Introduction
Polyphenols are antioxidant molecules that exist in
a wide variety of fruits and vegetables. These compounds are able
to interact with defense systems of the cell, and upregulate genes
containing a cis-acting element in their promoter region
known as antioxidant response element/electrophile response element
(ARE/EpRE) (1). When oxidative
stress occurs in the cells, nuclear factor (erythroid-derived
2)-like 2 (Nrf2) is released from the cytoplasm and translocates to
the nucleus. Nrf2 binds the ARE in the regulatory regions of
antioxidant target genes and activates transcription, which
ultimately provides protection against a number of pathologies in
various organs, including the liver, intestines, lungs, skin and
nervous system (2).
Quercetin is one of the most frequently consumed
dietary flavonoids, and serves multiple biologically significant
functions, including antioxidant, anti-carcinogenic,
anti-inflammatory, cardioprotective and radioprotective properties
(3–5). Quercetin is able to affect the ARE
activation pathway, and molecular studies have indicated the
upregulation of Nrf2 through the regulation of both transcriptional
and post-transcriptional sites of Nrf2. Enhanced repression of
Kelch-like ECH-associated protein 1 (Keap1) was also demonstrated
by affecting the post-transcriptional site, which revealed a number
of substantial differences between oxidative inducers (1).
A plethora of studies associating antioxidant
properties with the ability to reduce cytotoxic effects of ionizing
radiation have been conducted. Healthy tissues can be damaged in
multiple ways by radiation, depending on the type of cells and
organs being irradiated, the dose and dose rate of radiation
exposure, and the time elapsed from radiation exposure to the study
of the effects. Chronic, low dose exposure to ionizing radiation
can result in the induction of antioxidant enzymes, but not in a
dose-dependent way. Otsuka et al (6) demonstrated that exposure of mice to
0.5 Gy at a dose rate of 1.2 mGy/h for 23 days increased the gene
expression of catalase and MnSOD by a factor of 2.5, while at
higher doses of 1.0 and 1.3 Gy at a similar dose rate, gene
expression either increased by only 1.4 or was not significantly
different from non-irradiated controls, respectively.
The nucleus, cell membrane and mitochondria are
considered sensitive targets for the reduction or prevention of
radiation damage by antioxidants (7). One of the most studied effects of
ionizing radiation is DNA damage. In order to exert preventive
effects on the immediate genotoxicity induced by ionizing
radiation, antioxidants must be able to access the nucleus and be
near the DNA strands at the time of irradiation. Antioxidant agents
may act via the scavenging of oxygen-based free radicals in
addition to competing with oxygen for chemically repairing DNA
damage by reacting with free radicals on the DNA (7). When ionizing radiation impacts on
lipid membranes, the resulting increase in the formation of lipid
radicals and peroxides can cause damage or the release of membrane
proteins, or it can promote the liberation of lipid-based
peroxidation products that will react with other cell structures
(8). Long-term cell survival
requires the availability of sufficient functional mitochondria to
meet energy needs, and these organelles are particularly
well-suited to withstand radiation damage due to their high
antioxidant capacity and the fact that their DNA is located in
multiple replicates (9).
Whole-body irradiation has been used in the clinical
treatment of malignancies to induce immunosuppression and prevent
allograft rejection, and it has been associated with significant
changes in liver function (10).
The aim of the present study was to evaluate the modifications in
the liver antioxidant system by X-irradiation in male Wistar rats
under whole-body irradiation with a single sub-lethal dose, and the
effects of quercetin supplementation prior to and following
irradiation. The assessment was performed at 7 and 30 days
following irradiation.
Materials and methods
Animals
Male Wistar rats (250 g), were initially housed in a
room maintained at 22°C with a relative humidity ranging from 45 to
55% and a 12-h dark/light cycle. The animals had free access to
food (standard diet from Panlab, Barcelona, Spain) and water, and
were not starved prior to experiments. All study protocols were
reviewed and approved by the University of León Animal Care
Committee (León, Spain) and were in accordance with the indications
of the current Spanish and European laws (RD 53/2013 and EU
Directive 2010/63/EU).
Experimental procedures
Animals were split into four groups of 8, based on
irradiation (non-irradiated controls and X-ray exposure group) and
time periods of analysis (7 and 30 days). On the assumption that
quercetin may have radiomitigative effects shortly following
irradiation exposure (11),
intragastric quercetin supplementation (50 mg/kg body weight) was
used, following a previous study by Kawai et al (12). Thus, quercetin (50 mg/kg body
weight in propylene glycol) was administered intragastrically for 4
days prior to and 6 days following irradiation, and
non-supplemented animals received the same volume of propylene
glycol solvent alone. The groups were then constituted as the CS
(controls, solvent-supplemented), CQ (controls,
quercetin-supplemented), RS (irradiated, solvent-supplemented) and
RQ (irradiated, quercetin-supplemented) groups.
Experimental irradiation with X-rays
RS and RQ animals were whole-body irradiated with a
single X-ray dose of 6 Gy administered over 15 min, at a
source-skin distance of 50 cm. In order to immobilize the animals,
anesthesia was induced by intraperitoneal administration of
pentobarbital 0.5% in saline (10 ml/kg body weight) at noon. This
was 15 min prior to irradiation in order to ensure the loss of
palpebral and plantar reflex activity, and spontaneous respiration
throughout the procedure. The animals were positioned decubitus
pronus on a plexiglas board, so that two animals were irradiated
simultaneously. CS and CQ animals underwent the same procedure,
excluding irradiation. The X-ray source was a Maxishot 200 (YXLON
International GmbH, Hamburg, Germany) operated by qualified staff
at the Instrumental Techniques Laboratory, University of León, in
accordance with current Spanish legislation on radiation equipment
use. X-ray filtration was accomplished in the Maxishot 200 machine
according to manufacturer’s instructions using 4-mm thick beryllium
and 3-mm thick aluminum filters in the X-ray tube unit. Uniform
total-body X-irradiation distribution was confirmed by dosimetry
using isodose curves for measurement. A test with a phantom (water
layer) was performed in order to check self-shielding without
changes in the dose distribution profile for the thickness
involved.
