Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory
autoimmune disease, characterized by synovial hyperplasia and local
invasion of the bone and cartilage (1). Defective apoptosis of the RA
fibroblast-like synoviocytes (FLS) is an important contributing
factor of synovial hyperplasia (2). Previous research has indicated that
hyperplastic RA FLS possess unique characteristics in RA
pathogenesis, and have a key role in the development of sustained
inflammation and angiogenesis in arthritic joints (3). Numerous studies have demonstrated
that normal apoptosis is rare in RA FLS, due to the aberrant
expression levels of apoptosis-associated genes, including B-cell
lymphoma 2 (Bcl-2) (4–6), Fas (7,8) and
various oncogenes (9,10). Therefore, impaired apoptosis may
have an important role in the pathogenesis of RA and restoration of
FLS apoptosis may improve the RA joint destruction.
Salvia miltiorrhiza (SM) is a well-known herb
used in traditional Chinese medicine. It has been successfully used
in the treatment and prevention of aging diseases, such as
cardiovascular and cerebrovascular diseases, and cancers, and has
previously been considered as a ‘Super grade’ drug, according to
Shen-Nung’s Pen-Ts’ao (11).
Currently, SM is approved and used in Japan, the United States and
some European countries (11–13).
Products derived from SM include a dripping pill and an injection
(SMI); these formulations have been developed and used clinically
in China and other Asian countries. The pharmacologically active
components of SM include the lipophilic diterpenoid tanshinones and
water-soluble phenolic acids (11). The active ingredients of SMI
include tanshinol/danshensu, salvianolic acid B, tanshinone,
dihydrotanshinone, ursolic acid and cryptotanshinone. Danshensu and
salvianolic acid B have the highest concentrations within SMI,
accounting for >1 and 3–5% of the total dry weight, respectively
(14,15). Previous reports have demonstrated
that SMI-derived compounds can inhibit the growth of tumor cells,
by inducing apoptosis (16–18).
RA FLS display certain unique features that are similar to
transformed cells. Observations of a tumor-like phenotype were
initially made by Fassbender and Simmling-Annefeld (19) in 1983, who noted the distinctive
morphological features of RA FLS, including: Abundant cytoplasm,
large pale nuclei with several prominent nucleoli and a dense rough
endoplasmatic reticulum. Whether SMI can induce RA FLS apoptosis
remains unknown. Furthermore, it has been reported that nuclear
factor-κB (NF-κB), which regulates the expression of apoptotic
genes, including the Bcl family (20) and Fas (21), is activated in RA FLS, where it has
an anti-apoptotic role (22,23).
Therefore, the aim of the present study was to examine the effects
of SMI on the proliferation and apoptosis of cultured human RA FLS,
and to investigate the underlying mechanisms.
Culture medium has an important role in the growth
of RA FLS. Serum is added to provide mitogenic peptide growth
factors, attachment factors and undefined factors for cell growth,
division and differentiation maintenance. Numerous types of basal
culture media supplemented with animal serum have been widely used
to provide basic nutrients for human RA FLS. However, the use of
animal serum does not reflect the internal environment of the human
body, because the cells and the serum are isolated from different
species. The culturing of human cells with human serum avoids the
interference of allogeneic serum, and ensures that the experimental
conditions are similar to the natural environment. Therefore, in
the present study, foetal bovine serum (FBS) was replaced with
serum from patients with RA (RPS) and normal human serum (NHS), in
the culturing of RA FLS. Furthermore, a concentration of SM was
added, which had previously been proved to induce apoptosis of RA
FLS cultured with FBS, to investigate the mechanism of inhibition
of RA FLS proliferation by SMI.
Materials and methods
Cell culture and treatment
Synoviocytes were isolated from four patients (three
female, one male) with long-standing advanced RA, as confirmed by
the 1987 American College of Rheumatology diagnostic criteria
(24). The patients were sourced
from the Affiliated Hospital of North Sichuan Medical College
(Sichuan, China) and their ages ranged between 41 and 72 years old,
with an average age of 60 years.
Human serum was obtained from the Department of
Clinical Laboratory, Affiliated Hospital of North Sichuan Medical
College. The NHS was isolated from a patient undergoing a health
examination, whose laboratory results were normal and excluded
diabetes, cardiovascular disease and autoimmune disease. Approval
for the present study was obtained from the Local Ethics Committee
of the Affiliated Hospital of North Sichuan Medical College and
written informed consent was obtained from the patients.
