Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS) are common and severe conditions, with
high mortality rates. ALI/ARDS most seriously affects those with
existing medical conditions, affecting both the quality of life and
chance of survival in patients (1). The pathogenesis of the condition has
not been fully elucidated; however, it is known that the
pathophysiological basis involves a loss of control of the
regulatory network consisting of cells, cytokines and inflammatory
mediators. This leads to the damage of target cells, including
pulmonary capillary endothelial and alveolar epithelial cells
(2–5). Previous studies (6–8) have
shown that ATP-binding cassette transporter A1 (ABCA1) has an
essential effect on the regulation of the inflammatory response.
ABCA1-mediated cholesterol efflux can reduce the inflammatory
signaling induced by lipopolysaccharide (LPS) in cell membrane. The
metabolic balance between cholesterol and phospholipids has an
important effect on the inflammatory response. Phospholipids are
key components of surface-active substances, and a decrease in
phospholipid content can aggravate lung collapse (9). Bates et al (10) indicated that ABCA1-knockout mice
manifest low levels of high-density lipoprotein and exhibit lung
morphological abnormalities. Lipid analysis of the alveolar lavage
fluid suggested that the quantity and metabolism of phospholipids
in the lung tissues were abnormal. Considering the high
distribution of ABCA1 in the lungs under physiological conditions,
it is speculated that ABCA1 may be involved in the pathogenesis of
ALI/ARDS. Triptolide is a diterpene lactone epoxide compound with
high biochemical activity. The compound is isolated from
Celastraceae tripterygium (Tripterygium wilfordii
Hook. f.) and has been shown to have extensive pharmacological
effects and a potent immunomodulatory capacity (11,12).
It has yet to be elucidated whether TP can recover
the balance of the systemic inflammatory response and the
compensatory anti-inflammatory response systems, relieve the
pathological changes of ALI/ARDS and improve prognosis by
regulating the transcription and protein expression of ABCA1 and
inhibiting or reducing the release of inflammatory cells,
transmitters and cytokines. The aim of the present study was to
generate a rat model of ALI mediated by LPS, in order to
investigate the in vivo changes in the transcription and
protein expression of ABCA1 and the protective effects of
triptolide on LPS-mediated rat ALI.
Materials and methods
Materials
LPS (E. coli O111:B4; lot no. L2630) and 1%
dimethylsulfoxide (DMSO, lot no. D5879) were purchased from Sigma
(St. Louis, MO, USA). Triptolide (dissolved with 1% DMSO; lot no.
20090321) was obtained from the Nanjing Skin Disease Prevention
Institute (Nanjing, China), and a blood gas analyzer was purchased
from Nova Biomedical (Waltham, MA, USA). A tumor necrosis factor-α
(TNF-α) ELISA kit (article no. HY12849E) was obtained from R&D
Systems (Minneapolis, MN, USA). ABCA1 monoclonal antibody (article
no. p3490RB) was purchased from R&D Systems (Minneapolis, MN,
USA), GAPDH antibody (article no. sz-293072) was obtained from
Santa Cruz Biotechnology, Inc., (Santa Cruz, CA, USA) and rabbit
anti-mouse immunoglobulin G (IgG) (article no. KTB4027) was
purchased from Shanghai Boyao Biological Technology Co., Ltd.,
(Shanghai, China). A LightCycler 480 fluorescent quantitative
polymerase chain reaction (qPCR) instrument was obtained from Roche
Diagnostics (Indianapolis, IN, USA), TRIzol™ RNA extraction
solution (article no. 15596018) was purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA), a bicinchoninic acid (BCA)
protein quantification kit (article no. SK3021-500) was ordered
from Shanghai Sangon (Shanghai, China) and SDS (article no. H10236)
was obtained from Sigma.
Laboratory animals and groups
Thirty male Sprague Dawley rats, weighing 200–250 g,
were provided by Shanghai Laboratory Animal Center, CAS (Shanghai,
China). The rats were randomly distributed into six groups as
follows: Normal (N group, n=5), Control (C group, n=5), LPS (L
group, n=5) and three triptolide dosage groups (TP1–3
groups, n=5/group). Rats in the L and TP1–3 groups were
injected with 5 mg/kg LPS (dissolved with normal saline) via the
vena caudalis, as previously described (13), and the TP1–3 groups were
additionally administered an intraperitoneal injection of
triptolide at 25, 50 or 100 μg/kg, respectively. Rats in the N
group were not treated. Rats in the C group were injected with
normal saline via the vena caudalis and were intraperitoneally
injected with 1% DMSO. The present study was approved by the Animal
Care and Use Committee of Soochow University (Suzhou, China).
Arterial blood gas analysis
Arterial blood samples (0.3 ml) were collected 1 h
before treatment administration and 1, 3, 6 and 12 h after
treatment for blood gas analysis. Data of the arterial oxygen
tension (PaO2) were collected.