Determination of lipid peroxidation
Aldehydic products generated by lipid peroxidation
were determined by the thiobarbituric acid (TBA) reaction with
malondialdehyde using the methods of a previous study (13). Liver (1 g) was homogenated in 9 ml
potassium phosphate (0.1 M, pH 7.4) and tubes were prepared with 1
ml fresh homogenate plus 2 ml of a solution containing 15%
trichloroacetic acid, 0.37% TBA, and 0.25 M HCl. After 30 min at
90°C, the tubes were cooled and centrifuged at 2,000 × g.
Supernatants were collected and their absorbance read at 532
nm.
Protein expression of copper/zinc
superoxide dismutase (Cu/Zn-SOD), catalase (CAT), NAD(P)H: quinone
oxidoreductase 1 (NQO1), Nrf2 and Keap1
Liver tissue was cut into small pieces and
homogenized in RIPA buffer [Tris-HCl 50 mM pH 7.4, KCl 150 mM,
sodium deoxycholate 0.5%, NP-40 0.1% and sodium dodecyl sulfate
(SDS) 0.1%] with protease/phosphatase cocktail (one tablet per 10
ml RIPA buffer) (Roche Farma S.A., Madrid, Spain).
The cytoplasmic fraction was obtained as the
supernatant from centrifugation of the liver extract (1 h, 4°C,
12,000 × g). The nuclear fraction was obtained from fresh liver
tissue, homogenized in buffer A (10 mM HEPES-NaOH pH 7.9, 1.5 mM
MgCl2 and 10 mM KCl) including 0.1% NP-40 (EMD
Millipore, Billerica, MA, USA) and centrifuged (10 min, 4°C, 1,000
× g). The pellet was resuspended in buffer A without NP-40,
centrifuged again, and then resuspended again in buffer B (20 mM
HEPES-NaOH pH 7.9, glycerol, 420 mM NaCl, 1.5 mM MgCl2
and 0.2 mM EDTA pH 8.0), prior to being centrifuged for 30 min at
4°C and 12,000 × g.
Samples containing 50–100 μg protein were separated
by SDS-polyacrylamide gel electrophoresis (12–14% acrylamide) and
transferred onto polyvinylidine fluoride membranes (EMD Millipore).
The membranes were subsequently immersed in Ponceau stain in order
to verify equal sample loading. Non-specific binding was blocked by
pre-incubation of the membranes in phosphate-buffered saline
containing 3–5% non-fat dried milk for 30 min at 37°C. Membranes
were then incubated overnight at 4°C with polyclonal
anti-Cu/Zn-SOD, anti-CAT, anti-NQO1, anti-Nrf2 and anti-Keap1
(Santa Cruz Biotechnology, Santa Cruz, CA, USA). Bound primary
antibody was detected using a horseradish peroxidase-conjugated
goat anti-mouse antibody (Dako, Glostrup, Denmark) by
chemiluminescence using an Amersham ECL kit (GE Healthcare,
Chalfont, UK) according to the manufacturer’s instructions.
Histology
For histopathological examination, samples of the
liver were trimmed and fixed by immersion in 10% buffered formalin
for 24 h. The blocks were dehydrated in a graded series of ethanol
