Introduction
Multidrug resistance (MDR) refers to the resistance
of cancer cells to multiple anticancer drugs and is now a
significant clinical challenge facing cancer chemotherapy (1,2).
P-glycoprotein (P-gp) is a member of the ATP-binding cassette
family, which exports compounds from cells through a process driven
by adenosine triphosphate hydrolysis (3). Several chemotherapeutic drugs are
known to exert anticancer activity by inducing apoptosis. However,
MDR tumor cells are generally resistant to the induction of
apoptosis and the resistance of leukemic cells to
chemotherapy-induced apoptosis remains the most serious problem in
the treatment of leukemia (4,5).
Currently, the preferred strategy for overcoming MDR
is to use sensitizer or reversal agents, which are combined with
chemotherapeutic drugs (6). Plants
have been utilized as medicines and they are an important source of
the mainstream pharmacopoeia (Ciwujia, Chinese Pharmacopoeia, 2010:
192–193). There has been substantial effort to identify reversal
agents from natural products, which has resulted in significant
success (7,8). Flavonoids, which are polyphenolic
compounds, are a class of plant secondary metabolites possessing a
broad spectrum of pharmacological activities, including anticancer,
antimicrobial and immunoregulatory activities (9–11).
Quercetin is a naturally occurring flavonoid with a broad spectrum
of bioactivities, including antiproliferative, anti-inflammatory
and antioxidant effects and effects on the immune system (12,13).
Quercetin can inhibit intestinal crypt cell proliferation and
aberrant crypt formation, by targeting cyclins and cyclin-dependent
kinases (14). Increasing evidence
suggests that quercetin can be effective in cancer treatment by
inducing cell apoptosis (15),
however the function of quercetin on MDR in human leukemia remains
to be elucidated. Therefore, the present study aimed to investigate
the effect of quercetin on human leukemic MDR K562/adriamycin (ADR)
cells.
Materials and methods
Reagents
Quercetin and ADR were purchased from Sigma (St.
Louis, MO, USA). RPMI-1640 culture medium, fetal bovine serum
(FBS), phosphate-buffered saline (PBS), penicillin-streptomycin and
0.25% (w/v) trypsin/1 mM ethylenediaminetetraacetic acid were
purchased from Gibco-BRL (Grand Island, NY, USA).
Cell culture
K562/ADR human leukemia cells, which were provided
by the Chinese Medical Science Research Institute of Hematology
(Tian Jin, China), were maintained in RPMI-1640 medium containing
10% (v/v) FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C in a humidified 5% CO2 incubator. The K562/ADR
cells were cultured in the medium containing 1 μg/ml ADR to
maintain MDR phenotype and were maintained in drug-free medium for
at least 2 days prior to use.
Cell proliferation assay
A cell counting kit-8 (CCK-8) assay was performed
for the analysis of cell proliferation. Briefly, the cells
(2×105/ml) were seeded into 6-well plates and left to
adhere overnight. The cells were then incubated for 12, 24 and 48 h
at 37°C with different concentrations of quercetin and ADR.
Subsequently, 20 μl CCK-8 was added prior to incubation in the dark
at 37°C for 4 h. The absorbance was determined using a microplate
reader (Thermo Fisher Scientific, Waltham, MA, USA). The
concentration of compound required for the proliferation of 50% of
cells to be inhibited (IC50) was determined by plotting
the percentage of cell growth inhibition against the compound
concentration.
Apoptotic assay
The K562/ADR cells were seeded into 12-well plates
and treated with different concentrations of quercetin and ADR, as
indicated, for 48 h. The apoptotic morphology of the cells was
evaluated by staining with hematoxylin and eosin and visualizing
under a light microscope (Leica Microsystems, Wetzlar, Germany;
magnification, ×200). The cells undergoing apoptosis were assessed
using an Annexin V-fluorescein isothiocyanate/propidium iodide (PI)
apoptosis detection kit according to the manufacturer’s
instructions (Becton-Dickinson, Franklin Lakes, NJ, USA). The
number of apoptotic cells were quantified using a flow cytometer
(FACSCalibur; BD Biosciences, San Jose, CA, USA) and analyzed using
CellQuest software (BD Biosciences).
Determination of mitochondrial membrane
potential
Changes in mitochondrial transmembrane potential
were measured following staining with rhodamine-123. The cells were
incubated with the indicated doses of quercetin (100 μg/ml), ADR
(12 μg/ml) and quercetin (100 μg/ml) with ADR (12 μg/ml) for 48 h.
Rhodamine-123 (2 μl; 100 μg/ml) was added 1 h prior to termination
of the experiment and the cells were collected and washed in PBS.
The fluorescence intensity was then analyzed using a FACSCalibur
flow cytometer (Becton-Dickinson).
Western blot analysis
For the western blot analysis of the total cell
lysates, the cells were harvested and washed with ice-cold PBS. The
protein concentration in the lysates was measured using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific)
according to the manufacturer’s instructions. Samples of cell
lysate (50 μg/lane) were separated using 10% SDS-PAGE and were then
transferred onto polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). The membranes were incubated overnight at 4°C
with the following antibodies: B-cell lymphoma (Bcl)-2-interacting
mediator of cell death [(BIM; cat. no. 2933, clone C34C5, rabbit
monoclonal antibody (mAb)], Bcl-2-associated death promoter (BAD;
cat. no. 9239, clone D24A9, rabbit mAb), Bcl-2-associated X protein
(BAX; cat. no. 5023, clone D2E11, rabbit mAb), Bcl-extra (xL; cat.
no. 2764, clone 54H6, rabbit mAb), Bcl-2 (cat. no. 2827, clone
50E3, rabbit mAb), c-Jun N-terminal kinase (JNK; cat. no. 9258,
clone 56G8, rabbit mAb),), phosphorylated (p-)JNK (Thr183/Tyr185;
cat. no. 4668, clone 81E11, rabbit mAb), p38 mitogen-activated
protein kinase (MAPK; cat. no. 9212, clone, rabbit mAb), p-p38 MAPK
(Thr180/Thr182; cat. no. 4631, clone 12F3, rabbit mAb),
extracellular-regulated kinase (ERK)1/2 (cat. no. 4695, clone
137F5, rabbit mAb), p-ERK1/2 (cat. no. 4376, clone 20G11, rabbit
mAb), caspase-3 (cat. no. 96686, clone 3G2, mouse mAb), caspase-8
(cat. no. 9746, clone 1C12, mouse mAb) and caspase-9 (cat. no.