Collection and storage of samples
Rats were anesthetized with 4% chloral hydrate at a
dose of 400 mg/kg, 12 h after treatment administration. A
thoracotomy was then performed and the trachea and left and right
bronchi were exposed and isolated. Animals were then sacrificed by
exsanguination, taking blood from the left heart 12 h after
administration. The left bronchus was clipped using a vascular
clamp. An intravenous 24G Y type trocar (BD Biosciences, San Jose,
CA, USA) was placed in casing along the tracheal ring gap, below
the middle section of the trachea. Normal saline at 4°C was used
for bronchoalveolar lavage, repeated three times, at a total dose
of 25 ml/kg. The bronchoalveolar lavage fluid (BALF) was obtained
and, following centrifugation at 241 × g for 10 min at 4°C, the
supernatant was stored at −20°C. The blood samples were
anticoagulated with heparin and then centrifuged at 671 × g for 20
min at 4°C. The supernatant was then stored at −20°C.. The
concentration of TNF-α in the serum and BALF was measured by
double-antibody sandwich ELISA (R&D Systems).
The rats were sacrificed and fresh lung tissues were
collected from the inferior lobe of the right lung. The tissues
were washed with normal saline at 4°C and then placed on ice.
Ophthalmic scissors soaked in diethylpyrocarbonate-treated water
were used to collect ~100 mg tissue, which was then transferred to
a centrifuge tube containing a five-fold volume of
RNAlater® (article no. 15596018; Invitrogen Life
Technologies). The tube was stored at room temperature for 1 h and
then incubated at 4°C overnight, followed by storage at −20°C. The
remaining left lung inferior lobe tissue was cut into small pieces,
which were placed in cryovials containing glycerol. The cryovials
were frozen in liquid nitrogen for 10 min and then stored at
−70°C.
Pathology of lung tissue and wet/dry
ratio (W/D)
The connective tissue was removed from the middle
lobe of the right lung. The tissue was washed with normal saline at
4°C and soaked in paraformaldehyde solution (Invitrogen
Biotechnology Co., Ltd., Shanghai, China) for 24 h, followed by
conventional dehydration and embedding to create a wax block for
microtome cutting. Continuous 4-μm slices were prepared and stained
with a hematoxylin and eosin staining kit according to the
manufacturer’s instructions. The pathology status of the lung
tissue was observed under an optical microscope and graded in
accordance with the criteria for the evaluation of the diffuse
alveolar damage (DAD) score (14,15)
(Table I). The W/D of the middle
lobe of the right lung of the rats was calculated.
 | Table IEvaluation criteria of diffuse
alveolar damage. |
Table I
Evaluation criteria of diffuse
alveolar damage.
| Diffuse alveolar
damage score |
|---|
|
|
|---|
| Pathological
damage | 0 | 1 | 2 | 3 |
|---|
| Widened alveolar
septum | Not widened | Slightly widened | Obviously
widened | Abnormal alveolar
structure |
| Hemorrhage in
alveolar space | No erythrocytes | Few erythrocytes | Many
erythrocytes | Alveolar space filled
with erythrocytes |
| Effusion of fibrin in
alveolar space | No effusion | Slight effusion | Obvious effusion | Alveolar space filled
with fibrin |
| Effusion of
neutrophils in alveolar space and alveolar septum | No effusion | Effusion of
neutrophils | Scattered
effusion | Obvious effusion or
focal distribution |
Detection of ABCA1 mRNA with reverse
transcription-fluorescent qPCR
Right lung inferior lobe tissue (100 mg), was
collected and stored at −20°C prior to extraction of total RNA by
the TRIzol™ method. PCR primers were designed with Primer 5.0
software (Primer, Inc., Ottawa, ON, Canada), and were synthesized
by Invitrogen Biotechnology Co., Ltd. (Shanghai, China). The
sequences were as follows: ABCA1 upstream primer,
5′-CCCAATCCCAAACACTCC-3′ and downstream primer,
5′-GCTACACTGGCACGAAGG-3′, product size 133 bp; β-actin upstream
primer,5′-CCCATC TATGAGGGTTACGC-3′ and downstream primer, 5′-TTT
AATGTCACGCACGATTTC-3′, product size 150 bp. The reaction conditions
for the PCR were as follows: Predenaturation at 95°C for 2 min;
denaturation at 95°C for 10 sec, annealing at 60°C for 15 sec and
elongation at 72°C for 20 sec (40 cycles in total). The
fluorescence detection point was set at 72°C. The cycle threshold
(CT) value for each amplicon was measured using a LightCycler 480
fluorescent quantitative PCR instrument (Roche Diagnostics). The
ΔΔCT method was used for relative quantification of the
target gene, with the following formula:
ΔΔCT=2-[(CT1–CT2)-(CT3–CT4)]. CT1 and CT2 were the
average CT values of the target and housekeeping gene in the
control group, respectively. CT3 and CT4 were the average CT values
of the corresponding genes in the experimental group
respectively.