The synoviocytes were isolated from the synovial
fluid obtained from the patients with RA, by knee joint aspiration.
The synovial fluid was centrifuged at 840 × g, 4°C and then washed
with cold phosphate-buffered saline (PBS). Cell aggregates were
removed using a 100 μm mesh nylon mesh filter. The isolated cells
were then resuspended in Dulbecco’s modified Eagle’s medium (DMEM)
with high glucose (HyClone Laboratories, Inc., Logan, UT, USA),
supplemented with 10% FBS (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd, Hangzhou, China), 100 U/ml penicillin and 100
μg/ml streptomycin. Following an overnight culture, the
non-adherent cells were removed and synovial fibroblasts were
obtained from the adherent cells. The medium was refreshed every
two days, until the cells approached confluence by seven days. The
synovial cells used for the following experiments were obtained
from the third to sixth passages.
Cells cultured with human serum
The stable grown cells were washed twice with cold
PBS and trypsinized. The cells (5×103/l,
5×104/l or 5×105/l) were resuspended in DMEM
supplemented with 10% NHS, RPS or FBS, as a control. The cells were
then cultured in six- or 96-well plastic culture plates, or a 25
cm2 plastic culture flask, in an automatically
controlled incubator with a humidified atmosphere of 5%
CO2, at 37°C for 24 h. According to previous results,
0.39 mg/ml of freeze-dried SMI (Batch no: 090313; Harbin
Pharmaceutical Group Co., Ltd., Harbin, China), was used to
stimulate the cells for a further 24 h. The morphology of the RA
FLS was confirmed using an Olympus BX51 optical microscope (Olympus
Corporation, Tokyo, Japan).
Cell density
MTT reagent (Amresco LLC Solon, OH, USA) was used to
measure the density of the cells. The cells were grown in 96-well
plates overnight and treated with different concentrations of SMI
(0, 0.195, 0.39, 0.78, 1.56 and 3.12 mg/ml). Following the 24 h
incubation the cells were centrifuged at 1,260 × g for 5 minutes,
the supernatant was removed and the cells were washed twice with
PBS. The labeling solution (20 μl) was then added to each well. The
solubilization solution (200 μl) was added to dissolve the purple
crystals produced by the MTT substrate, following a 4 h incubation
in a CO2 incubator. The absorbance was measured at 450
nm, using a microplate reader (Bio-Rad Laboratories, Hercules, CA,
USA).
Apoptosis analysis
The synoviocytes were cultured in 25 mm plastomer
culture flasks. Following a 24 h incubation, different
concentrations of SMI were added (0. 0.195 and 0.39 mg/ml) and the
cells were cultured for a further 24 h, at 37°C. The cells were
trypsinized and collected for apoptosis detection, using an Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Keygen
Biotech, Nanjing, China). Briefly, the cells were washed twice with
cold PBS and resuspended in 500 ml of binding buffer (10 mM
HEPES/NaOH pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), at a
concentration of 1×106 cells/ml. Following the addition
of 5 μl Annexin V-FITC solution and propidium iodide (PI) (1
mg/ml), the cells were incubated for 15 min at room temperature and
analyzed using a flow cytometer (Beckman Coulter, Brea, CA, USA)
(25).