9508, clone C9, mouse mAb) (all 1:1,000; all obtained from Cell
Signaling Technology, Inc., Danvers, MA, USA). The membranes were
washed three times with tris-buffered saline with Tween 20 and
incubated for 1 h at room temperature with the appropriate
secondary polyclonal anti-rabbit IgG HRP-linked antibody (1:3,000;
cat.no. 7074; Cell Signaling Technology, Inc.). Immunoreactive
bands were then detected using an enhanced chemiluminescence kit a
ChemiGenius bioimaging system (Syngene, Frederick, MD, USA).
Western blots were quantified by calculating the gray ratio of
target protein:β-actin.
Determination of the expression of P-gp
using flow cytometric analysis
The cells were seeded into a 12-well plate at a
density of 2×105/ml and treated with either quercetin
(100 μg/ml), ADR (12 μg/ml) or quercetin (100 μg/ml) with ADR (12
μg/ml). The cells were then collected, washed in PBS and fixed
using 70% cold ethanol following incubation with mouse-anti-human
P-gp monoclonal antibody (cat.no 340555). Goat-anti-mouse
polyclonal fluorescent antibody (cat.no 20010; Biotium, Inc.,
Hayward, CA, USA) was used as a secondary antibody and flow
cytometry was performed to measure the fluorescence intensity
(Becton-Dickinson).
Statistical analysis
For statistical analysis, all the data are expressed
as the means ± standard deviation of at least triplicate
determinations and statistical analysis was performed using SPSS
software (SPSS, Inc., St. Louis, MO, USA). Comparison between
groups was made using analysis of variance. P<0.05 was
considered to indicate a statistically significant difference.
Results
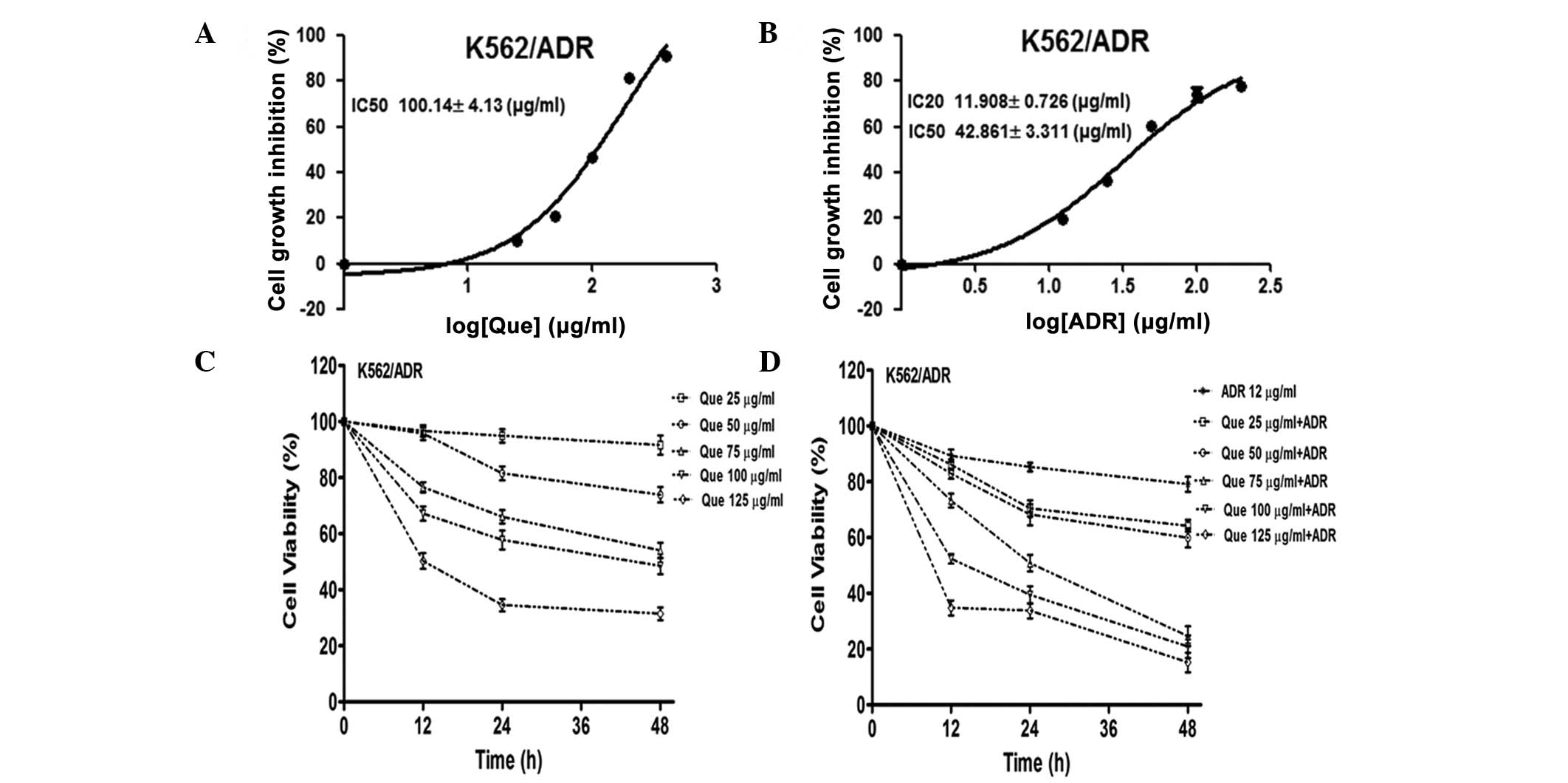
Quercetubg and ADR inhibit leukemia cell
proliferation
The cytotoxicity of quercetin and ADR in the
K562/ADR human leukemia cell line was assessed using a CCK-8 assay.
