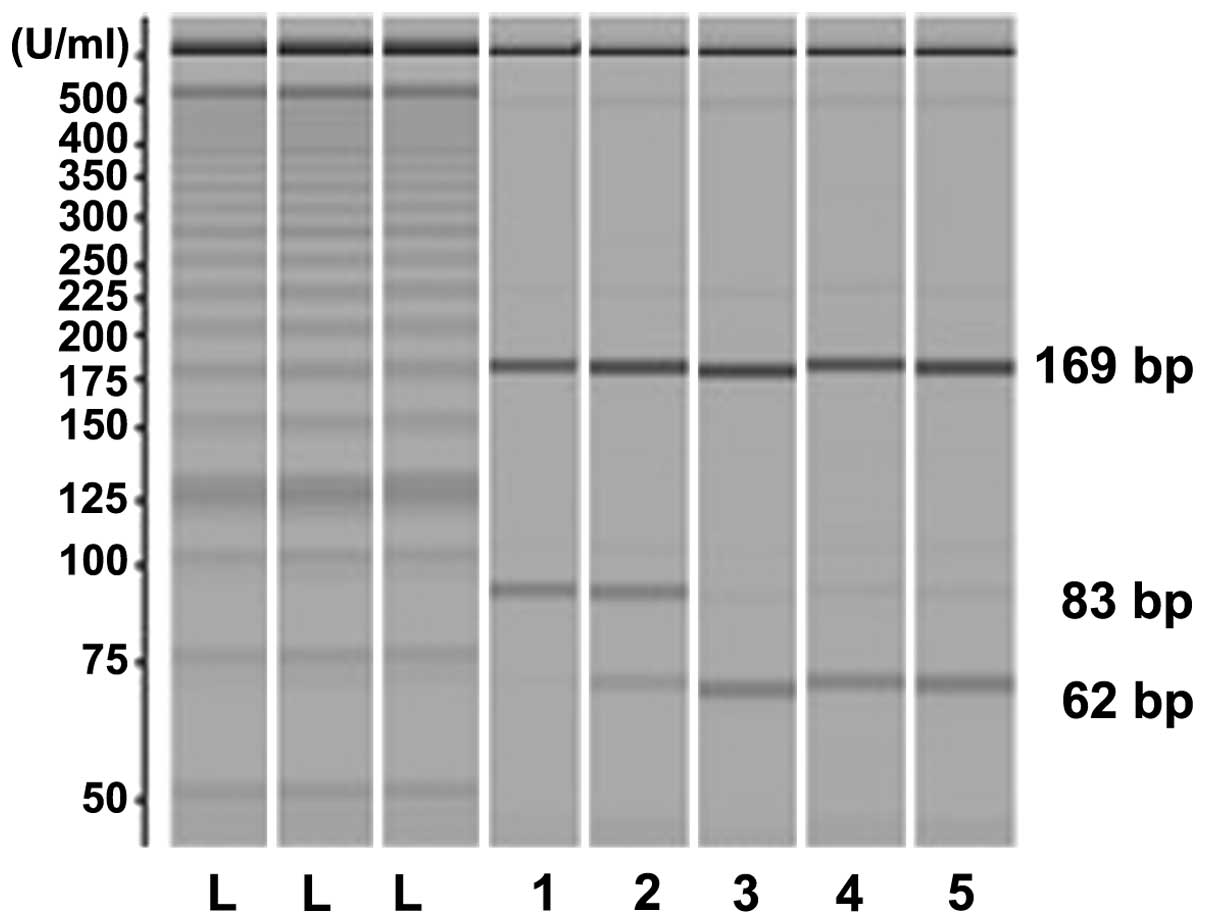
the Microchip Electrophoresis system for DNA/RNA Fragment Analysis
MCE®-202 MultiNA (Shimadzu, Japan). The common
SRD5A2-89V allele retained the RsaI restriction site
at the polymorphic region and generated 21-, 62- and 169-bp
fragments. The allelic variant SRD5A2-89L generated two
bands of 83 and 169 bp since the RsaI site was lost. The
PCR-RFLP of the SRD5A2 polymorphism was analyzed by
MCEA®-202 MultiNA (Shimadzu, Japan) and the PCR-RFLP
product samples are shown in Fig.