solutions (50, 70, 96 and 100%) and embedded in paraffin wax.
Serial 4-μm sections were stained with hematoxylin and eosin and
evaluated blindly by a pathologist. Photomicrographs were performed
on an Olympus Provis AX-70 microscope (Olympus Corporation, Tokyo,
Japan) fitted with a DXM 1200 digital camera (Nikon Corporation,
Tokyo, Japan).
Statistical analysis
Results are expressed as the mean ± standard error.
The data were compared by analysis of variance; significant
differences in the means were compared with the Newman-Keuls test.
Values were analyzed using the statistical package Statistica,
version 8.0 (Statsoft, Inc., Tulsa, OK, USA).
Results
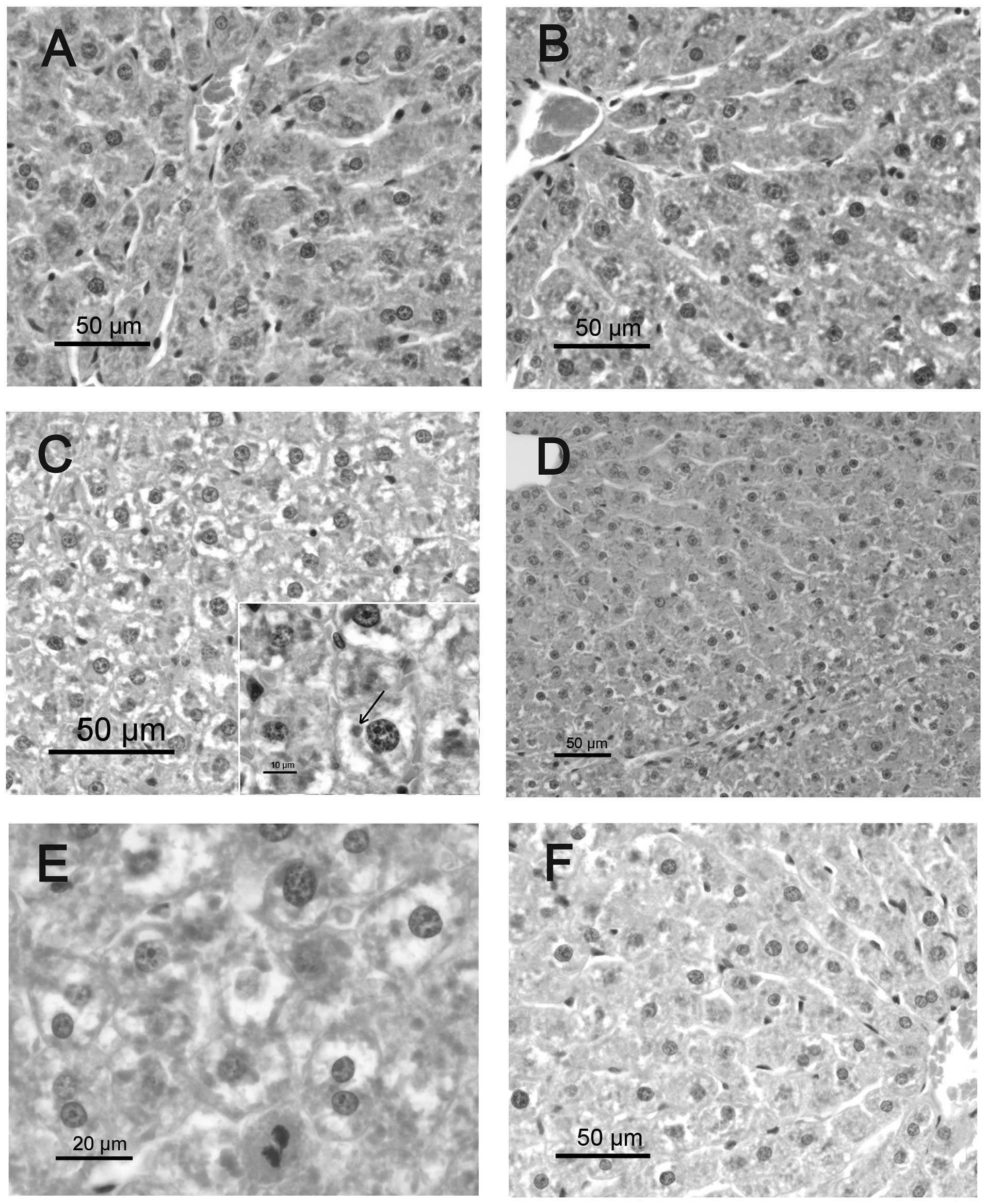
Histological findings
The livers of the CS group exhibited normal cell
structure. In the RS group, the hepatocytes were swollen and
enlarged (ballooned cells) 7 days following irradiation, due to
marked hydropic degeneration, mainly in the mid-zonal and
periportal areas. Hyaline inclusions (Mallory bodies) were observed
within the ballooning hepatocytes. Occasional inflammatory cell
infiltration (mainly lymphocytes) was observed in the portal triads
of the RS group. The RQ group exhibited a reduction in hepatic
pathological changes compared with the RS group, and mild hydropic
change was observed in periportal hepatocytes. No significant
hepatic pathological changes were observed in the CQ group compared
with solvent-supplemented controls.
The hepatocellular degeneration in irradiated
animals was more diffuse at 30 days post-irradiation, and some
small portal infiltrates consisting mainly of lymphocytes were
identified. Hepatocyte cytoplasm appeared diffusely vacuolized with
a central nucleus, which is a feature compatible with fluid
metabolism alteration (Fig. 1).
Mitotic activity of hepatocytes and a high proportion of binucleate
cells were evident. No differences were identified between RQ
animals and the RS group. Thirty days following irradiation,
untreated animals also displayed discrete portal infiltration with
lymphocytes and macrophages. Hepatocytes, mainly from periportal
areas, exhibited dense cytoplasmic inclusions, which may have been
protein-based (hyaline droplets or Mallory bodies).
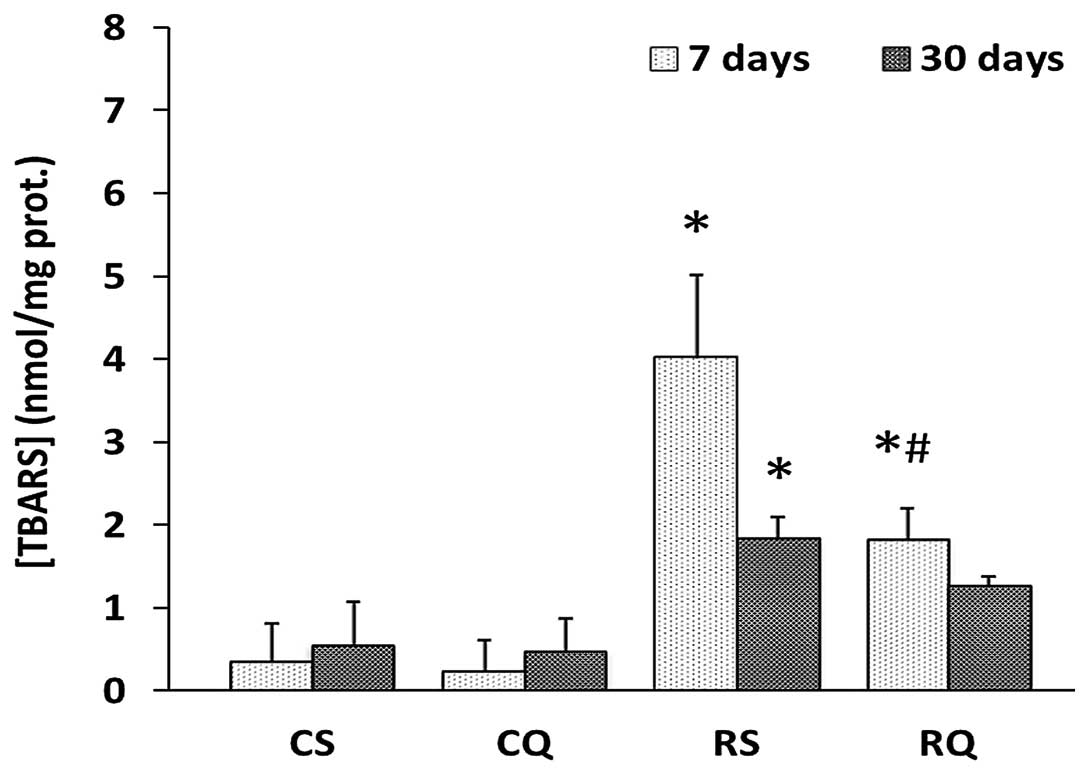
Oxidative stress markers
The cytoplasmic concentration of thiobarbituric acid
reactive substance (TBARS), a marker for lipid peroxidation, was
significantly increased in the RS group compared with the CS group,
at 7 and 30 days post-irradiation (39.89 and 4.21%, respectively).
In RQ animals, the cytoplasmic concentration of TBARS increased to
a lesser degree than in RS rats (Fig.
2).