The IC50 values of quercetin alone were 100.14±4.13
μg/ml in the K562/ADR cells following 48 h of treatment (Fig. 1A). The IC50 and
IC20 values of ADR along were 42.86±3.31 and 11.91±0.73
μg/ml in the K562/ADR cells following 48 h treatment, respectively
(Fig. 1B). The CCK-8 assay
revealed that quercetin treatment caused concentration-dependent
inhibition of cell proliferation (Fig.
1C). In addition, the combination of quercetin and ADR
synergistically inhibited cell proliferation (Fig. 1D). Together, these findings
demonstrated that treatment of the K562/ADR cells with a
combination of quercetin and ADR potentiated the cytotoxicity.
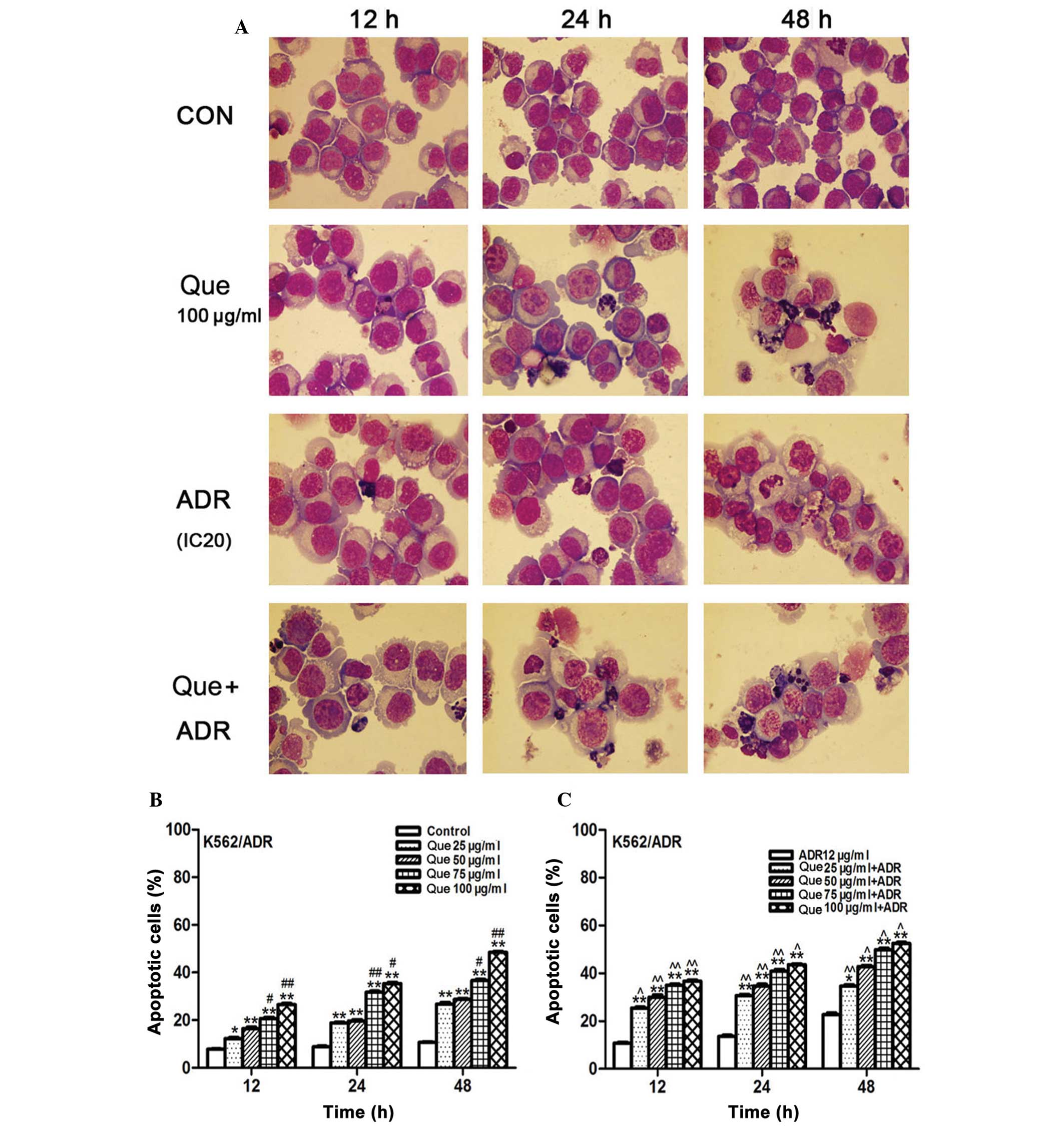
Quercetin and ADR synergistically promote
cell apoptosis
Treatment of K562/ADR human leukemia cells for the
indicated periods of time with either quercetin and ADR alone or in
combination caused nuclear condensation, fragmentation of nuclei
and formation of scattered apoptotic bodies, while no clear change
in morphology was observed in the nuclei of untreated cells
(Fig. 2A). In addition, flow
cytometric analysis demonstrated that quercetin promoted cell
apoptosis in a concentration- and time-dependent manner (Fig. 2B). Furthermore, a synergistic
effect on apoptosis was observed following combination treatment
with ADR and quercetin at different concentrations (Fig. 2C).