Analysis of ABCA1 protein expression by
western blotting
Right lung inferior lobe tissue (400 mg) stored at
−70°C was used for the extraction of total proteins from the lung
tissue subsequent to lysis. The concentration of the extracted
proteins was measured using a BCA protein quantification kit. A
total of 50 μg protein solution was dissolved in 2× SDS sample
loading buffer. The samples were then boiled at 100°C for 5 min,
followed by separation by 10% SDS-PAGE. Following electrophoresis,
the proteins were transferred to a polyvinylidene difluoride (PVDF)
membrane. Blocking solution was made with 10 g skimmed milk powder
added to 200 ml phosphate-buffered saline. The mixture was filtered
to prepare 5% sealing solution. The PVDF membrane was incubated in
sealing solution and agitated in a shaker for 2 h, followed by
further incubation at 4°C overnight. Monoclonal ABCA1 and GAPDH
antibodies were added and incubated on the membranes at 37°C for 2
h before washing with Tris-buffered saline with Tween®
20, three times. Horseradish peroxidase-labeled rabbit anti-mouse
IgG was added to the membranes for 1 h. Following the western
blotting, the membranes were developed and scanned and the optical
density of the bands was measured using a gel image analysis
system. The ratio of ABCA1 to GAPDH optical density was calculated.
The band intensity of each group was compared with that of GADPH,
and the ratio represented ABCA1 protein expression.
Statistical analysis
The data were processed with Statistical Analysis
System (SAS) version 8 software (SAS Institute, Inc., Cary, NC,
USA). Measurement data are expressed as the mean ± standard
deviation. One-way analysis of variance was used for comparisons
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Pathological changes and DAD score of
lung tissue, and W/D of the right lung middle lobe
As shown in Fig. 1,
the lung tissue structure of rats in the N group was normal. The
lungs of rats in the C group had a normal structure and no notable
widening of the alveolar septum. The alveolar septum of rats in the
L group was widened significantly, and alveolar trapping and
effusion in the alveolar space were observed. Similarly, the
alveolar septum of rats in the TP1 group was widened,
and alveolar trapping and effusion in the alveolar space were
observed. As compared with the N and C groups, the differences in
the DAD score of pathological changes and the W/D of the middle
lobe of the right lung of the TP1 group were
statistically significant (P<0.05). As compared with the L
group, the differences were not statistically significant
(P>0.05). Observations by optical microscopy indicated that the
alveolar structure of the TP2 group was normal, with the
presence of interstitial edema. Angiotelectasis, congestion and