Expression of antioxidant enzymes (CAT,
Cu/ZN-SOD, NQO1)
The expression of antioxidant enzymes was quantified
by the measurement of protein levels using western blotting. CAT
expression at 7 days post-irradiation was increased in irradiated
animals, supplemented or unsupplemented with quercetin (47 and 53%,
respectively). No changes were observed in CAT enzyme expression at
30 days post-irradiation between any of the experimental groups. An
increased expression level of Cu/Zn-SOD was observed in all
irradiated animals (RS and RQ) at 7 and 30 days post-irradiation.
This increase was significantly reduced by quercetin
supplementation, but Cu/Zn-SOD expression did not return to control
levels (Fig. 3). The expression of
the NQO1 enzyme increased in all irradiated animals at 7 and 30
days post-irradiation with or without quercetin supplementation
(Fig. 3).
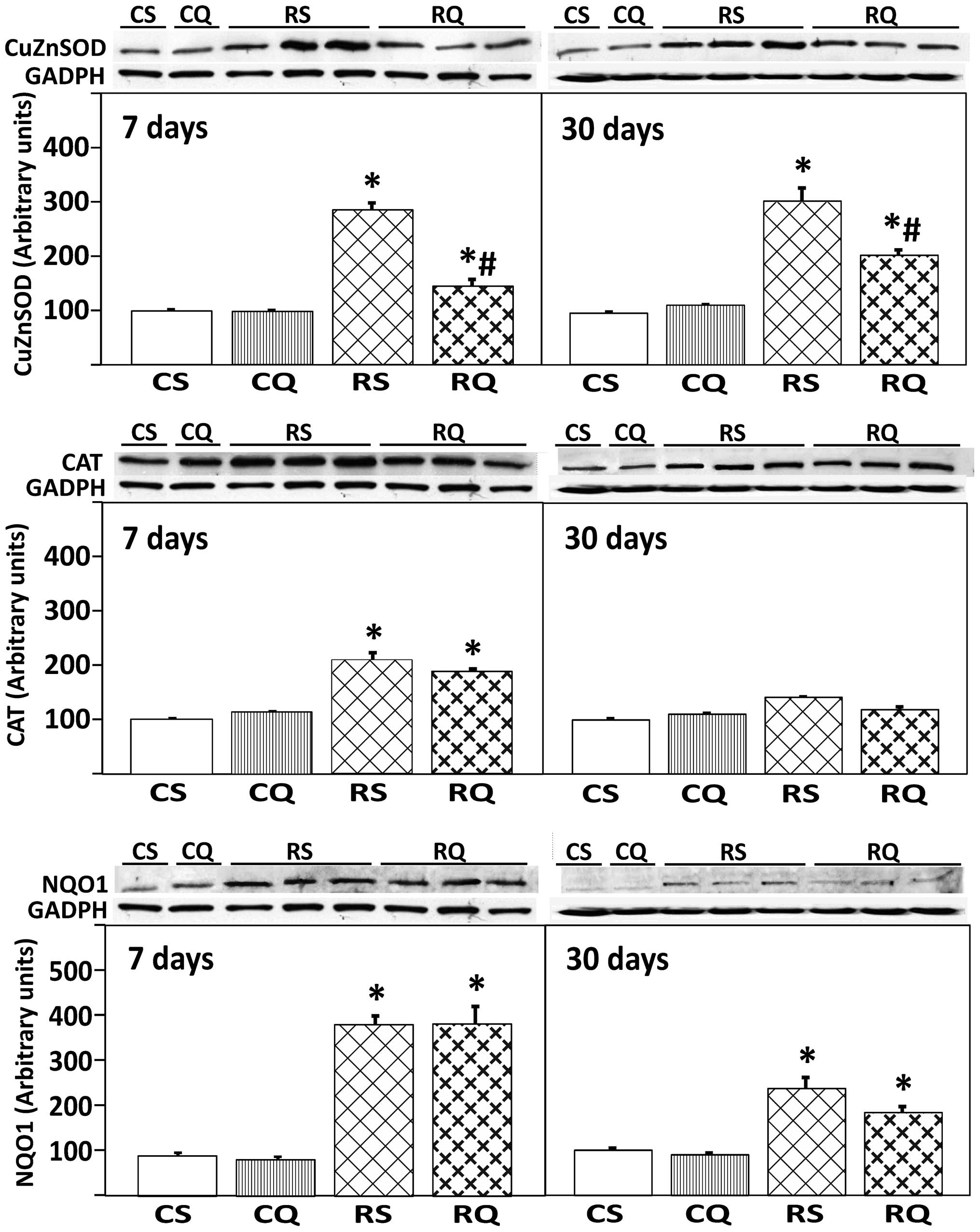 | Figure 3Protein expression of Cu/Zn-SOD, CAT
and NQO1 in livers from the different experimental groups at 7 and
30 days following irradiation. At the top, representative Western
blot photographs are shown. Data are presented as the mean value ±
standard error, n=8 in each group. GADPH was used as loading
control. *P<0.05 vs. CS, #P<0.05 vs.
RS. Cu/Zn-SOD, copper/zinc superoxide dismutase; CAT, catalase;
NQO1, NAD(P)H: quinone oxidoreductase 1; CS, control
solvent-supplemented; CQ, control quercetin-supplemented; RS,
irradiated solvent-supplemented; RQ, irradiated
quercetin-supplemented. |
Expression of transcription factor Nrf2
and protein Keap1
As presented in Fig.
4, irradiation in quercetin-supplemented and unsupplemented
animals induced a significant increase (59 and 70%, respectively)
of nuclear expression levels of Nrf2 at 7 days post-irradiation.
This activation was less marked at 30 days post-irradiation
(Fig. 4, upper panel). By
contrast, cytoplasmic Nrf2 expression levels remained unchanged
throughout the experiment, irrespective of quercetin
supplementation (Fig. 4, middle
panel). The expression of Keap1 protein in the liver cytoplasmic
fraction significantly increased in all irradiated animals (RQ and
RS, vs. CS) at 7 and 30 days post-irradiation (Fig. 4). This increase in Keap1 protein
expression level was reduced in RQ animals at 30 days, to a level
that was not significantly higher than the level of the CS
group.