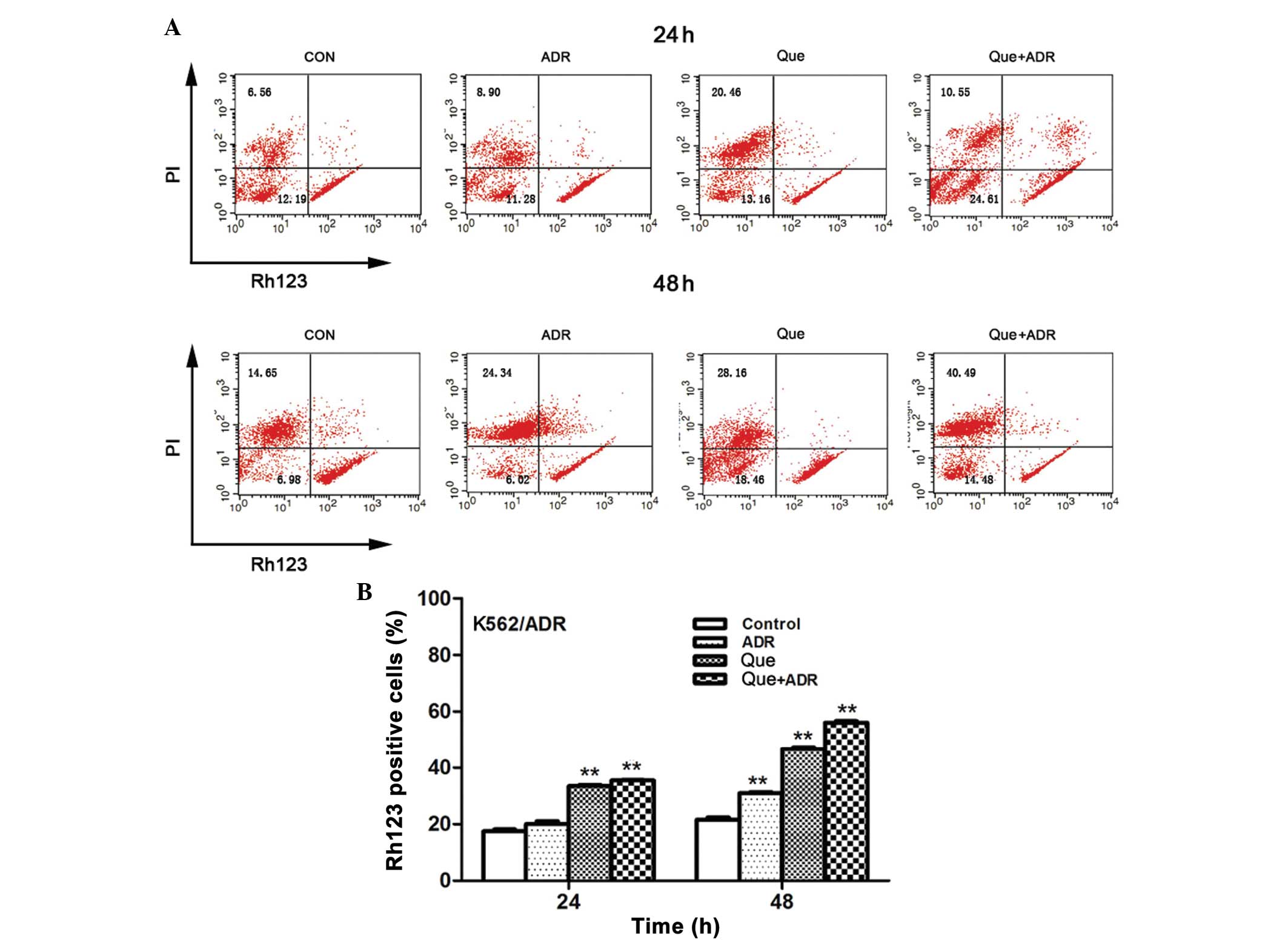
Loss of mitochondrial membrane potential
is induced by quercetin and ADR
Mitochondrial damage to cells results in
perturbation of mitochondrial membrane potential (16). The present study analyzed the loss
in mitochondrial potential in the K562/ADR cells using
rhodamine-123 dye. The results demonstrated that 24 h quercetin
treatment, at a concentration of 100 μg/ml, resulted in a
significant increase in Rh123 fluorescence intensity. The
combination of quercetin and ADR further increased the number of
Rh123 positive cells. Additionally, a more marked Rh123 positive
cell rate was observed following treatment with quercetin alone
(46.60±1.13%) and in combination with ADR (56.08±0.99%) after 48 h.
Collectively, these results demonstrated that drug combination
induced a loss of mitochondrial membrane potential in a synergistic
manner (Fig. 3).