inflammatory cell infiltration were observed. In the TP2
group, the DAD score, representing pathological change, was higher
than that in the N and C groups, but lower than that in the L
group. The difference in the DAD score between the TP2
and the L group was statistically significant (P<0.05) (Table II). In the TP3 group,
the alveolar septum was slightly widened, and partial alveolar
trapping, interstitial edema, partial angiotelectasis, congestion
and inflammatory cell infiltration were observed. As compared with
the L group, the differences in the DAD score and W/D of the middle
lobe of the right lung in the TP3 group were
statistically significant (P<0.05). However, as compared with
the TP2 group, the differences were not statistically
significant (P>0.05) (Table
II).
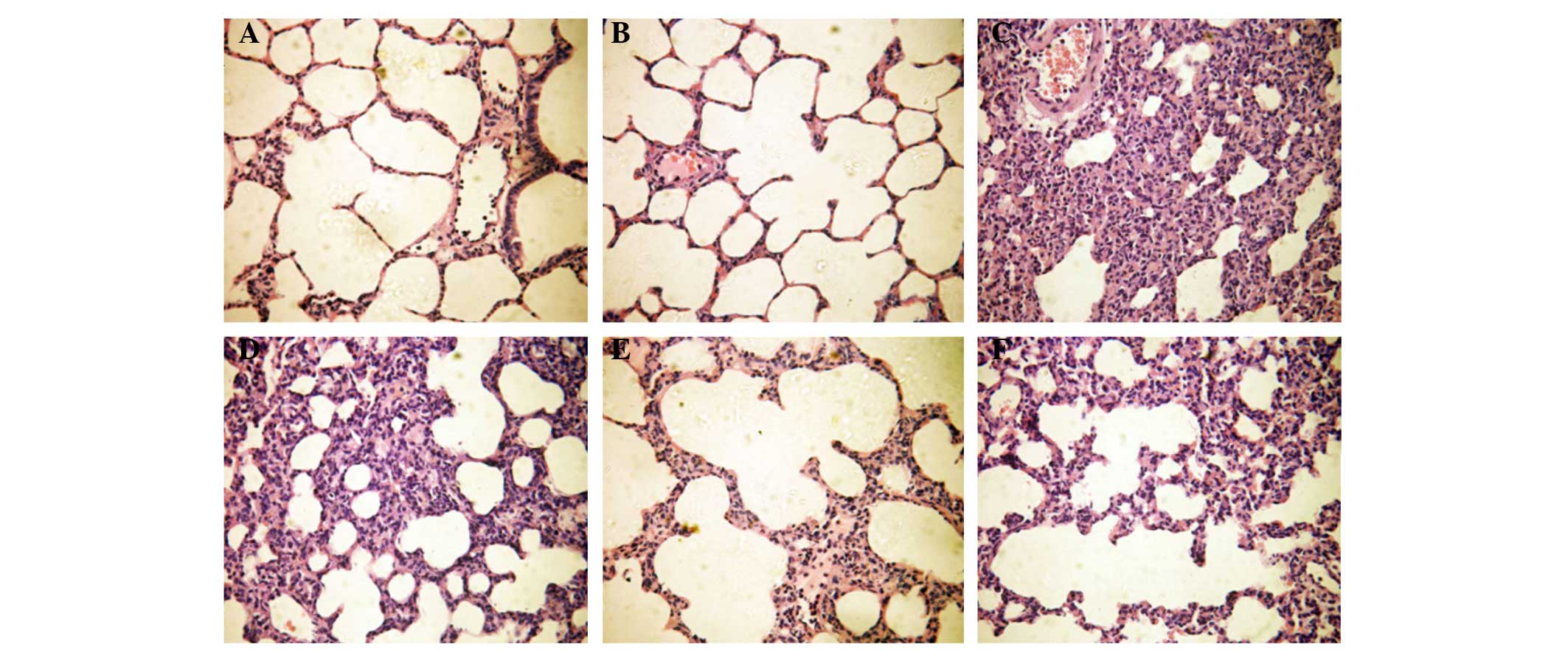 | Figure 1Pathological changes in the lungs of
rats in all groups (hematoxylin and eosin staining; magnification,
×400). (A) The lung tissue structure of rats in the N group was
normal. (B) The alveolar septum of rats in the C group was not
notably widened and the structure was basically normal. (C) A
notably widened alveolar septum, alveolar trapping and effusion in
the alveolar space were observed in the L group. (D) A widened
alveolar septum, alveolar trapping and effusion in the alveolar
space were observed in the TP1 group. (E) Under the
optical microscope, the alveolar structure in the TP2
group was basically normal, although interstitial edema, partial
angiotelectasis, congestion and inflammatory cell infiltration were
observed. (F) A slightly widened alveolar septum, edema, partial
alveolar trapping, angiotelectasis, congestion and inflammatory
cell infiltration were observed in the TP3 group. N,
normal group; C, control group; L, LPS-treated group;
TP1–3, triptolide treated group at 25, 50 or 100 μg/kg,
respectively. |
 | Table IIComparison of pathological score and
W/D of rats in all groups. |
Table II
Comparison of pathological score and
W/D of rats in all groups.
| Group | DAD score | W/D |
|---|
| N | 2.68±0.36 | 4.38±0.12 |
| C | 2.55±0.45 | 4.29±0.13 |
| L | 9.68±1.32a | 6.43±0.71a |
| TP1 | 9.46±1.35a | 6.92±0.68a |
| TP2 | 5.53±0.86a,b | 5.18±0.52b |
| TP3 | 5.58±0.83a,b | 4.99±0.73b |
Arterial blood gas analysis
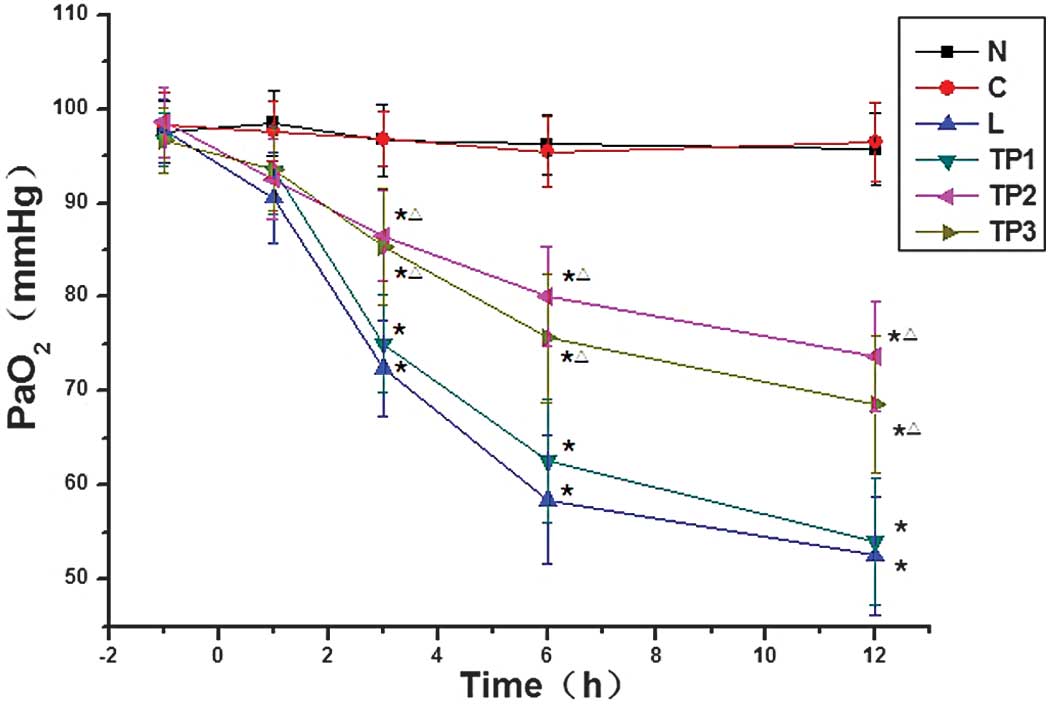
The differences in PaO2 at 3, 6 and 12 h
between the L and TP1 groups and the N and C groups were
statistically significant (P<0.05). In addition, the differences
in PaO2 at 3, 6 and 12 h between the TP2 and
TP3 groups and the N and C groups were statistically
significant (P<0.05). The PaO2 in the TP2
and TP3 groups was relatively high as compared with that
in the L and TP1 groups, and the differences were
statistically significant (P<0.05) (Fig. 2).