1.
Statistical analysis
Basic descriptive statistics and cross tabulations
for frequencies with χ2 tests were performed. The
measure of choice was the odds ratio (OR) with its corresponding
95% confidence intervals (95% CIs) that was used to determine the
association between the SRD5A2 gene polymorphism and
prostate cancer. All the data were analyzed with SPSS software
(version 19.0; SPPS Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference and
all the P-values presented are two-sided. The distribution of the
genotypes in the control group was tested for the deviation from
Hardy-Weinberg equilibrium (HWE) at the P<0.05 level using an
online tool for case-control studies (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl).
Results
Characteristics of the study
population
Selected characteristics of the study population,
which consisted of 260 patients with prostate cancer and 196
controls, are presented in Table
I. The cases and controls were age-matched with a mean age of
63.6 years and 62.3 years, respectively. PSA level was measured in
control group at the time of patient first visit. The mean serum
PSA levels, measured at the time of diagnosis averaged to 27.39
ng/ml in the prostate cancer group and 1.05 ng/ml in the controls
(P<0.001).
Genotype variants
The frequencies of the V and L allele in the
controls were consistent with HWE (Pearson coefficient, P=0.266);
however, there was significant deviation of genotype distribution
from HWE in the prostate cancer group (Pearson coefficient,
P=0.037) (data not shown).
The frequencies of the V89L genotype variants and
the correspondent ORs (95% CI) for the risk of prostate cancer
development that are valid for particular allele variants are
listed in Table II. The
difference in distribution of three genotype variants resulting
from the polymorphism at codon 89 between the case and control
groups was not statistically significant (χ2 test,
P=0.842). There was no significant allelic or genotypic association
between the SDR5A2 V89L polymorphism and the risk of
developing prostate cancer. Males with the VL genotype had a
0.95-fold reduction in the risk of developing prostate cancer (95%
CI 0.63–1.44), the LL genotype was associated with a 1.11-fold
increased risk of developing prostate cancer (95% CI 0.66–1.86) and
the VL + LL genotype with a 0.99-fold reduced risk of developing
prostate cancer (95% CI 0.68–1.46) when compared with the VV
genotype.
 | Table IIDistribution of the SRD5A2
genotype variants. |
Table II
Distribution of the SRD5A2
genotype variants.
|
Genotype/allele | Cases, n (%) | Controls, n
(%) | OR (95% CI) | P-value |
|---|
| VV | 97 (37.3) | 73 (37.2) | 1.00
(reference) | - |
| VL | 110 (42.3) | 87 (44.4) | 0.95
(0.63–1.44) | NS |
| LL | 53 (20.4) | 36 (18.4) | 1.11
(0.66–1.86) | NS |
| VL + LL | 163 (62.7) | 123 (62.8) | 0.99
(0.68–1.46) | NS |
| Allele V | 304 (58.5) | 233 (59.4) | 1.00
(reference) | - |
| Allele L | 216 (41.5) | 159 (40.6) | 1.04
(0.80–1.36) | NS |
Analysis according to the Gleason score. An analysis
according to the Gleason score and the SRD5A2 V89L
polymorphism was performed in 242 patients with prostate cancer
(Table III). No significant
difference was identified in the frequencies of genotype variants
with respect to tumor aggressiveness according to the Gleason score
(χ2 test, P=0.971). Therefore, no significant increase
in OR for developing highly aggressive prostate cancer for any
particular genotype variant was identified when compared with the
wild type VV variant (ORs ranged from 0.95–1.04, P>0.05).
 | Table IIIGleason score in patients with
prostate cancer with SRD5A2 genotypes (n=242). |
Table III
Gleason score in patients with
prostate cancer with SRD5A2 genotypes (n=242).