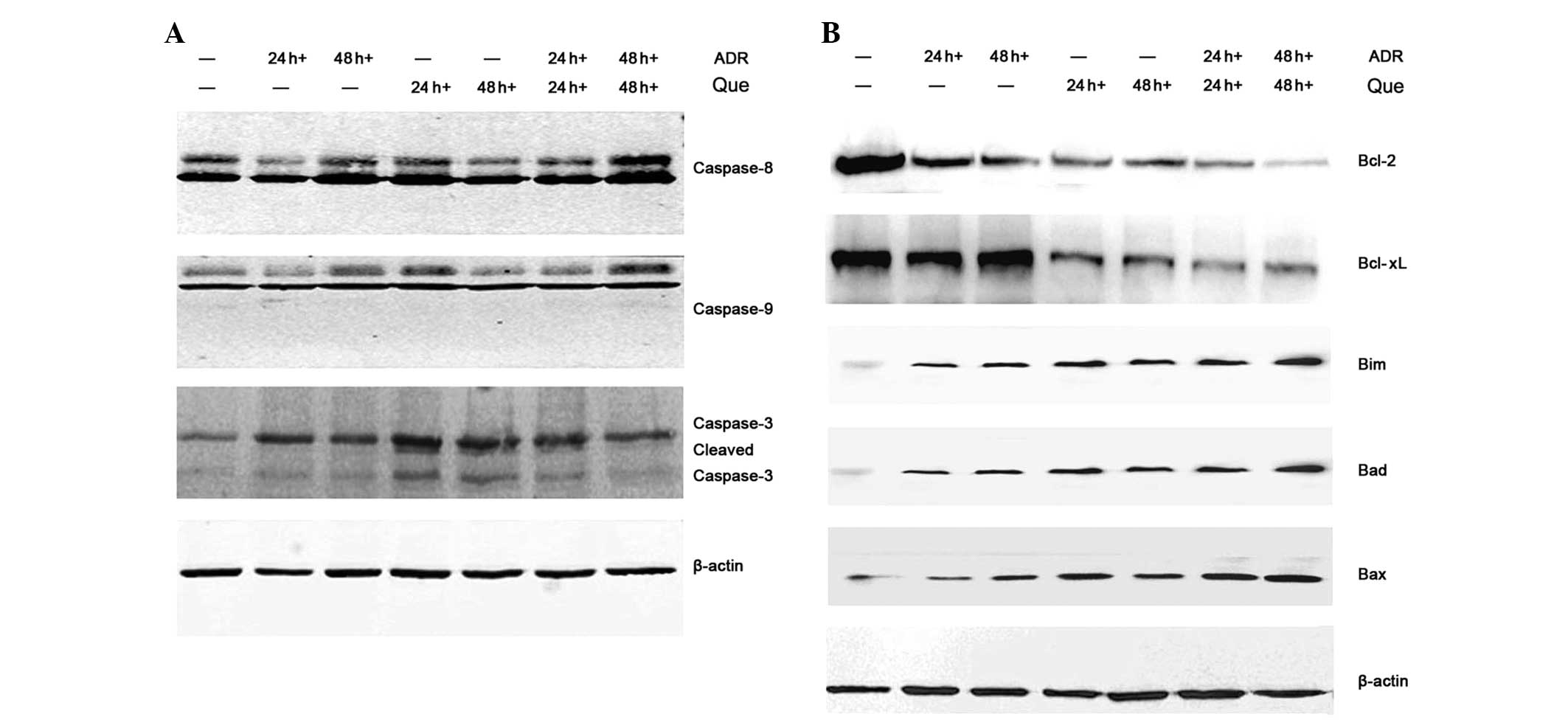
Induction of apoptosis signaling cascade
by quercetin and ADR
Apoptosis can be triggered by extrinsic or intrinsic
pathways. The extrinsic pathway involves the cleavage of caspase-8,
while the intrinsic apoptotic pathway involves the activation of
procaspase-9 (17). Western blot
analysis was performed to detect changes in the expression of
proteins involved in the apoptotic signaling pathway. The results
demonstrated that quercetin and ADR induced the protein expression
of a series of caspases, including caspase-8, -9 and -3 (Fig. 4A). The effect of these compounds on
the expression of mitochondrial-dependent apoptotic proteins in the
K562/ADR cells was also examined. Quercetin and ADR significantly
decreased the expression of anti-apoptotic proteins Bcl-2 and
Bcl-xL and increased the expression of pro-apoptotic proteins Bim,
Bad and Bax in the K562/ADR cells (Fig. 4B). Taken together, these findings
suggested that quercetin and ADR promoted cell apoptosis by the
extrinsic and intrinsic pathways.
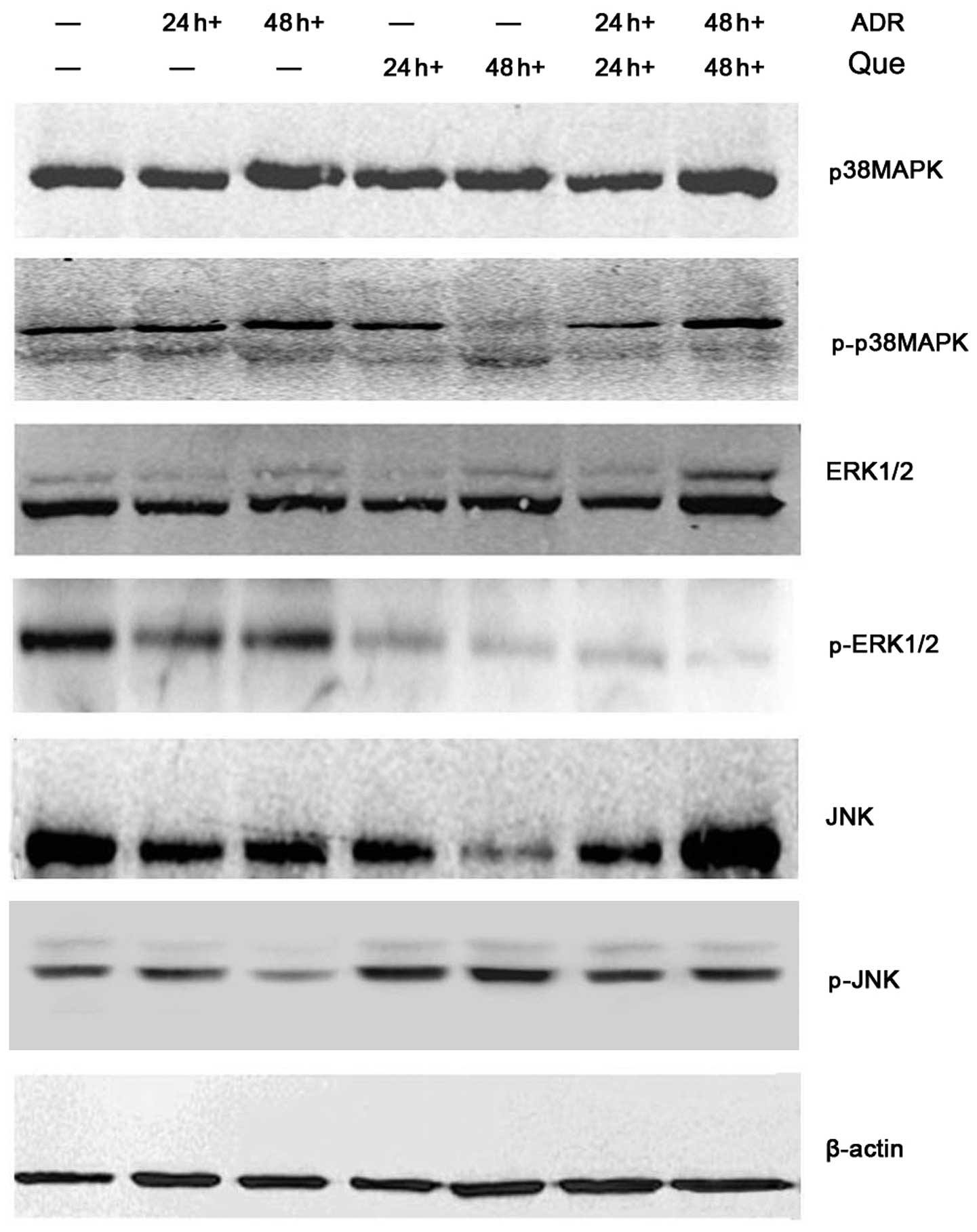
Effect of quercetin and ADR on the
MAPK/ERK/JNK signaling pathway
To further characterize the mechanisms involved in
the pro-apoptotic or proliferation-inhibiting actions of quercetin