Reverse transcription (RT) and
quantitative polymerase chain reaction (qPCR)
RT and qPCR were conducted as described by previous
methods (26,27). Briefly, the RA FLS were cultured in
six-well plates with FBS (5×105 cells/well). Following a
24 h incubation, SMI was added at different concentrations (0,
0.195, 0.39 mg/ml) and the cells were cultured at 37°C, for an
additional 24 h. Total RNA was extracted using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) and was
reverse transcribed into cDNA using the Improm-II Reverse
Transcription system (Promega Corporation, Madison, WI, USA). The
RT and qPCR analyses were performed using the 2.5× Taq PCR
MasterMix and 2× Taq real-PCR MasterMix (Tiangen Biotech Co., Ltd.,
Beijing, China), respectively. The following primer pairs were
used: Human Bcl-2 forward, 5′-TCC CAT CAA TCT TCA GCA CTC T-3′
reverse, 5′-TCG ATC TGG AAA TCC TCC TAA T-3′; human Bcl-2
associated X protein (Bax) forward, 5′-TGT CGC CCT TTT CTA CTT
TGC-3′ reverse, 5′-GCT CCC GGA GGA AGT CCA AT-3′; human Fas
forward, 5′-CCT CCC ATC CTC CTG ACC ACC G-3′ reverse, 5′-CTG GTT
GCC TTG GTA GGA TTG-3′; human Fas ligand (FasL) forward, 5′-CTG GTT
GCC TTG GTA GGA TTG-3′ reverse, 5′-TCA CTC GTA AAC CGC TTC CCT
C-3′; and human β-actin forward, 5′-AGC ACT GTG TTG GCG TAC AG-3′
and reverse, 5′-TCC CTG GAG AAG AGC TAC GA-3′. The densitometry of
the PCR electrophoresis bands for Bcl-2, Bax and Fas mRNA from the
RT reaction were analyzed using Quantity One software (Bio-Rad,
Hercules, CA, USA) and it was determined that the levels of Bcl-2
mRNA changed significantly after SMI stimulation, but those of Bax
and Fas mRNA did not changed significantly (data were not shown).
Hence, qPCR was used to detect the expression levels of Bax and Fas
mRNA. qPCR was performed on 2 μl cDNA, 0.25 μl of each primer (10
pmol/μl) and 10 μl 2× SYBR Green Master mix (Tiangen Biotech Co.,
Ltd.) to obtain a final reaction volume of 20 μl. Triplicate
amplification reactions were performed with an ABI 7900TH Sequence
Detection system (Applied Biosystems, Foster City, CA, USA). The
primers’ sequences were as described previously. The data was
analyzed using the cycle threshold (Ct) 2−ΔΔCt method
(28). Furthermore, based on the
results of Bax mRNA expression in the cells cultured with FBS, the
level of Bax mRNA in the cells cultured with RPS and NHS was
detected by qPCR as described above.
Western blot analysis
A total of 50 μg of protein was separated using a
12% SDS containing PAGE and transferred onto polyvinylidene
fluoride membranes (GE Healthcare Life Sciences, Chalfont, UK). The
membranes were blocked with 5% non-fat milk and then incubated with
the following primary antibodies: Rabbit-anti-p65 or -p50 (1:200
dilution; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
overnight at 4°C, or mouse-anti-β-actin (1:1,000 dilution; Santa
Cruz biotechnology Inc.) for 1 h at room temperature. The membranes
were then incubated with a horseradish peroxidase-conjugated rabbit
secondary antibody (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at a 1:2,000 dilution for 1 h. The blots were developed using
electrochemical luminescence (Engreen Biosystem Co. Ltd., Beijing,
China) and an X-ray technique. The densitometry of the bands of
western blot were analyzed using Quantity One software (Bio-Rad,
Hercules, CA, USA)
Determination of TNF-α
The secretion levels of TNF-α in the cell
supernatants were determined using an ELISA (BD Biosciences,
Franklin Lakes, NJ, USA), according to the manufacturer’s
instructions.