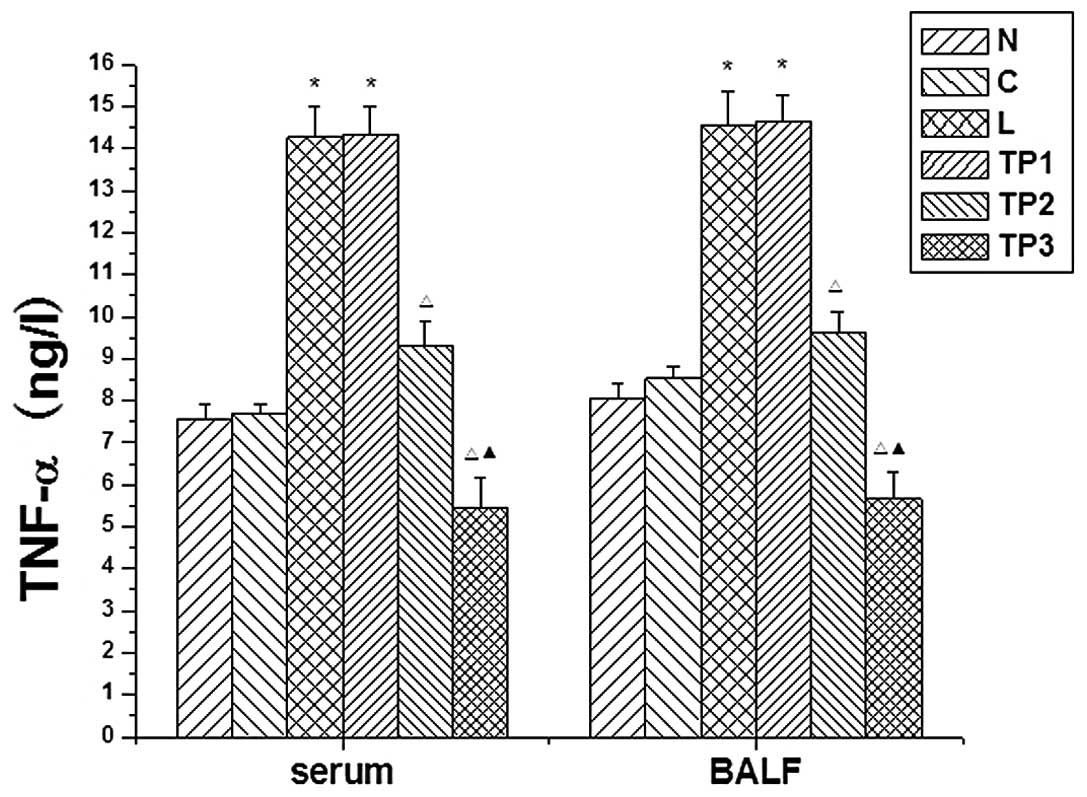
Changes in TNF-α expression in serum and
BALF
The expression levels of TNF-α in the serum and BALF
were increased at 12 h in the L and TP1 groups. As
compared with the N and C groups, the differences were
statistically significant (P<0.05). The TNF-α expression in the
serum and BALF at 12 h in the TP2 and TP3
groups was decreased as compared with that in the L and
TP1 groups. These differences were statistically
significant (P<0.05). As compared with the N and C groups, the
expression of TNF-α in the serum and BALF in the TP3
group was significantly decreased (P<0.05) (Fig. 3).