and ADR, the present study analyzed the effect on the main
signaling pathways associated with proliferation and the regulation
of apoptosis. Western blot analysis was performed to investigate
the effect of these compounds on the MAPK/ERK/JNK signaling
pathway. The results demonstrated that quercetin and ADR
upregulated the content of p-JNK and p-p38 MAPK and downregulated
the expression of p-ERK (Fig. 5).
These results indicated that the quercetin and ADR-induced cell
apoptosis was associated with MAPK/ERK/JNK signaling regulation in
the K562/ADR cells.
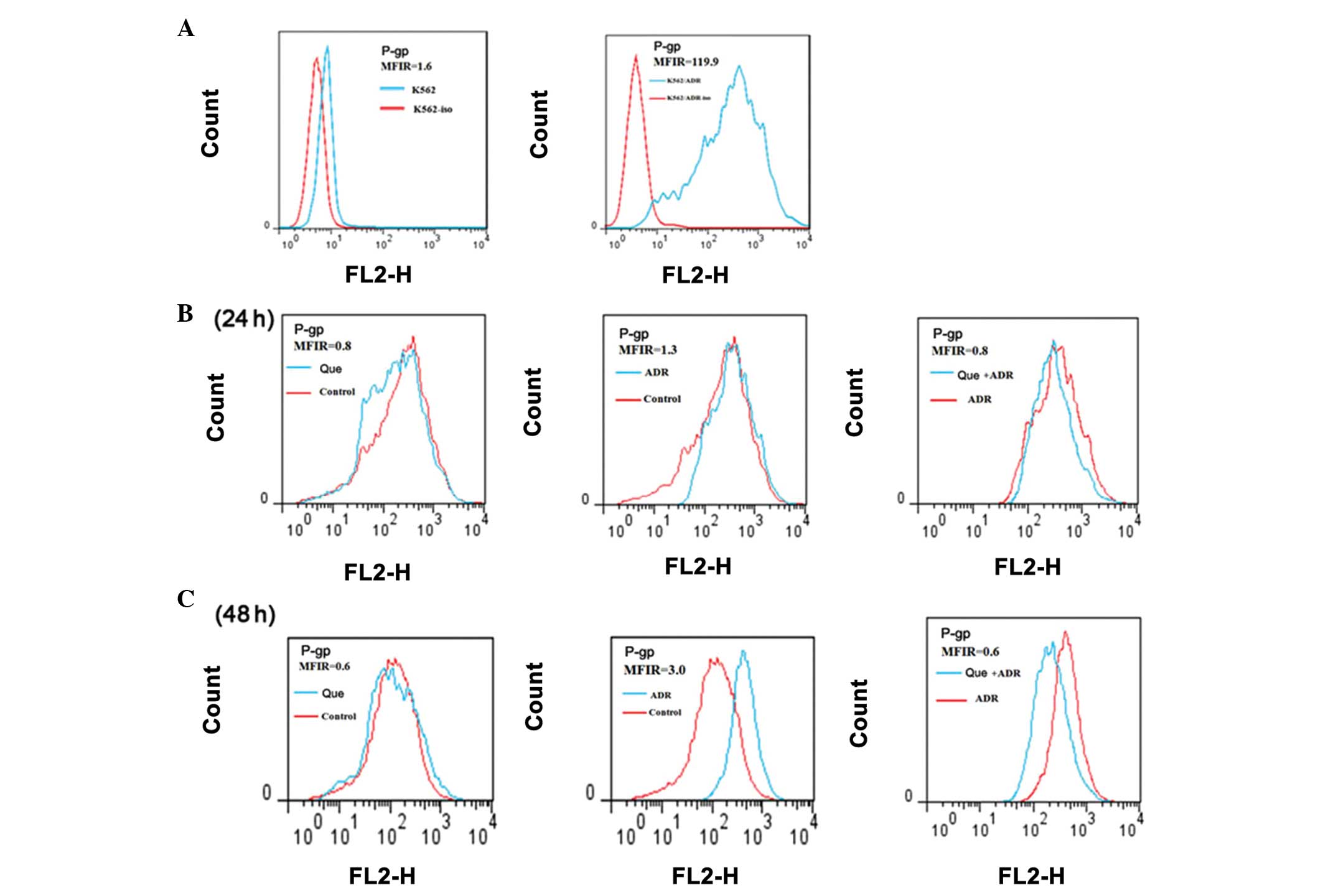
Effect of quercetin and ADR on the
expression of P-gp
In order to examine the reversal effect of
quercetin, flow cytometric analysis was performed to measure the
expression of P-gp in the K562/ADR cells. The results indicated
that the mean fluorescence intensity ratio in the K562/ADR cells
increased significantly (119.9) compared with that in the K562
cells (1.6). However, the expression of P-gp was significantly
reduced following 24 h treatment with either quercetin or ADR,
either alone or in combination. The reversal effect on the
expression of P-gp was also observed following 48 h treatment
(Fig. 6).