| No. of cases
(%) | | |
|---|
|
| | |
|---|
|
Genotype/allele | Gleason <7 | Gleason ≥7 | OR (95% CI) | P-value |
|---|
| VV | 31 (37.3) | 59 (37.1) | 1.00
(reference) | - |
| VL | 37 (44.6) | 73 (45.9) | 1.04
(0.58–1.86) | NS |
| LL | 15 (18.1) | 27 (17.0) | 0.95
(0.44–2.04) | NS |
| VL + LL | 52 (62.7) | 100 (62.9) | 1.01
(0.58–1.76) | NS |
| Allele V | 99 (59.6) | 191 (60.1) | 1.00
(reference) | - |
| Allele L | 67 (40.4) | 127 (39.9) | 0.98
(0.67–1.44) | NS |
Additionally, no significant difference was
identified in the genotype distribution leading to an increased or
decreased risk of developing prostate cancer regardless of the age
of disease onset when comparing LL and VV variants (under and over
65 years of age) with OR=1.09 (95% CI 0.57–2.09, P>0.05) and
OR=1.08 (95% CI 0.43–2.72, P>0.05), respectively (data not
shown). Considering 65 years of age as early onset of disease, no
difference in genotype distribution leading to early onset of
prostate cancer with comparable OR for LL and VV variants was
observed (OR=1.09, 95% CI 0.57–2.09, P>0.05 and OR=1.08, 95% CI
0.43–2.72, P>0.05, respectively) (data not shown).
Discussion
Androgens are critical in prostate gland growth and
prostate cancer development. It has been hypothesized that
variation in the genes involved in androgen biosynthesis and
metabolism may be a risk factor for prostate cancer. Genetic
research has aimed to identify genetically determined differences
in androgen metabolism, which may explain a number of the
differences in the risk of prostate cancer (35,36).
Results of such studies, including clinical, epidemiological and
biochemical research are presented with conflicting outcomes.
SRD5A2 has long been considered as a strong candidate gene
for prostate cancer development due to its key role in the androgen
metabolism. Polymorphisms in the SRD5A2 gene have been
investigated extensively. The V89L substitution mutation results in
an almost 30% reduction in enzyme activity in vitro and
in vivo when compared with the wild type gene (37). Thus, this polymorphism may alter
the intraprostatic level and activity of DHT and consequently, the
risk of prostate cancer.
To the best of our knowledge, this is the first
study to investigate the association between prostate cancer risk
and the functional polymorphism of the SRD5A2 gene involved
in the sex hormone pathways. The results do not indicate a
significant effect of the SRD5A2 V89L polymorphism on the
risk of developing prostate cancer. Allele frequencies of the
SRD5A2 gene follow diverse ethnicity and/or
geography-specific patterns. According to data from the SNP
database (38), the frequency of
the leucine allele ranges from 19–23% in North American and
European Caucasian populations and 40–50% in Asians.
Correspondingly, the LL phenotype frequency is low in Caucasians
(3–6%) and higher in Asians (27–30%) (20). The prevalence rate of VL
heterozygosity and LL homozygosity was 44.4 and 18.4%,
respectively, in the Caucasian control subjects. However, the
distribution of genotypes in the control group was consistent with
HWE.
The results of the present study are generally in
agreement with the results of several published case-control
studies and meta-analyses (12,30,28).
Li et al (28) conducted a
meta-analysis of 31 studies with SRD5A2 genotyping in 14,726
patients with prostate cancer and 15,802 controls, revealed no
association between prostate cancer risk and the L allele compared
with the V allele with an OR of 1.02 (95% CI 0.98–1.06, P=0.38).
Their results were consistent across regardless of race. This study
also observed no association between the L allele and the risk of
prostate cancer regardless of the grade of cancer. The results of
the present study showed no changes in the risk of prostate cancer
development for different allele variants (LL, VL, LL + VL)
compared with the wild type VV variant (ORs ranged from
0.95–1.11).