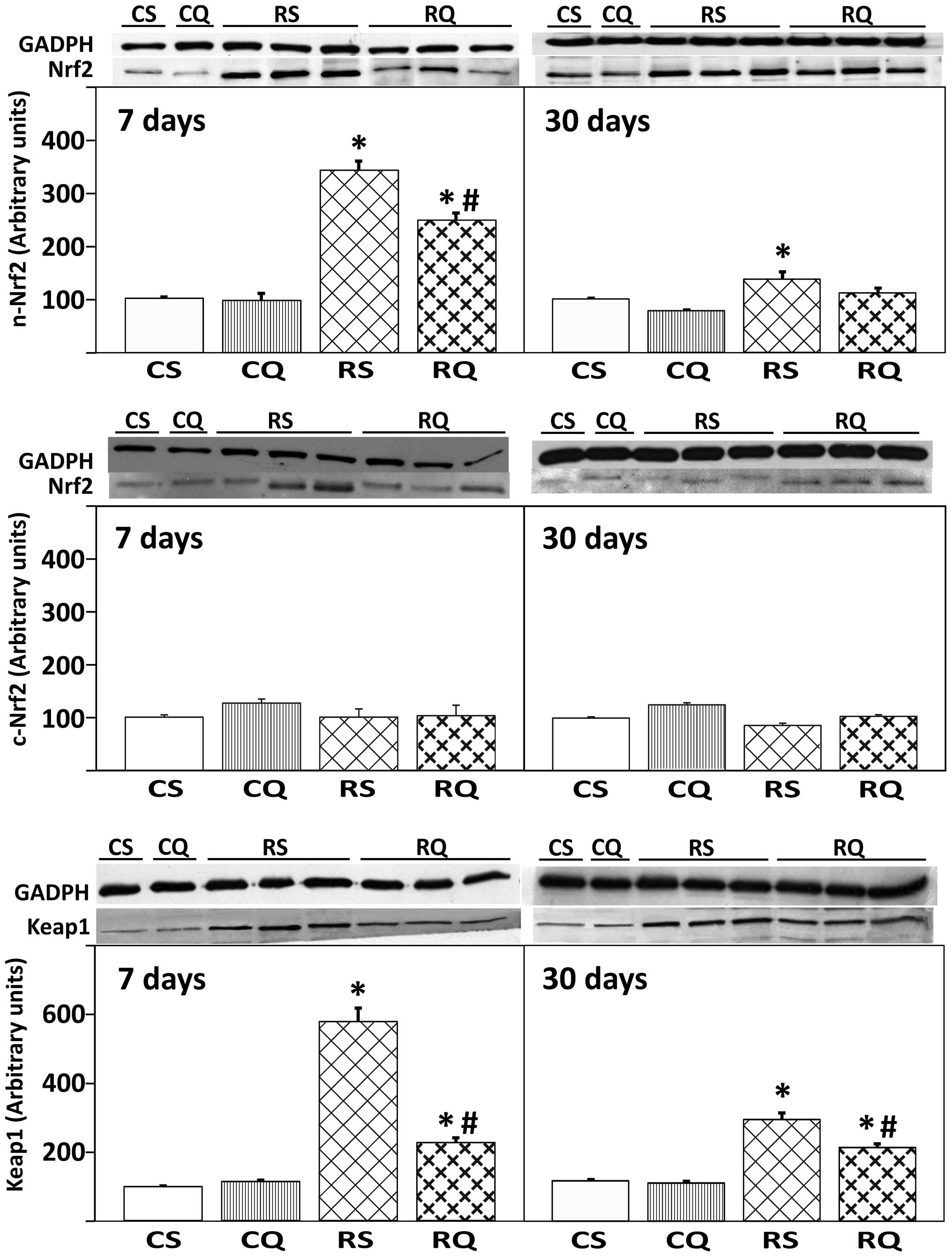 | Figure 4Protein expression of Nrf2 in
cytoplasmic (c-Nrf2) and nuclear (n-Nrf2) compartments, and
cytoplasmic Keap1 in livers from the four experimental groups at 7
and 30 days post-irradiation. At the top, representative western
blot photographs are presented. Data are expressed as the mean
value ± standard error, n=8 in each group. GADPH was used as
loading control. *P<0.05 vs. CS,
#P<0.05 vs. RS. Nrf2, nuclear factor
(erythroid-derived 2)-like 2; Keap1, Kelch-like ECH-associated
protein 1; CS, control solvent-supplemented; CQ, control
quercetin-supplemented; RS, irradiated solvent-supplemented; RQ,
irradiated quercetin-supplemented. |
Discussion
Experimental quercetin supplementation has been used
to counteract oxidative stress in a number of forms, including
dissolved in water (14), ethanol
(15) or propylenglycol (12), or food-supplemented (12). Quercetin has been used as
pre-treatment to radiation in mice (16) and other flavonoids have been tested
both prior to and following irradiation (17). The present study has demonstrated
the radiomitigative effects of quercetin when administered for four
days prior to and six days following whole-body X-irradiation.
Hepatic injury induced by radiation is characterized
by the loss of parenchymal hepatocytes and the distortion of the
lobular architecture, which is accompanied by periportal fibrosis
(18). Previous studies on human
liver have indicated that the reaction of the liver to irradiation
is largely dependent upon parameters such as the type of
irradiation, dose, dose rate, fractionation schedule and irradiated
volume, and the liver function is not normally compromised unless
radiation doses >35 Gy are used (19). The sensitivity of hepatocytes to
ionizing radiation has been observed to be similar in human and rat
liver tissues (20). The results
of the present study suggest a marked hydropic degeneration at 7 or
30 days post-irradiation (6 Gy), which is most likely due to an
intra-cytoplasmic accumulation of fluid as a result of the
disturbed integrity of the cell membrane. Mallory bodies are
accepted to represent degenerate cytoskeleton in damaged
hepatocytes and are a consequence of cellular injury (21).
Quercetin supplementation may protect cells from the
damage induced by ionizing radiation, since the data of the present
study indicated that hydropic change was less extended and limited
to periportal areas in quercetin-supplemented rats, compared with
controls.