Statistical analyses
Statistical significance was assessed by one-way
analysis of variance, or a two-sided Student’s t-test using SPSS
version 17.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
SMI promotes apoptosis of RA FLS cultured
in vitro
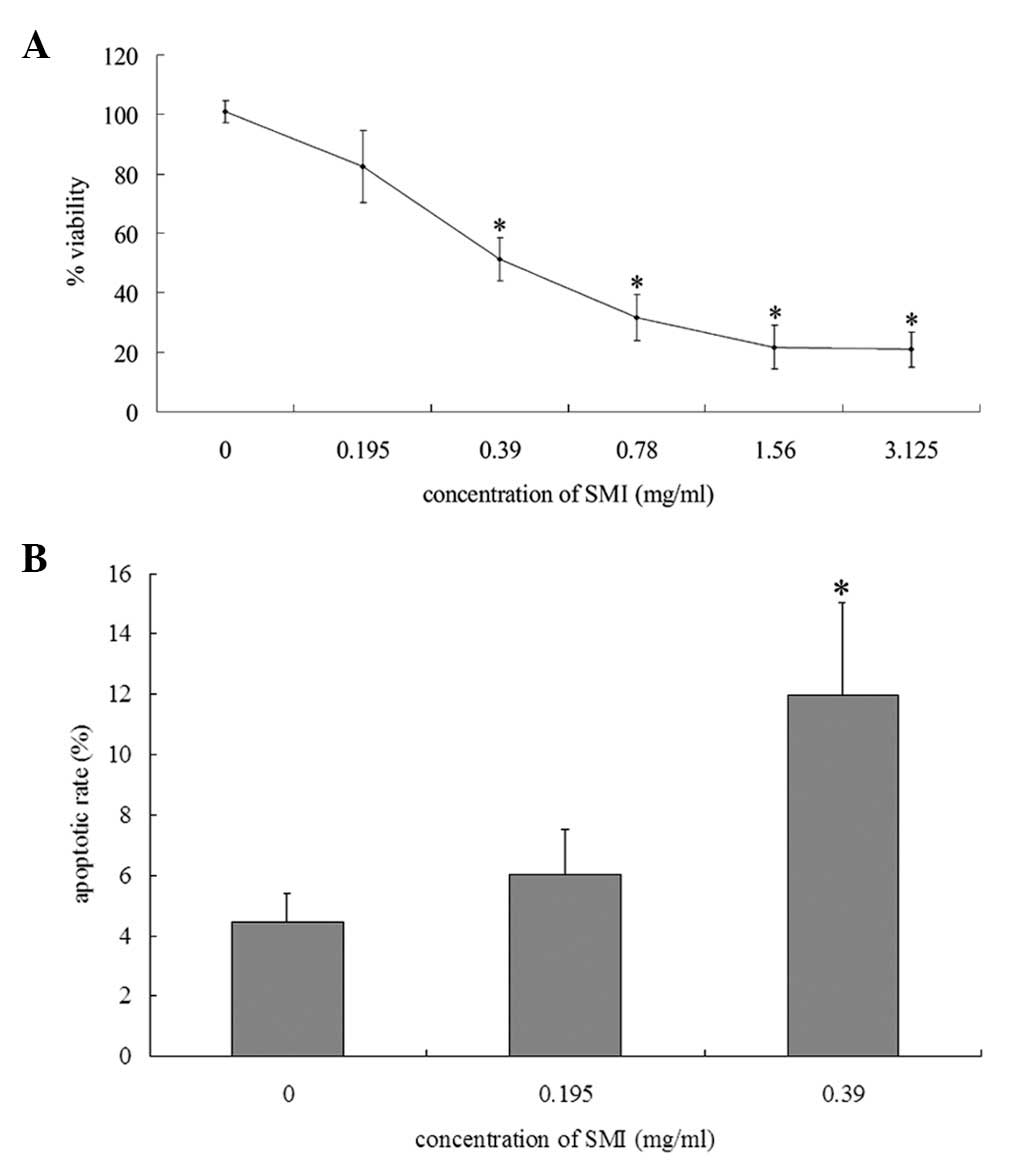
To determine whether SMI altered the proliferation
of RA FLS, an MTT assay was performed to assess the viability of
the cells. SMI significantly decreased the viability of the RA FLS
cultured with FBS, in a dose-dependent manner (Fig. 1A). To investigate whether the
reduction in viability was due to the induction of apoptosis,
Annexin V-FITC/PI double staining was used to assess the apoptotic
rate. Treatment with 0.39 mg/ml SMI resulted in a significant
increase in the rate of apoptosis of the RA FLS cultured with FBS,
as compared with the cells cultured in the absence of SMI (Fig. 1B). These results indicate that SMI
induced apoptosis of the RA FLS.