Additionally, an earlier meta-analysis by Wang et
al (30) indicated that the
SRD5A2 V89L polymorphism only exhibited a low-penetrant role
in prostate cancer risk among Europeans and individuals younger
than 65 years. In Europeans the small increase in prostate cancer
risk was observed in the dominant model (LL + VL vs. VV) with an OR
of 1.11 (95% CI, 1.03–1.19; P<0.01). A significant association
with an increased prostate cancer risk in men aged 65 years was
observed in codominant allelles, i.e. LL vs. VV (OR, 1.70; 95% CI
1.09–2.66, P=0.02) and recessive, i.e. LL vs. VV + VL (OR, 1.75;
95% CI, 1.14–2.68;P=0.01) models. However, no significant
associations were found in Asians and Africans or in the overall
analysis.
The exact role of androgens in the pathogenesis of
prostate cancer has been a contentious issue; thus, serum hormone
levels may not provide definitive proof for the presence or absence
of a genetic predisposition to prostate cancer (17). Certain studies report associations
between the SRD5A2 V89L polymorphism and plasma androgen
levels, including testosterone and 5a-androstane-3a-17a-diol
glucuronide (3a-diolG), which is a direct metabolite of DHT and may
be an indicator of 5-α-reductase, type II activity (22,39).
It has been reported that males with the VL and LL genotypes in
comparison with those with the VV genotype exhibited a lower serum
level of 3a-diolG (22).
Conversely, Hayes et al (40) found no evidence of association
between the SRD5A2 V89L polymorphism and testosterone,
androstenedione, sex hormone-binding globulin or circulating levels
of 3a-diolG and the risk of prostate cancer. The results of the
study by Hayes et al (40)
are concurrent with the results of the European Prospective
Investigation into Cancer and Nutrition study performed in 2001
(41), which revealed that
SRD5A2 V89L was associated with changes in the serum
androgen levels, while another SRD5A2 A49T polymorphism
caused a 24% reduction of circulating levels of 3a-diolG in
carriers of the GA genotype compared with the GG genotype, leading
to a 60% higher risk of developing prostate cancer (24,40).
The most significant clinical features of prostate
cancer that influence the choice of treatment include the age of a
patient at the time of diagnosis (early vs. late onset of disease)
and prostate tumor aggressiveness. The best result of genotyping
studies in an individual patient would be the ability to predict
early onset of highly aggressive prostate cancer with establishment
of an appropriate therapy. Scariano et al (20) genotyped 33 males with early onset
prostate cancer and found that the expression of at least one
SRD5A L allele in young males with prostate cancer was
associated with a more significant disease at the time of
presentation as was defined by the pretreatment PSA level, clinical
staging and Gleason score when compared with affected subjects
harboring the more common SRD5A2 V variant. Similar results
were published by other studies (34,42).
Cicek et al (34) confirmed
the association between the SRD5A2 polymorphism and
increased risk of prostate cancer in males diagnosed at an earlier
age or with more aggressive disease (OR, 2.35; 95% CI, 1.41–3.92;
P=0.001 and OR, 1.63; 95% CI, 0.98–2.72; P=0.06, respectively). By
contrast, in our cohort no significant difference was identified in
the genotype distribution leading to increased risk of prostate
cancer regardless of disease onset when comparing LL and VV
variants (data not shown). The hypothesis that any allele variant
can drive the development of high risk prostate cancer was not able
to be proven. The cut-off value (equal to 7) for the Gleason score
was selected with respect to the indubitable significance of such a
value for clinical decision making in a treatment scenario. Results
similar to ours, regarding the non-significant association between
SRD5A2 V89L polymorphism and Gleason score are also
presented in a meta-analysis published by Li et al (28).