Ionizing radiation is established to induce
oxidative stress through the generation of reactive oxygen species,
resulting in an imbalance in the pro-oxidant/antioxidant status of
the cells (22–24). Data of the current study indicate
an apparent increase in lipid peroxidation at 7 days
post-irradiation. The presence of oxidative stress in the
experimental model was confirmed by increased levels of TBARS in
the liver in addition to the increase in the expression of the
enzymatic antioxidant system. In the current results, lipid
peroxidation reduced 30 days following irradiation to levels half
of those observed 7 days following irradiation, but were still
significantly higher than the non-irradiated controls. This is
consistent with previous reports of decreased oxidative stress in
rats 68 days after 8 Gy irradiation compared with 10 days
post-irradiation (25). The
present results indicate that quercetin may prevent oxidative
stress, reducing the increase in lipid peroxidation markers and
maintaining the expression of antioxidant enzymes.
Similar to previous reports (26), Nrf2 expression levels in the
nuclear fraction were increased in response to X-irradiation,
confirming the important role of this nuclear factor in the
antioxidant cell response. In contrast, no significant effects were
observed following analysis of cytoplasmic Nrf2 expression levels.
The present study also demonstrated that X-irradiation is able to
activate Nrf2 in order to increase ARE-dependent gene expression 7
days following irradiation, but activated it to a lesser extent at
30 days post-irradiation. An accompanying increase in the
expression of several antioxidant enzymes (CAT, Cu/Zn-SOD, NQO1)
was also observed. Nrf2 expression levels were significantly
reduced in the RQ animals compared with the RS group. These results
may be interpreted as follows: By decreasing the levels of free
radicals following quercetin supplementation, the activation of
Nrf2 is reduced in accordance with the presently lower oxidation
status. This idea is reinforced by the findings of McDonald et
al (27) who indicated
activation of Nrf2 following single doses of ionizing radiation
from 2–8 Gy in breast cancer cells in a dose-dependent manner, but
only following a delay of 5 days. As a result of the exogenous
antioxidants glutathione and PEG-SOD (but not PEG-CAT) being
administered post-irradiation, they demonstrated partially reduced
ARE reporter activity after 5 days. This is also consistent with
the finding of the present study, which indicated that Cu/Zn-SOD
expression levels, but not those of CAT or NQO1, were reduced in RQ
rats. However, other authors have reported increased Nrf2
expression levels following antioxidant administration. Liu et
al (28) did not observe
increased Nrf2 levels in mouse brain after 12 h of a single 4 Gy
radiation dose, but did observe an increase with increasing
concentrations of melatonin (1–10 mg/kg). Similarly, following
dimethylnitrosamine-induced ROS increase, melatonin (50 mg/kg)
administration over 14 days was able to double the nuclear levels
of Nrf2 (29).
It is accepted that Keap1 is a protein involved in
detecting oxidative stress via oxidative modification of distinct
cysteine residues, leading to conformational changes that allow for
the release of Nrf2, but Nrf2 itself may behave as a redox sensor.
As the concentration of oxidants becomes higher, more Nrf2 proteins
translocate into the nucleus at a higher speed. This graded nuclear
translocation of Nrf2 indicates that Nrf2 can transmit the presence
of oxidative stress and also the intensity of oxidative stress
(30). An autoregulation interplay
cycle has been reported between Nrf2 and Keap1, in that the
increase in Nrf2 expression levels may induce the inducing activity
of the Keap1 gene by binding to an ARE in the reverse strand of the
proximal promoter of the Keap1 gene (31). This positive feedback may aid in
explaining the parallel increases in nuclear Nrf2 and cytoplasmic
Keap1 protein expression observed at 7 days post-irradiation, in
addition to the reduced levels observed as a result of quercetin
supplementation.
The Cu/Zn-SOD gene promoter contains an antioxidant
responsive element (32). The
transcription of the Cu/Zn-SOD gene has been reported to be
upregulated in human HepG2 hepatoma cells following dioxin
treatment used to induce ROS production and the activation of Nrf2
signalling (33), and also after
irradiation in human oral cancer following preoperative radiation
therapy (34) and human prostate
cancer cells (35). In the results
of the current study, the observed increase in Cu/Zn-SOD expression
levels following whole-body irradiation is consistent with the
activation of Nrf2 by increased oxidative stress. Quercetin
supplementation reduced Cu/Zn-SOD expression levels, but they
remained at a significantly higher level than non-irradiated
controls, possibly as a consequence of the reduction in Nrf2
expression levels.
The current study indicated that NQO1 expression was
significantly increased at 7 days post-irradiation, and it remained
unchanged following quercetin supplementation, while Nrf2
expression levels were significantly reduced in the RQ group. In
order to explain these findings it may be important to consider the
free radical-scavenging activity of quercetin, and that it produces
oxidized derivatives such as quercetin ortho-quinone/quinone
methide, which is a substrate that interacts with NQO1 (36). The fact that NQO1 expression was
not altered in RQ rats may be attributed to the additional need for
NQO1 to inactivate the potentially harmful oxidation products
derived from quercetin antioxidant activity. Further research is
required to clarify the dynamics of quercetin-derived adducts and
their role in the antioxidant regulatory pathways.
The distinct changes in antioxidant enzyme
expression observed in the present study can be interpreted by
considering that ionizing radiation induces multiple molecular
changes in the cells, depending on the dose and the time of
testing. Transcription factors such as AP-1 and nuclear factor
(NF)-κB are activated only 24 h after irradiation (37), while MnSOD is simultaneously
induced through the NF-κB pathway. It has also been reported that
human lymphoblasts exposed at 3 Gy display increased levels of CAT
and glutathione-peroxidase, but no other enzymes, at 20 h
post-irradiation, suggesting that a low dose of radiation induces
an increase in the expression of antioxidant enzymes as a
radiomodifying response (38).
Similarly, McDonald et al (27) demonstrated increased activation of
Nrf2 after 5 days exposure of several cell lines to 8 Gy
irradiation.
Quercetin has been successfully tested as an
antioxidant in several in vitro studies using cell cultures
(16,39,40),
but its in vivo activity has been questioned. Studying
vitamin E-deficient rats supplemented with vitamin E or a variety
of flavonoids, including quercetin, Duthie et al (41) concluded that in vivo
supplementation with these antioxidant compounds (100 mg/kg diet)
is ineffective in restoring lipid peroxidation indices in plasma
and liver. However, another study has indicated that serum
malondialdehyde levels are inversely correlated to the oral daily
quercetin dose (0.01–1.00 g/kg body weight) after 22 days of
treatment in otherwise normal Wistar rats (42); and this is in line with the results
from the current study on lipid peroxidation.