Discussion
The identification of drugs from medicinal plants
has been important in the treatment of cancer. Quercetin is a
naturally occurring flavonoid, which has antiproliferative,
anti-inflammatory and immunoregulatory activities (12,13).
In the present study, the effect and associated mechanism of
quercetin on K562/ADR human leukemic MDR cells was investigated.
The CCK-8 assay revealed that quercetin had a significant
inhibitory effect on the K562/ADR cells in a
concentration-dependent manner. The combination of quercetin (100
μg/ml) and ADR (IC20; 12 μg/ml) resulted in potentiation
of the cytotoxicity.
Apoptosis is a complex process, characterized by
morphological and biochemical changes in the nucleus and the
formation of apoptotic bodies (18,19).
The present study observed that quercetin and ADR induced nuclear
condensation, fragmentation of nuclei and formation of scattered
apoptotic bodies. Flow cytometric analysis also revealed that
quercetin promoted cell apoptosis in a concentration- and
time-dependent manner and the synergistic effect on apoptosis was
more marked following combination treatment with ADR and quercetin
at different concentrations.
Apoptosis can be activated by extrinsic or intrinsic
signaling pathways (20). The
extrinsic pathway involves the cleavage of caspase-8, while the
intrinsic apoptotic pathway involves procaspase-9, which triggers
downstream mitochondrial pro-apoptotic events. Following the
activation of initiator caspases, procaspase-3 becomes activated,
which induces cell apoptosis (21,22).
In the present study, quercetin and ADR induced the loss of
mitochondrial membrane potential. Several studies have suggested
that abrogation of mitochondrial membrane potential leads to the
activation of caspases (23,24).
As demonstrated in the present study, quercetin and ADR led to the
activation of a series of caspases, including caspase-8, -9 and -3
in the K562/ADR cells. A marked reduction in the expression levels
of anti-apoptotic proteins Bcl-2 and Bcl-xL and increase in the
expression levels of pro-apoptotic proteins Bim, Bad and Bax were
also observed in the K562/ADR cells. Taken together, these findings
suggested that quercetin and ADR promoted cell apoptosis through
extrinsic and intrinsic apoptotic pathways in the human leukemia
K562/ADR cells.
It has been suggested that the apoptosis of tumor
cells involves the activation of JNK and the inactivation of ERK
(25). Various studies have
demonstrated that activation of the JNK signaling pathway is
important in regulation of the expression of pro-apoptotic proteins
(26,27). The JNK downstream transcriptional
factors phosphorylate the Bcl-2 family members and are involved in
various pathophysiological processes, including embryonic
development, immune regulation and tumorigenesis (28,29).
The results of the present study revealed that quercetin combined
with ADR increased the expression of p-JNK and p-p38 MAPK and
decreased the expression of p-ERK in a synergistic way, thereby
promoting the apoptosis of K562/ADR cells.
Drug resistance in leukemia cells is associated with
the increased expression of resistance proteins (30). P-gp, encoded by the MDR 1 gene, is
one of the MDR-associated proteins (31). The present study found that
quercetin combined with ADR markedly reduced the expression of
P-gp.
In conclusion, the findings of the present study
suggested that the combination of quercetin and ADR in MCF-7/ADR
cells inhibited cell proliferation, promoted apoptosis via
regulation in MAPK/ERK/JNK signaling and decreased the expression
of P-gp. Therefore, quercetin is of important clinical significance
in the MDR of tumor therapy and may be developed into a new
reversal agent for cancer chemotherapy.
Acknowledgements
The authors would like to thank the all members of
the Leukemia Research Institute in Renji Hospital. This study was
supported by the Shanghai Municipal Bureau of Health Major Subject,
the Class of Traditional Chinese Medicine (no. ZYSNXD-CC-ZDYJ001)
and the TCM Guide Project from the Natural Science Foundation of
Shanghai of China (no. 12401906700).
References
|
1
|
Fang S, Zhu W, Zhang Y, Shu Y and Liu P:
Paeoniflorin modulates multidrug resistance of a human gastric
cancer cell line via the inhibition of NF-κB activation. Mol Med
Rep. 5:351–356. 2012.PubMed/NCBI
|
|
2
|
Cho S, Lu M, He X, et al: Notch1 regulates
the expression of the multidrug resistance gene ABCC1/MRP1 in
cultured cancer cells. Proc Natl Acad Sci USA. 108:20778–20783.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stępień KM, Tomaszewski M, Tomaszewska J
and Czuczwar SJ: The multidrug transporter P-glycoprotein in
pharmacoresistance to antiepileptic drugs. Pharmacol Rep.
64:1011–1019. 2012.PubMed/NCBI
|
|
4
|
Assef Y, Rubio F, Coló G, del Mónaco S,
Costas MA and Kotsias BA: Imatinib resistance in
multidrug-resistant K562 human leukemic cells. Leuk Res.
33:710–716. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higgins CF: Multiple molecular mechanisms
for multidrug resistance transporters. Nature. 446:749–757. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tai DJ, Jin WS, Wu CS, et al: Changes in
intracellular redox status influence multidrug resistance in
gastric adenocarcinoma cells. Exp Ther Med. 4:291–296.