The present study and results of previous studies do
not clearly confirm the exact role of the SRD5A2 V89L
polymorphism in prostate cancer susceptibility. This requires a
complex approach and we hypothesized that the activity of
5-α-reductase, type II can be affected not only by this and/or
other polymorphisms, but also by: i) Changes in the expression of
the SRD5A2 gene; ii) differences in the local concentrations
of growth factors and other androgens that are also involved in the
regulation of this enzyme activity in vivo; and iii)
environmental factors, such as diet that may also influence the
androgen levels and may be partly mediated through the different
activity of 5-α-reductase, type II. In addition, circulating
androgen levels are likely to be only weakly correlated with
androgen levels within the prostate gland and can only provide a
limited view of the complexity of physiological events that
regulate 5-α-reductase, type II activity (41).
Furthermore, the etiology of such a multigenetic
disease cannot be explained by allelic variability at a single
locus and it is supposed that high throughput genotyping may aid in
the identification of novel genes, which could be suitable targets
for analysis in association studies.
Acknowledgements
This study was supported by the Ministry of Health
of the Slovak Republic under the project 2007/45-UK-10 ‘Genetic
polymorphism of xenobiotic metabolising enzymes and susceptibility
to prostate cancer in the Slovak population’ (grant no. MH SR
2007/57-UK-17). This study was supported by the ‘Center of
Excellence for Research on Personalized Therapy (CEVYPET)’ project,
code 2622012053, co-financed from EU sources and the European
Regional Development Fund. The authors would like to thank Mrs. M.
Martinčeková for her technical assistance.
References
|
1
|
Grönberg H: Prostate cancer epidemiology.
Lancet. 361:859–864. 2003.PubMed/NCBI
|
|
2
|
Ondrušová M and Ondruš D: The occurence
and mortality from prostate cancer in Slovakia and abroad.
Onkológia (Bratisl.). 4:272–274. 2009.(In Slovak).
|
|
3
|
Plesko I, Obsitníkova A, Cuninkova M,
Tomasek L, Stefanakova D and Kubik A: Increasing occurrence of
urological cancers in Slovakia. Neoplasma. 51:248–254.
2004.PubMed/NCBI
|
|
4
|
Khan N, Afaq F and Mukhtar H: Lifestyle as
risk factor for cancer: evidence from human studies. Cancer Lett.
293:133–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stein QP and Flanagan JD: Genetic and
familial factors influencing breast, colon, prostate and lung
cancers. S D Med. 16–22. 2010.PubMed/NCBI
|
|
6
|
Sánchez-Visconti G, Herrero L, Rabadán M,
Pereira I and Ruiz-Torres A: Ageing and prostate: age-related
changes in androgen receptors of epithelial cells from benign
hypertrophic glands compared with cancer. Mech Ageing Dev.
82:19–29. 1995.PubMed/NCBI
|
|
7
|
Brawley OW, Jani AB and Master V: Prostate
cancer and race. Curr Probl Cancer. 31:211–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sivonova MK, Dobrota D, Matakova T,
Dusenka R, Grobarcikova S, et al: Microsomal epoxide hydrolase
polymorphisms, cigarette smoking and prostate cancer risk in the
Slovak population. Neoplasma. 59:79–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Imamoto T, Suzuki H, Utsumi T, Endo T,
Takano M, et al: Association between serum sex hormone levels and
prostate cancer: effect of prostate cancer on serum testosterone
levels. Future Oncol. 5:1005–1013. 2009. View Article : Google Scholar
|
|
10
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van der Kwast TH, Schalken J, Ruizeveld de
Winter JA, van Vroonhoven CC, et al: Androgen receptors in
endocrine-therapy-resistant human prostate cancer. Int J Cancer.