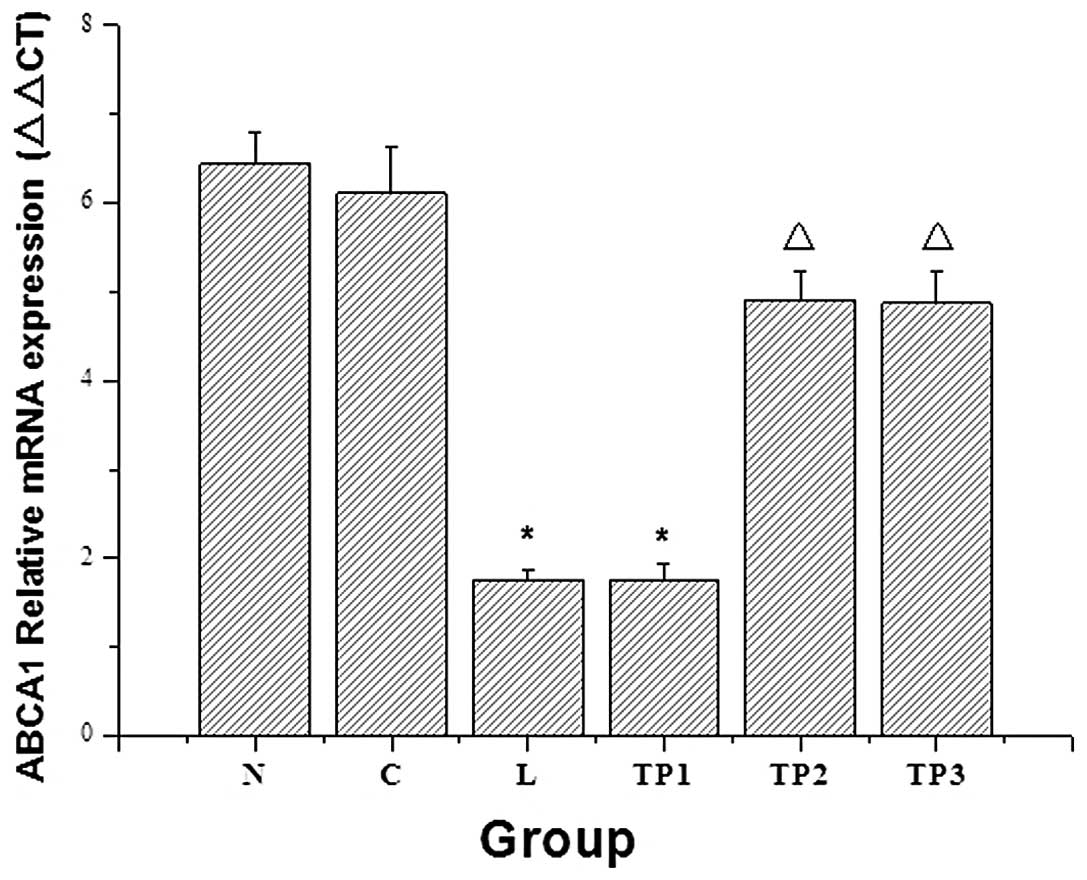
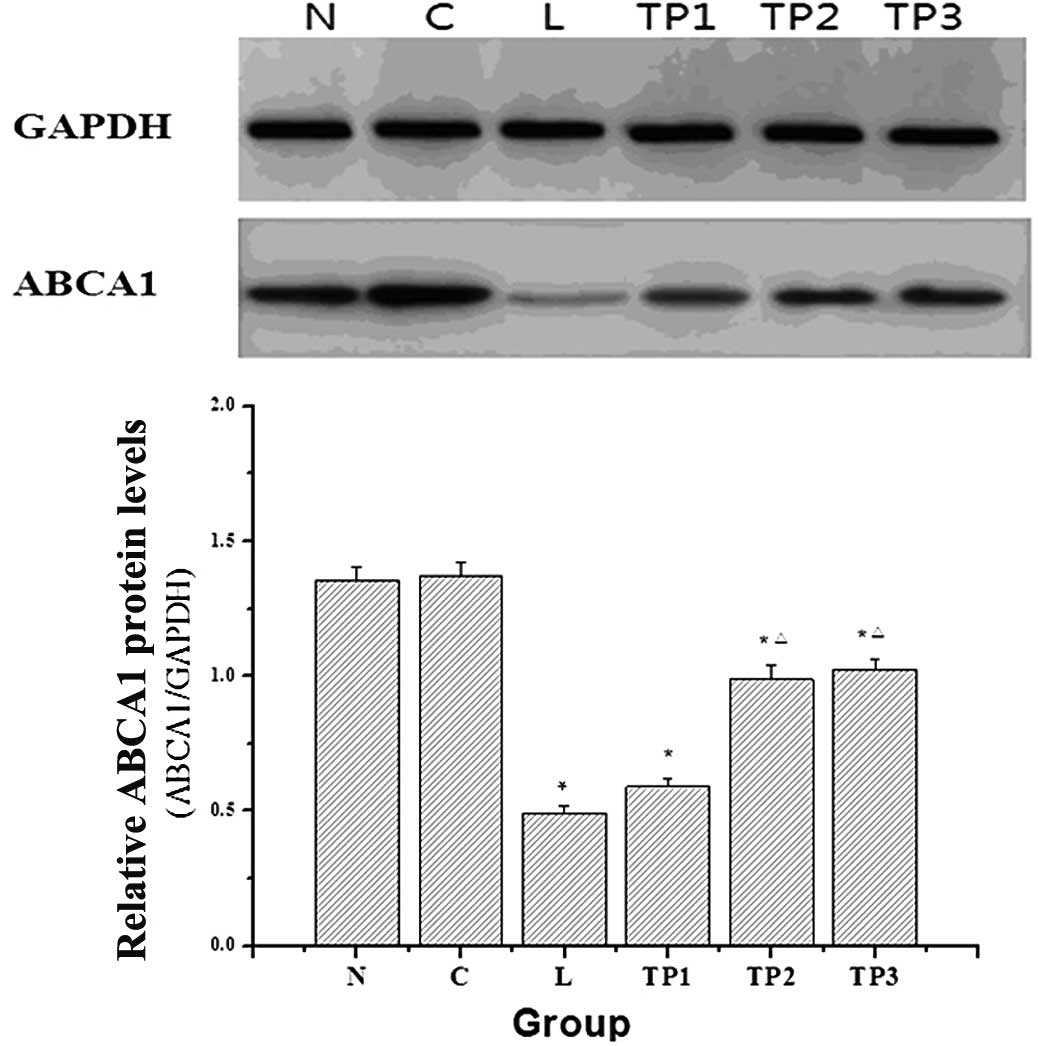
Analysis of ABCA1 expression in lung
tissue
The mRNA and protein expression levels of ABCA1 in
the L group were significantly decreased as compared with those in
the N and C groups (P<0.05). With the increasing doses of
triptolide, the mRNA and protein expression of ABCA1 in the
TP2 and TP3 groups showed an increasing
trend. As compared with the L group, the corresponding expression
of ABCA1 in the TP2 group was significantly increased
(P<0.05). As compared with the TP2 group, the mRNA
and protein expression levels of ABCA1 in the TP3 group
were not significantly different (P>0.05) (Figs. 4 and 5).
Discussion
The inflammatory response and the changes in the
expression of active substances on the lung surface play an
important role in the pathogenesis of LPS-mediated ALI. The
regulation of signaling factors at the molecular level, in addition
to the termination of the cytokine chain reaction (16–20),
may be areas in which targeted therapies could be designed. ABCA1
is a transmembrane protein that is most highly expressed in the
lung. In the lung, the protein expression of ABCA1 is closely
associated with the inflammatory response (6,7,10,11),
and ABCA1-mediated cholesterol transport activity is correlated
with the anti-inflammatory activity of ABCA1. Adjustments to the
lipid composition in the lipid raft region may be the potential
mechanism underlying the ABCA1-mediated inflammatory regulation.
LPS stimulates alveolar macrophages in ABCA1-knockout rats, which
leads to an increase in the expression of pro-inflammatory
cytokine, as well as the activation of the nuclear factor
κ-light-chain-enhancer of activated B cells-mitogen-activated