Overexpression of Nrf2 can result in enhanced
resistance of tumoral cells to chemotherapeutic agents, while
downregulation of Nrf2 has been translated into more susceptibility
to these drugs (43). These
findings suggest that cancer cells can protect themselves from the
stress-inducing conditions of the tumoral environment: An active
Nrf2 pathway may promote a favorable redox balance and upregulate
antioxidant gene products in order to enhance their survival; by
contrast, normal cells do not have such high metabolic requirements
and Nrf2 levels are not expected to be elevated to such a level.
Supporting this view, in HepG2 cells incubated with 0–40 μM
quercetin, this flavonoid enhanced the steady-state level of Nrf2
and also reduced the steady-state level of Keap1 through 26S
proteasome-independent degradation (1). The study also concluded that
quercetin is able to increase the protein level of Nrf2 and
simultaneously inhibit its ubiquitination, while Keap1 expression
is reduced. While these effects have been observed in cell cultures
without additional treatment, it has been recently reported that
the combination of quercetin plus X-irradiation can significantly
increase the tumoral radiosensitivity in vitro and in
vivo (44). The data from the
current study have demonstrated that quercetin in vivo
supplementation influences the antioxidant system by reducing lipid
peroxidation and modifying the activation of Nrf2 and various
antioxidant enzymes. By administering quercetin to X-irradiated
animals, its antioxidant status was improved; the present study
demonstrated that the oxidative stress-associated increase in Nrf2
expression levels in X-irradiated rats was diminished following
quercetin supplementation. This approach could be utilized to avoid
favoring cancer cell survival (by hindering the increase in Nrf2
expression levels), so quercetin may produce effects that reduce
the oxidative stress-associated increase in Nrf2 expression levels,
which may be beneficial in the prevention of cancer growth.
Acknowledgements
The authors are grateful for the financial support
provided by Fundación Mapfre, and to Dra. María Teresa Ribas Ariño
for her helpful assistance in histopathological interpretation.
Abbreviations:
|
ARE
|
antioxidant response element
|
|
CAT
|
catalase
|
|
Cu/ZnSOD
|
copper/zinc superoxide dismutase
|
|
EpRE
|
electrophile response element
|
|
Keap1
|
Kelch-like ECH-associated protein
1
|
|
MnSOD
|
manganese superoxide dismutase
|
|
NQO1
|
NAD(P)H: quinone oxidoreductase 1
|
|
Nrf2
|
nuclear factor (erythroid-derived
2)-like 2
|
|
PEG-CAT
|
polyethylene glycol-conjugated
catalase
|
|
PEG-SOD
|
polyethylene glycol-conjugated
superoxide dismutase
|
|
QQ
|
quercetin ortho-quinone/quinone
methide
|
|
TBARS
|
thiobarbituric acid reactive
substance
|
References
|
1
|
Tanigawa S, Fujii M and Hou D: Action of
Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free
Rad Biol Med. 42:1690–1703. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aleksunes LM and Manautou JE: Emerging
role of Nrf2 in protecting against hepatic and gastrointestinal
disease. Toxicol Pathol. 35:459–473. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Middleton EJ, Kandaswami C and Theoharides
T: The effects of plants flavonoids on mammalian cells:
implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
4
|
González-Gallego J, García-Mediavilla MV,
Sánchez-Campos S and Tuñón MJ: Fruit polyphenols, immunity and
inflammation. Br J Nutr. 104:S15–S27. 2010.PubMed/NCBI
|
|
5
|
Hosseinimehr SJ: Trends in the development
of radioprotective agents. Drug Discov Today. 12:794–805. 2007.
View Article : Google Scholar
|
|
6
|
Otsuka K, Koana T, Tauchi H and Sakai K:
Activation of antioxidative enzymes induced by low-dose-rate
whole-body gamma irradiation: adaptive response in terms of initial
DNA damage. Radiat Res. 166:474–478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okunieff P, Swarts S, Keng P, et al:
Antioxidants reduce consequences of radiation exposure. Adv Exp Med
Biol. 614:165–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marnett LJ: Oxy radicals, lipid
peroxidation and DNA damage. Toxicology. 181–182:219–222.
2002.PubMed/NCBI
|
|
9
|
Wallace DC: The mitochondrial genome in
human adaptive radiation and disease: on the road to therapeutics
and performance enhancement. Gene. 354:169–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nwokocha CR, Nwokocha M, Mounmbegna P, et
al: Proteins and liver function changes in rats following
cumulative total body irradiations. West Indian Med J. 61:773–777.
2012.PubMed/NCBI
|
|
11
|
Citrin D, Cotrim AP, Hyodo F, et al:
Radioprotectors and mitigators of radiation-induced normal tissue
injury. Oncologist. 15:360–371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kawai Y, Saito S, Nishikawa T, et al:
Different profiles of quercetin metabolites in rat plasma:
comparison of two administration methods. Biosci Biotechnol
Biochem. 73:517–523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Willis ED: Evaluation of lipid
peroxidation in lipids and biological membranes. Biochemical
Toxicology: A Practical Approach. Snell KB and Mullock B: IRL
Press; Oxford: pp. 407–420. 1987
|
|
14
|
Jung JH, Kang JI and Kim HS: Effect of
quercetin on impaired immune function in mice exposed to
irradiation. Nutr Res Pract. 6:301–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kanter M, Aktas C and Erboga M: Protective
effects of quercetin against apoptosis and oxidative stress in
streptozotocin-induced diabetic rat testis. Food Chem Toxicol.
50:719–25. 2012. View Article : Google Scholar
|
|
16
|
Benkovic V, Knezevic AH, Dikic D, et al:
Radioprotective effects of quercetin and ethanolic extract of
propolis in gamma-irradiated mice. Arh Hig Rada Toksikol.