48:189–193. 1991.PubMed/NCBI
|
|
12
|
Li X, Huang Y, Fu X, Chen C, Zhang D, et
al: Meta-analysis of three polymorphisms in the
steroid-5-α-reductase, α polypeptide 2 gene (SRD5A2) and risk of
prostate cancer. Mutagenesis. 26:371–383. 2011.PubMed/NCBI
|
|
13
|
Wilbert DM, Griffin JE and Wilson JD:
Characterization of the cytosol androgen receptor of the human
prostate. J Clin Endocrinol Metab. 56:113–120. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coffey DS: The Molecular Biology of the
Prostate. Prostate Diseases. Lepor H and Lawson RK: Saunders;
Philadelphia, PA: pp. 28–56. 1993
|
|
15
|
Ross RK, Bernstein L, Lobo RA, Shimizu FZ,
Stanczyk FZ, et al: 5-α-reductase activity and risk of prostate
cancer among Japanese and US white and black males. Lancet.
339:887–889. 1992.
|
|
16
|
Wu AH, Whittemore AS, Kolonel LN, John EM,
Gallagher RP, et al: Serum androgens and sex-hormone binding
globulins in relation to lifestyle factors in older
African-American, white, and Asian men in the United States and
Canada. Cancer Epidemiol Biomarkers Prev. 4:735–741. 1995.
|
|
17
|
Ntais C, Polycarpou A and Ioannidis JP:
SRD5A2 gene polymorphisms and the risk of prostate cancer: a
meta-analysis. Cancer Epidemiol Biomarkers Prev. 12:618–624.
2003.PubMed/NCBI
|
|
18
|
Montgomery RB, Mostaghel EA, Vessella R,
Hess DL, Kalhorn TF, et al: Maintenance of intratumoral androgens
in metastatic prostate cancer: a mechanism for castration-resistant
tumor growth. Cancer Res. 68:4447–4454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reichardt JK, Makridakis N, Henderson BE,
Yu MC, Pike MC and Ross RK: Genetic variability of the human SRD5A2
gene: implications for prostate cancer risk. Cancer Res.
55:3973–3975. 1995.PubMed/NCBI
|
|
20
|
Scariano JK, Treat E, Alba F, Nelson H,
Ness SA and Smith AY: The SRD5A2 V89L polymorphism is associated
with severity of disease in men with early onset prostate cancer.
Prostate. 68:1798–1805. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Boger-Megiddo I, Weiss NS, Barnett MJ,
Goodman GE and Chen CH: V89L polymorphisms of the 5α-reductase Type
II gene (SRD5A2), endogenous sex hormones, and prostate cancer
risk. Cancer Epidemiol Biomarkers Prev. 17:286–291. 2008.
|
|
22
|
Hsing AW, Chen C, Chokkalingam AP, Gao YT,
Dightman DA, et al: Polymorphic markers in the SRD5A2 gene and
prostate cancer risk: a population-based case-control study. Cancer
Epidemol Biomarkers Prev. 10:1077–1082. 2001.PubMed/NCBI
|
|
23
|
Makridakis NM, di Salle E and Reichardt
JK: Biochemical and pharmacogenetic dissection of human steroid
5a-reductase type II. Pharmacogenetics. 10:407–413. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Allen NE, Forrest MS and Key TJ: The
association between polymorphisms in the CYP17 and 5α-reductase
(SRD5A2) genes and serum androgen concentrations in men. Cancer
Epidemiol Biomarkers Prev. 10:185–189. 2001.
|
|
25
|
Febbo PG, Kantoff PW, Platz EA, et al: The
V89L polymorphism in the 5α-reductase type 2 gene and risk of
prostate cancer. Cancer Res. 59:5878–5881. 1999.
|
|
26
|
Giwercman YL, Abrahamsoon PA, Giwercman A,
Gadaleanu V and Ahlgren G: The 5α-reductase type II A49T and V89L
high-activity allelic variants are more common in men with prostate
cancer compared with the general population. Eur Urol. 48:679–685.
2005.
|
|
27
|
Berndt SI, Chatterjee N, Huang WY, et al:
Variant in sex hormone-binding globulin gene and the risk of
prostate cancer. Cancer Epidemiol Biomarkers Prev. 16:165–168.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li J, Coates RJ, Gwinn M, Khoury MJ, et
al: Steroid 5-α-reductase Type 2 (SRD5A2) gene polymorphisms and
risk of prostate cancer: a HuGE review. Am J Epidemiol. 171:1–13.