protein kinase pathway (21,22).
In this study, rats were injected with endotoxin
(LPS) through the caudal vein. Twelve hours after the LPS
challenge, the PaO2 of each group was analyzed. The
PaO2 of each of the LPS-treated groups was observed to
be significantly decreased 3, 6 and 12 h after injection compared
with that of the N and C groups (P<0.05). Additionally, the BALF
and TNF-α serum expression levels, the pathological changes in the
rat lung tissue and the W/D were concordant with the criteria of an
animal model of ALI as compared with the C group. Compared with the
C and N groups, ABCA1 gene and protein expression in the L group
was respectively reduced, and these differences were statistically
significant (P<0.05). These data preliminarily suggest that
ABCA1 is involved in the formation and development of ALI/ARDS.
The results of this study showed that ABCA1 mRNA and
protein expression levels in the TP2 and TP3
groups increased with the increasing dose of triptolide. Compared
with the L group, the ABCA1 mRNA and protein expression levels were
significantly different (P<0.05). When 50 μg/kg was exceeded,
there was no notable change. The analysis of PaO2 at 3,
6 and 12 h after injection indicated that PaO2 was
significantly higher in the TP2 and TP3
groups as compared with that in the L group (P<0.05). The W/D in
the TP2 and TP3 groups was lower than that in
observed in the L group (P<0.05). The degree of pathological
change in the lung was low in the TP2 and TP3
groups and, compared with the L group, the differences in
pathological score were statistically significant (P<0.05).
TNF-α expression in the serum and BALF in the TP2 and
TP3 groups was significantly decreased 12 h after
injection, as compared with the L group (P<0.05).