SMI induces RA FLS apoptosis through
NF-κB activation
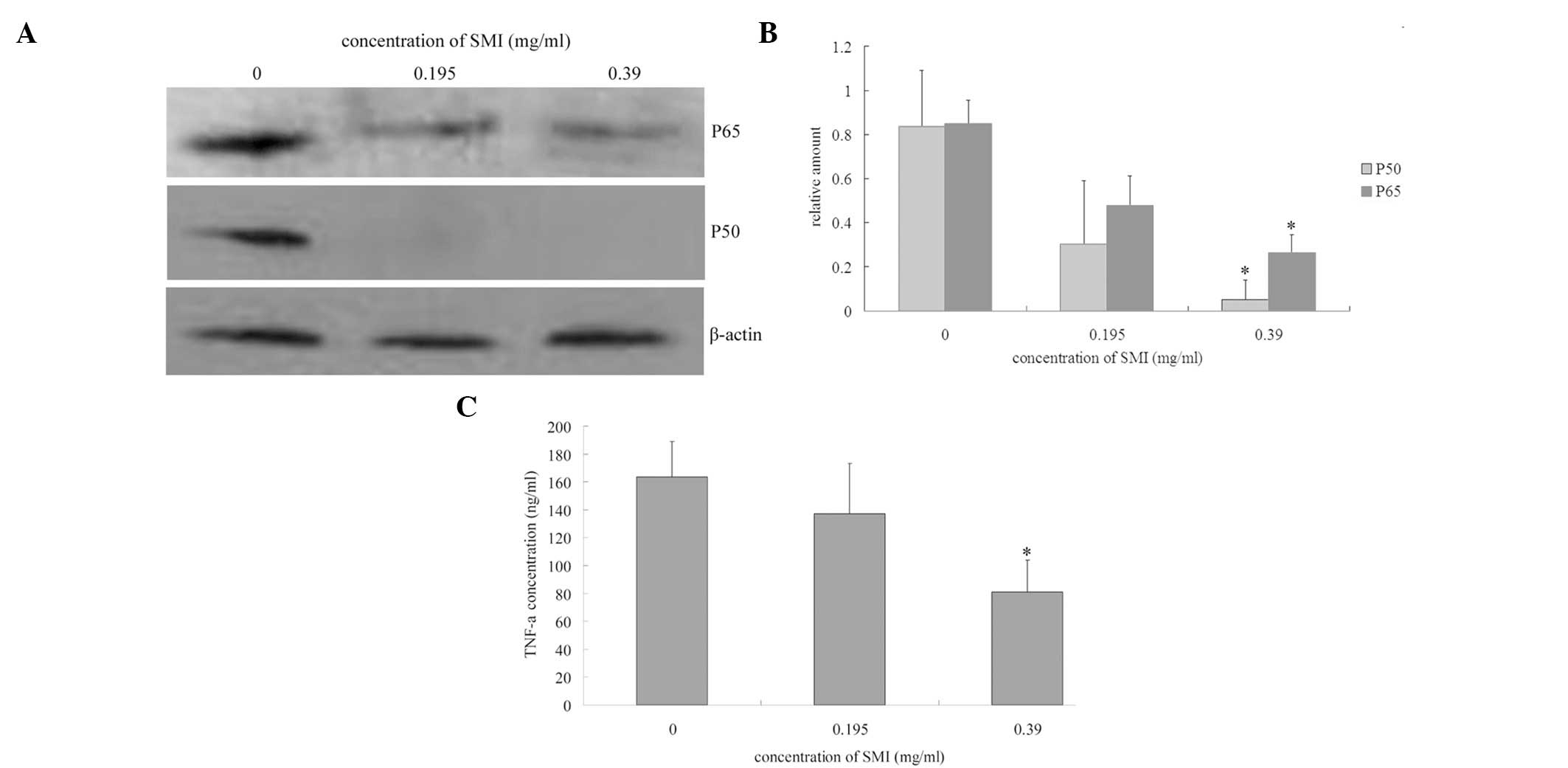
NF-κB activation has previously been reported to
initiate both cell survival and death pathways (28). Therefore the expression levels of
the NF-κB subunits p65 and p50, were assessed in the nucleus of the
RA FLS by western blot analysis. SMI decreased the p65 and p50
protein expression levels in the RA FLS nucleus extracts (Fig. 2A and B). As NF-κB activation has
been reported to stimulate TNF-α production (29–31),
the levels of TNF-α in the cell supernatants were also analyzed, by
ELISA. The incubation of the RA FLS with 0.39 mg/ml SMI for 24 h
reduced TNF-α production (Fig.