2010.
|
|
29
|
Schatzl G, Madersbacher S, Gsur A, et al:
Association of polymorphisms within androgen receptor,
5α-reductase, and PSA genes with prostate volume, clinical
parameters, and endocrine status in elderly men. Prostate.
52:130–138. 2002.
|
|
30
|
Wang CH, Tao W, Chen Q, Hu H, Wen XY, et
al: SRD5A2 V89L polymorphism and prostate cancer risk:
Meta-analysis. The Prostate. 70:170–178. 2010.PubMed/NCBI
|
|
31
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; ISUP Grading Committee. The 2005 International Society
of Urological Pathology (ISUP) Consensus Conference on Gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deantoni EP: Age-specific reference ranges
for PSA in the detection of prostate cancer. Oncology (Williston
Park). 11:475–482. 485–486. 1997.PubMed/NCBI
|
|
33
|
Ausubel F, Brent R, Kingston R, Moore D,
Seidman JG, Smith J and Struhl K: Short Protocols in Molecular
Biology. 3rd edition. (Unit 21)John Wiley & Sons; New York, NY:
pp. 2–3. 1995
|
|
34
|
Cicek MS, Conti DV, Curran A, Neville PJ,
Paris PL, et al: Association of prostate cancer risk and
aggressiveness to androgen pathway genes: SRD5A2, CYP17, and the
AR. The Prostate. 59:69–76. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lunn RM, Bell DA, Mohler JL and Taylor JA:
Prostate cancer risk and polymorphism in 17 hydroxylase (CYP17) and
steroid reductase (SRD5A2). Carcinogenesis. 20:1727–1731. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ross RK, Bernstein L, Lobo RA, Shimizu H,
Stanczyk FZ, et al: 5a-reductase activity and risk of prostate
cancer among Japanese and U.S. white and black males. Lancet.
339:887–889. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Makridakis N, Ross RK, Pike MC, Chang L,
Stanczyk FZ, et al: A prevalent missense substitution that
modulates activity of prostatic steroid 5a-reductase. Cancer Res.
57:1020–1022. 1997.PubMed/NCBI
|
|
38
|
Zeigler-Johnson CM, Walker AH, Mancke B,
et al: Ethnic differences in the frequency of prostate cancer
susceptibility alleles at SRD5A2 and CYP3A4. Hum Hered. 54:13–21.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang J, Tang NL, Ohlsson C, Eriksson AL,
Vandenput L, et al: Association of SRD5A2 variants and serum
androstane-3α, 17beta-diol glucuronide concentration in Chinese
elderly men. Clin Chem. 56:1742–1749. 2010.PubMed/NCBI
|
|
40
|
Hayes VM, Severi G, Padilla EJD and Morris
HA: 5α-reductase type 2 gene variant associations with prostate
cancer risk, circulating hormone levels and androgenetic alopecia.
Int J Cancer. 120:776–780. 2006.
|
|
41
|
Allen NE, Forrest MS and Key TJ: The
association between polymorphisms in the CYP17 and 5alpha-reductase
(SRD5A2) genes and serum androgen concentrations in men. Cancer
Epidemiol Biomarkers Prev. 10:185–189. 2001.
|
|
42
|
Forrest MS, Edwards SM, Houlston R,
Kote-Jarai Z, Key T, et al: Association between hormonal genetic
polymorphisms and early-onset prostate cancer. Prostate Cancer and
Prostatic Diseases. 8:95–102. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Allen NE, Reichardt JK, Nguyen H and Key
TJ: Association between two polymorphisms in the SRD5A2 gene and
serum androgen concentrations in British men. Cancer Epidemiol
Biomarkers Prev. 12:578–81. 2003.PubMed/NCBI
|