The results of the present study suggest that
triptolide is capable of promoting the expression of ABCA1,
reducing the secretion of inflammatory factors and relieving the
lung pathological injury associated with ALI. Therefore, it is
speculated that the regulation of ABCA1 gene and protein expression
by triptolide may be a potential strategy for lung protection.
Within a range of 25–100 μg/kg triptolide, the regulation of ABCA1
gene and protein expression was dose-dependent, however, 100 μg/kg
was considered to be the threshold dose for dose-dependent
effects.
References
|
1
|
Leaver SK and Evans TW: Acute respiratory
distress syndrome. BMJ. 335:389–394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goodman RB, Strieter RM, Martin DP, et al:
Inflammatory cytokines in patients with persistence of the acute
patients with persistence of the acute respiratory distress
syndrome. Am J Respir Crit Care Med. 154:602–611. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beck-Schimmer B, Schwendener R, Pasch T,
et al: Alveolar macrophages regulate neutrophil recruitment in
endotoxin-induced lung injury. Respir Res. 6:612005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong L and Millar AB: Relative
production of tumour necrosis factor alpha and interleukin 10 in
adult respiratory distress syndrome. Thorax. 52:442–446. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhatia M and Moochhala S: Role of
inflammatory mediators in the pathophysiology of acute respiratory
distress syndrome. J Pathol. 202:145–146. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu X, Lee JY, Timmins JM, et al:
Increased cellular free cholesterol in macrophage-specific ABCA1
knock-out mice enhances pro-inflammatory response of macrophages. J
Biol Chem. 283:22930–22941. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yin K, Liao DF and Tang CK: ATP-binding
membrane casette transporter A1 (ABCA1): A possible link between
inflammation and reverse cholesterol transport. Mol Med.
16:438–449. 2010.PubMed/NCBI
|
|
8
|
Tang C, Liu Y, Kessler PS, et al: The
macrophage cholesterol exporter ABCA1 functions as an
anti-inflammatory receptor. J Biol Chem. 284:32336–32343. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ying J, Ma YM and Pei WY: Research
progress and application of pulmonary surfacetant. Chin Pham J.
19:1449–1453. 2005.(In Chinese).
|
|
10
|
Bates SR, Tao JQ, Collins HL, et al:
Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J
Physiol Lung Cell Mol Physiol. 289:L980–L989. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao G, Vaszar LT, Qiu D, et al:
Anti-inflammatory effects of triptolide in human bronchial
epithelial cells. Am J Physiol Lung Cell Mol Physiol.
279:L958–L966. 2000.PubMed/NCBI
|
|
12
|
Tengchaisri T, Chawengkirttikul R,
Rachaphaew N, et al: Antitumor activity of triptolide against
cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett.
133:169–175. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Y, Yang Q, Xu J, et al: Effects of
HMGB1 on PMN apoptosis during LPS-induced acute lung injury. Exp
Mol Pathol. 85:214–222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoyama T, Tomiguchi S, Nishi J, et al:
Hyperoxia-induced acute lung injury using a pig model: correlation
between MR imaging and histologic results. Radiat Med. 19:131–143.
2001.PubMed/NCBI
|
|
15
|
Gao JL, Chen J, Zhan Y and Wang LN:
Effects of triptolide on lipopolysaccharide-induced acute lung
injury in rats. Chin JAnesthesiol. 31:1245–1248. 2011.
|
|
16
|
Metz C and Sibbald WJ: Anti-inflammatory
therapy for acute lung injury. A review of animal and clinical
studies. Chest. 100:1110–1119. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
St John RC and Dorinsky PM: Immunologic
therapy for ARDS, septic shock, and multiple organ failure. Chest.
103:932–943. 1993.PubMed/NCBI
|
|
18
|
Downey GP and O’Brodovich HM: Mechanisms
of acute lung injury and repair. Pediatric Respiratory Medicine.
Taussig L: Mosby; St. Louis, MO: pp. 76–91. 1997
|
|
19
|
Downey GP, Dong Q, Kruger J, et al:
Regulation of neutrophil activation in acute lung injury. Chest. (1
Suppl)116:46S–54S. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Heering P, Morgera S, Schmitz FJ, et al:
Cytokine removal and cardiovascular hemodynamics in septic patients
with continuous venovenous hemofiltration. Intensive Care Med.
23:288–296. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu X, Owen JS, Wilson MD, et al:
Macrophage ABCA1 reduces MyD88-dependent Toll-like receptor
trafficking to lipid rafts by reduction of lipid raft cholesterol.
J Lipid Res. 51:3196–3206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu X, Lee JY, Timmins JM, et al:
Increased cellular free cholesterol in macrophage-specific Abca1
knock-out mice enhances pro-inflammatory response of macrophages. J
Biol Chem. 283:22930–22941. 2008. View Article : Google Scholar : PubMed/NCBI
|