2012.PubMed/NCBI
|
|
7
|
Sun L, Chen W, Qu L, Wu J and Si J:
Icaritin reverses multidrug resistance of HepG2/ADR human hepatoma
cells via downregulation of MDR1 and P-glycoprotein expression. Mol
Med Rep. 8:1883–1887. 2013.PubMed/NCBI
|
|
8
|
Limtrakul P, Anuchapreeda S and Buddhasukh
D: Modulation of human multidrug-resistance MDR-1 gene by natural
curcuminoids. BMC Cancer. 4:132004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maeda J, Roybal EJ, Brents CA, Uesaka M,
Aizawa Y and Kato TA: Natural and glucosyl flavonoids inhibit
poly(ADP-ribose) polymerase activity and induce synthetic lethality
in BRCA mutant cells. Oncol Rep. 31:551–556. 2014.PubMed/NCBI
|
|
10
|
Kawai Y, Nishikawa T, Shiba Y, et al:
Macrophage as a target of quercetin glucuronides in human
atherosclerotic arteries: implication in the anti-atherosclerotic
mechanism of dietary flavonoids. J Biol Chem. 283:9424–9434. 2008.
View Article : Google Scholar
|
|
11
|
Hu QF, Zhou B, Huang JM, et al: Cytotoxic
oxepinochromenone and flavonoids from the flower buds of Rosa
rugosa. J Nat Prod. 76:1866–1871. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Umathe SN, Dixit PV, Kumar V, Bansod KU
and Wanjari MM: Quercetin pretreatment increases the
bioavailability of pioglitazone in rats: involvement of CYP3A
inhibition. Biochem Pharmacol. 75:1670–1676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Punithavathi VR and Prince PS:
Pretreatment with a combination of quercetin and alpha-tocopherol
ameliorates adenosine triphosphatases and lysosomal enzymes in
myocardial infarcted rats. Life Sci. 86:178–184. 2010. View Article : Google Scholar
|
|
14
|
Gee JM, Hara H and Johnson IT: Suppression
of intestinal crypt cell proliferation and aberrant crypt foci by
dietary quercetin in rats. Nutr Cancer. 43:193–201. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Duo J, Ying GG, Wang GW and Zhang L:
Quercetin inhibits human breast cancer cell proliferation and
induces apoptosis via Bcl-2 and Bax regulation. Mol Med Rep.
5:1453–1456. 2012.PubMed/NCBI
|
|
16
|
Wu YM, Xia XY, Pan LJ, et al: Evaluation
of sperm mitochondrial function using Rh123/PI dual fluorescent
staining. Zhonghua Nan Ke Xue. 12:803–806. 2006.(In Chinese).
|
|
17
|
Sun c, Guo XX, Zhu D, et al: Apoptosis is
induced in cancer cells via the mitochondrial pathway by the novel
xylocydine-derived compound JRS-15. Int J, Mol Sci. 41:850–870.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li T, Kon N, Jiang L, et al: Tumor
suppression in the absence of p53-mediated cell-cycle arrest,
apoptosis, and senescence. Cell. 149:1269–1283. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Ren X, Alt E, et al:
Chemoprevention of colorectal cancer by targeting APC-deficient
cells for apoptosis. Nature. 464:1058–1061. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tassi E, Zanon M, Vegetti C, et al: Role
of Apollon in human melanoma resistance to antitumor agents that
activate the intrinsic or the extrinsic apoptosis pathways. Clin
Cancer Res. 18:3316–3327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franklin EE and Robertson JD: Requirement
of Apaf-1 for mitochondrial events and the cleavage or activation
of all procaspases during genotoxic stress-induced apoptosis.
Biochem J. 405:115–122. 2007.
|
|
22
|
Tsuruma K, Nakagawa T, Morimoto N, et al:
Glucocorticoid modulatory element-binding protein 1 binds to
initiator procaspases and inhibits ischemia-induced apoptosis and
neuronal injury. J Biol Chem. 281:11397–11404. 2006. View Article : Google Scholar
|
|
23
|
Li J, Li PF, Dietz R and von Harsdorf R:
Intracellular superoxide induces apoptosis in VSMCs: role of
mitochondrial membrane potential, cytochrome C and caspases.
Apoptosis. 7:511–517. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu CY, Chiang RL, Chang TH, Liao CL and
Lin YL: The interferon stimulator mitochondrial antiviral signaling
protein facilitates cell death by disrupting the mitochondrial
membrane potential and by activating caspases. J Virol.
84:2421–2431. 2010. View Article : Google Scholar
|
|
25
|
Kumar A, Byun HS, Bittman R and Saba JD:
The sphingolipid degradation product trans-2-hexadecenal induces
cytoskeletal reorganization and apoptosis in a JNK-dependent
manner. Cell Signal. 23:1144–1152. 2011. View Article : Google Scholar
|
|
26
|
Lei K and Davis RJ: JNK phosphorylation of
Bim-related members of the Bcl2 family induces Bax-dependent
apoptosis. Proc Natl Acad Sci USA. 100:2432–2437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ha Thi HT, Lim HS, Kim J, Kim YM, Kim HY
and Hong S: Transcriptional and post-translational regulation of
Bim is essential for TGF-β and TNF-α-induced apoptosis of gastric
cancer cell. Biochim Biophys Acta. 1830:3584–3592. 2013.PubMed/NCBI
|
|
28
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Platanias LC: Map kinase signaling
pathways and hematologic malignancies. Blood. 101:4667–4679. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niedermeier M, Hennessy BT, Knight ZA, et
al: Isoform-selective phosphoinositide 3′-kinase inhibitors inhibit
CXCR4 signaling and overcome stromal cell-mediated drug resistance
in chronic lymphocytic leukemia: a novel therapeutic approach.
Blood. 113:5549–5557. 2009.
|
|
31
|
Ebert SP, Wetzel B, Myette RL, et al:
Chalcogenopyrylium compounds as modulators of the ATP-binding
cassette transporters P-glycoprotein (P-gp/ABCB1) and multidrug
resistance protein 1 (MRP1/ABCC1). J Med Chem. 55:4683–4699. 2012.
View Article : Google Scholar : PubMed/NCBI
|