2C). To further explore whether the inhibition of NF-κB
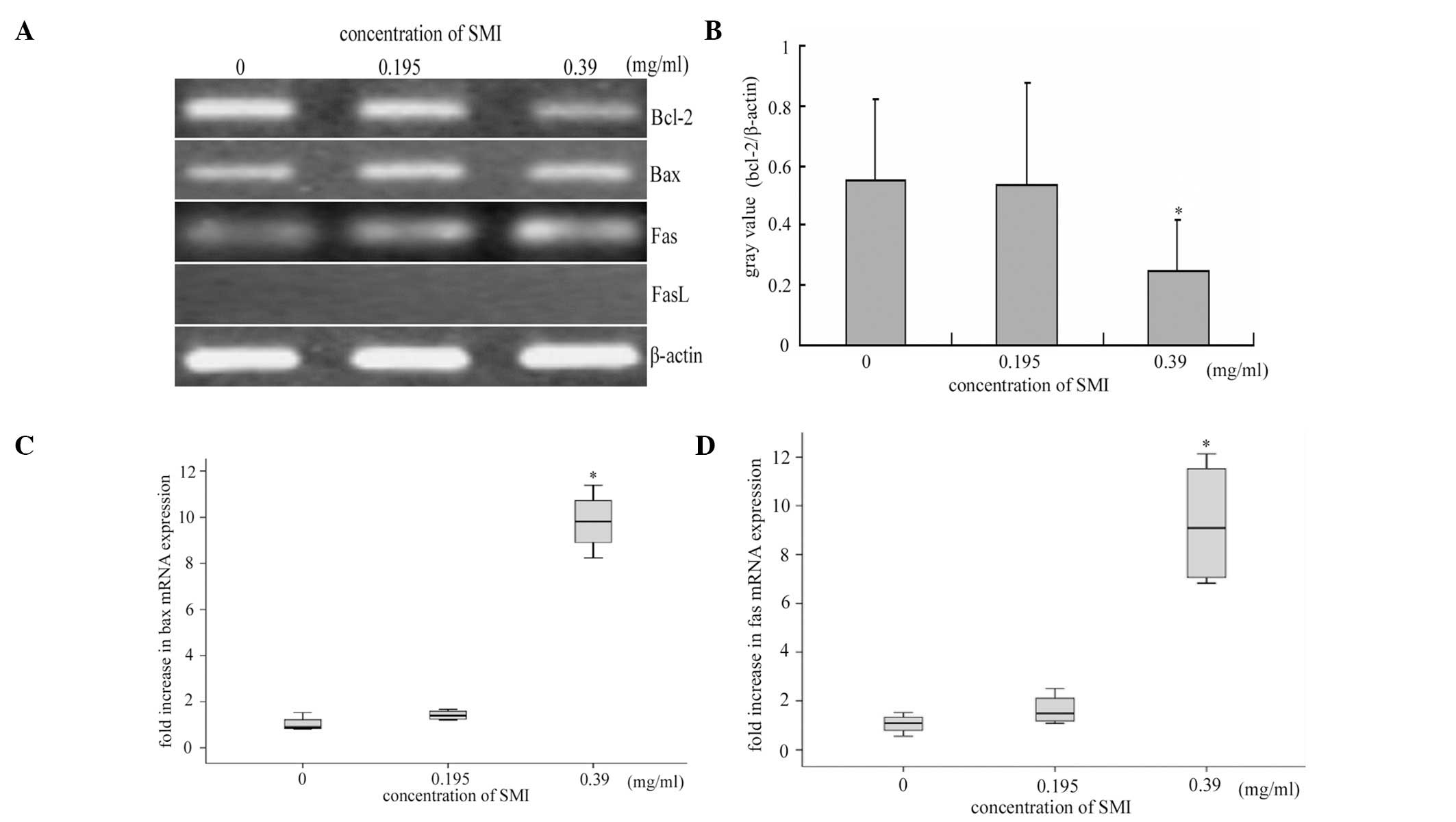
activation resulted in altered expression levels of
apoptosis-related genes, RT and qPCR analyses were used to
determine the mRNA expression levels of Bcl-2, Bax, Fas and Fas L.
Treatment with SMI triggered a significant downregulation of Bcl-2
(Fig. 3A and B), and upregulation
of Bax (Fig. 3C) mRNA expression
levels in the RA FLS cultured with FBS, as compared with the cells
cultured in the absence of SMI. FasL expression was not detected in
the RA FLS, neither in the presence nor absence of SMI, however Fas
mRNA expression levels were significantly increased following SMI
stimulation (Fig. 3A and D). These
results demonstrate that the inhibition of NF-κB activation by SMI,
is crucial for SMI-induced RA FLS apoptosis.