60:129–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sezer A, Usta U, Kozak Z and Yagci MA: The
effect of a flavonoid fractions diosmin + hesperidin on
radiation-induced acute proctitis in a rat model. J Cancer Res
Ther. 7:152–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
An JH, Kim J and Seong J: Redox signaling
by ionizing radiation in mouse liver. Ann NY Acad Sci. 1030:86–94.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cromheecke M, Konings AW, Szabo BG and
Hoekstra HJ: Liver tissue tolerance for irradiation: experimental
and clinical investigations. Hepatogastroenterology. 47:1732–4170.
2000.PubMed/NCBI
|
|
20
|
Alati T, Van Cleef M, Strom SC and Jirtle
RL: Radiation sensitivity of adult human parenchymal hepatocytes.
Radiat Res. 115:152–160. 1988. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pei RJ, Danbara N, Tsujita-Kyutoku M, et
al: Immunohistochemical profiles of Mallory body by a panel of
anti-cytokeratin antibodies. Med Electron Microsc. 37:114–118.
2004.PubMed/NCBI
|
|
22
|
Bhosle SM, Huilgol NG and Mishra KP:
Enhancement of radiation-induced oxidative stress and cytotoxicity
in tumor cells by ellagic acid. Clin Chim Acta. 359:89–100. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karran P: DNA double strand break repair
in mammalian cells. Curr Opin Genetics Dev. 10:144–150. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrade ER, Cruz IB, Andrade VV, et al:
Evaluation of the potential protective effects of ad libitum black
grape juice against liver oxidative damage in whole-body acute
X-irradiated rats. Food Chem Toxicol. 49:1026–1032. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meydan D, Gursel B, Bilgici B, Can B and
Ozbek N: Protective effect of lycopene against radiation-induced
hepatic toxicity in rats. J Int Med Res. 39:1239–1252. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukimoto M, Tamaishi N, Homma T and
Kojima S: Low-dose gamma-ray irradiation induced translocation of
Nrf2 into nuclear in mouse macrophague RAW264.7. J Radiat Res.
51:349–353. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McDonald JT, Kim K, Norris AJ, et al:
Ionizing radiation activates the Nrf2 antioxidant response. Cancer
Res. 70:8886–8895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y, Zhang L, Zhang H, et al: Exogenous
melatonin modulates apoptosis in the mouse brain induced by
high-LET carbon ion irradiation. J Pineal Res. 52:47–56. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung KH, Hong SW, Zheng HM, Lee DH and
Hong SS: Melatonin downregulates nuclear erythroid 2-related factor
2 and nuclear factor-kappaB during prevention of oxidative liver
injury in a dimethylnitrosamine model. J Pineal Res. 47:173–183.
2009. View Article : Google Scholar
|
|
30
|
Li W, Khor TO, Xu C, et al: Activation of
Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory
response and elicits apoptosis. Biochem Pharmacol. 76:1485–1489.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee OH, Jain AK, Papusha V and Jaiswal AK:
An auto-regulatory loop between stress sensors INrf2 and Nrf2
controls their cellular abundance. J Biol Chem. 282:36412–36420.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Milani P, Gagliardi S, Cova E and Cereda
C: SOD1 Transcriptional and Posttranscriptional Regulation and Its
Potential Implications in ALS. Neurol Res Int.
2011:4584272011.PubMed/NCBI
|
|
33
|
Park EY and Rho HM: The transcriptional
activation of the human copper/zinc superoxide dismutase gene by
2,3,7,8-tetrachlorodibenzo-p-dioxin through two different regulator
sites, the antioxidant responsive element and xenobiotic responsive
element. Mol Cell Biochem. 240:47–55. 2002. View Article : Google Scholar
|
|
34
|
Terakado N, Shintani S, Nakahara Y, et al:
Expression of Cu/Zn-SOD, Mn-SOD and GST-pi in oral cancer treated
with preoperative radiation therapy. Oncol Rep. 7:1113–1117.
2000.PubMed/NCBI
|
|
35
|
Vucic V, Isenovic ER, Adzic M, Ruzdijic S
and Radojcic MB: Effects of gamma-radiation on cell growth, cycle
arrest, death, and superoxide dismutase expression by DU 145 human
prostate cancer cells. Braz J Med Biol Res. 39:227–236. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boots AW, Bast A and Haenen GR: No role of
DT-diaphorase (NQO1) in the protection against oxidized quercetin.
FEBS Lett. 579:677–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orlowski RZ and Baldwin AS Jr: NF-kappaB
as a therapeutic target in cancer. Trends Mol Med. 8:385–389. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bravard A, Luccioni C, Moustacchi E and
Rigaud O: Contribution of antioxidant enzymes to the adaptative
response to ionizing radiation of human lymphoblasts. Int J Radiat
Biol. 75:639–645. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Crespo I, Garcia-Mediavilla MV, Almar M,
et al: Differential effects of dietary flavonoids on reactive
oxygen and nitrogen species generation and changes in antioxidant
enzyme expression induced by proinflammatory cytokines in Chang
Liver cells. Food Chem Toxicol. 46:1555–1569. 2008. View Article : Google Scholar
|
|
40
|
Kook D, Wolf AH, Yu AL, et al: The
protective effect of quercetin against oxidative stress in the
human RPE in vitro. Invest Ophthalmol Vis Sci. 49:1712–1720. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Duthie G and Morrice P: Antioxidant
capacity of flavonoids in hepatic microsomes is not reflected by
antioxidant effects in vivo. Oxid Med Cell Longev.
2012:1651272012.PubMed/NCBI
|
|
42
|
Nakamura Y, Ishimitsu S and Tonogai Y:
Effects of quercetin and rutin on serum and hepatic lipid
concentrations, fecal steroid excretion and serum antioxidant
properties. J Health Sci. 46:229–240. 2000. View Article : Google Scholar
|
|
43
|
Wang XJ, Sun Z, Villeneuve NF, et al: Nrf2
enhances resistance of cancer cells to chemotherapeutic drugs, the
dark side of Nrf2. Carcinogenesis. 29:1235–1243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin C, Yu Y, Zhao HG, et al: Combination
of quercetin with radiotherapy enhances tumor radiosensitivity in
vitro and in vivo. Radiother Oncol. 104:395–400. 2012. View Article : Google Scholar : PubMed/NCBI
|