Serum source is associated with
SMI-restored apoptosis of RA FLS
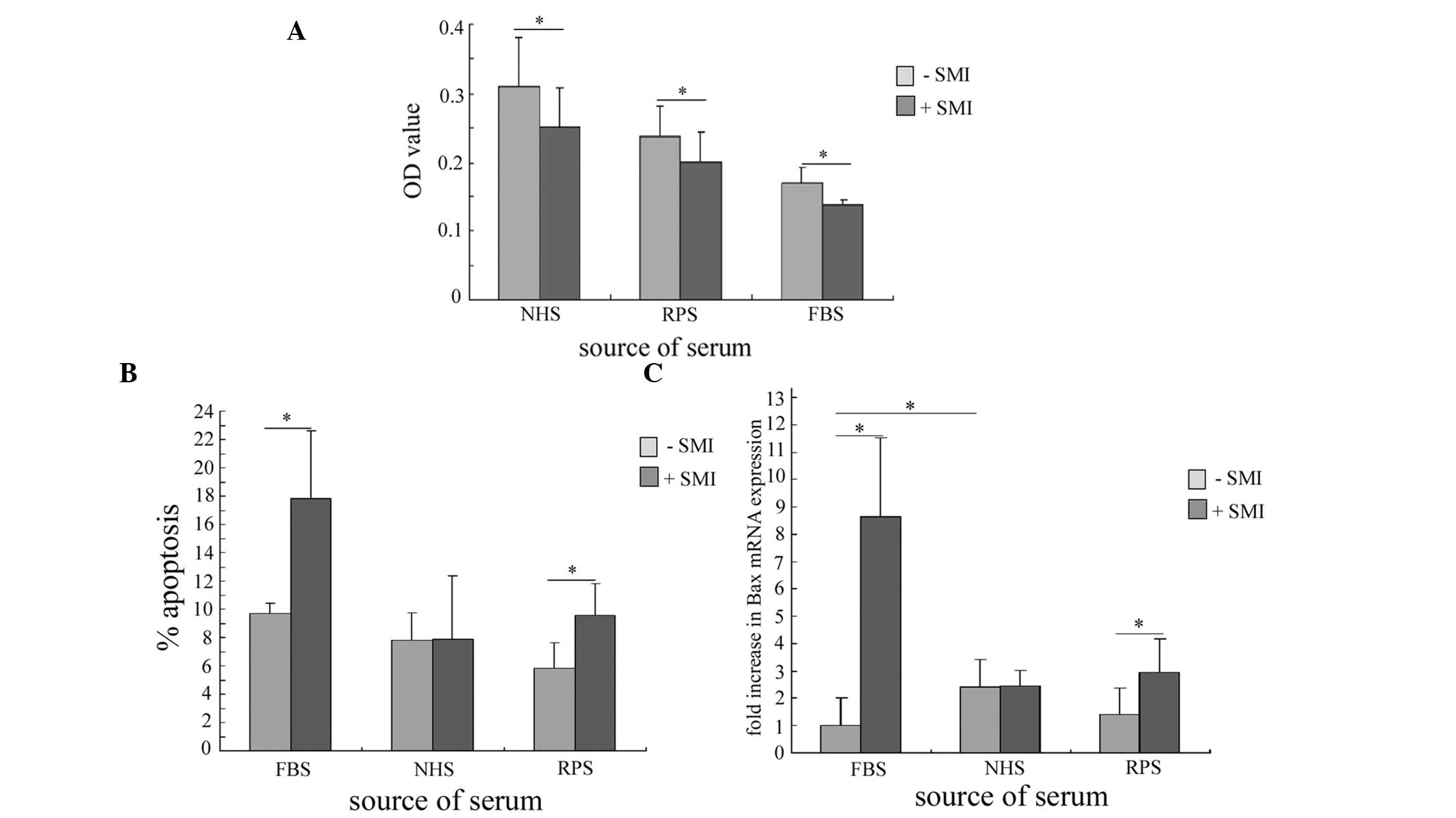
To further investigate whether SMI altered the
serum-sensitivity of RA FLS, the effects of several serums were
assessed on cell viability, in the presence and absence of SMI. A
concentration of SMI (0.39 mg/ml) was selected that had previously
resulted in ~50% inhibition of cell viability, as determined by MTT
assay (Fig. 1A). The addition of
SMI significantly decreased the viability of the RA FLS cultured
with all of the serums tested: NHS, RPS and FBS, as compared with
the RA FLS cultured with serum alone (Fig. 4A). This finding indicates that SMI
can inhibit the proliferation of RA FLS and sensitize the cells
towards the serum they are cultured with.
To gain insight into the involvement of the serum in
the SMI-mediated apoptosis of RA FLS, the apoptotic rate of the RA
FLS cultured with the differently sourced serums was determined, in
the presence or absence of SMI. The RPS group had the lowest
apoptotic rate in the absence of SMI stimulation. However, the
addition of SMI significantly increased the apoptotic rate of the
RA FLS cultured with RPS and FBS, and the apoptotic rate of the
RPS-cultured cells was restored to the NHS-cultured level. SMI
could not induce apoptosis of the RA FLS cultured with NHS
(Fig. 4B).
SMI-induced NF-κB activation was shown to upregulate
the expression levels of Bax, which contributed to FBS-cultured RA
FLS apoptosis. Therefore, the expression levels of Bax in the RA
FLS, cultured with NHS or RPS were determined by qPCR. SMI did not
alter the expression levels of Bax in the RA FLS cultured with NHS;
however the Bax expression levels were significantly upregulated in
the RPS-cultured SMI-stimulated cells, as compared with the cells
cultured in the absence of SMI (Fig.
4C). Following SMI stimulation, the Bax expression levels in
the RPS-cultured RA FLS were restored to almost the level observed
in the NHS-stimulated cells. These results suggest that SMI
restored apoptosis of the RA FLS cultured with RPS to a normal
level.
Discussion
RA FLS have been identified as being responsible for
the invasion and destruction of cartilage and bone (32,33).
Furthermore, RA FLS have shown evidence of transformation,
indicated by excessive proliferation, loss of contact inhibition
and increased migration (34).
Therefore, the inhibition of FLS proliferation may provide a
potential treatment strategy for RA. The pharmacologically active
compounds of SMI have been shown to significantly inhibit the
proliferation of cancer cells, including HepG cells (16), and head and neck squamous cell
carcinomas (17,18). The present study was the first, to
the best of our knowledge, to demonstrate that SMI significantly
inhibited the proliferation of human RA FLS, cultured with FBS, by
promoting apoptosis in a NF-κB-dependent manner. SMI stimulation
was shown to significantly inhibit the proliferation of RA FLS and
promote apoptosis. Furthermore, the addition of SMI inhibited the
activation of NF-κB and the secretion of TNF-α, resulting in a
significant downregulation of Bcl-2 and upregulation of Bax and Fas
mRNA expression levels. These findings are consistent with the
hypothesis that SMI triggers NF-κB-dependent apoptosis signaling
pathways in RA FLS.
The Bcl-2 family is essential for the maintenance of
mitochondrial homeostasis and cell density in FLS (5). SMI stimulation increased the mRNA
expression levels of the pro-apoptotic protein Bax, and decreased
the mRNA expression levels of the anti-apoptotic protein Bcl-2, in
RA FLS. These results indicate that SMI likely induced apoptosis
through modulating the balance of pro- and anti-apoptotic factors.
Rheumatoid synoviocytes also undergo Fas-mediated apoptosis
(23,35). The results of the present study
revealed that FasL expression was not detected under any of the
experimental conditions, which is consistent with the observations
of other studies (36), however
Fas expression levels were increased in response to SMI
stimulation. These results indicate that SMI may also have the
ability to interact with Fas, in order to promote RA FLS
apoptosis.
The present study is the first, to the best of our
knowledge, to demonstrate that the source of serum is important in
SMI-induced RA FLS apoptosis in vitro. The apoptotic rate of
the RA FLS was increased in the RPS-cultured cells in response to
SMI stimulation, however this SMI-induced increase was not observed
in the NHS-cultured cells. The Bax mRNA expression levels were
significantly upregulated by SMI stimulation (0.39 mg/ml) in the RA
FLS cultured with RPS, however there was no SMI-stimulated
difference in the RA FLS cultured with NHS. These results suggest
that: RA FLS can adequately grow and proliferate when cultured in
human serum. The proliferation of RA FLS cultured with NHS was
inhibited by 0.39 mg/ml SMI, however the apoptotic rate and Bax
mRNA expression levels were not significantly altered, indicating
that the inhibition of RA FLS proliferation, by SMI, may be due to
changes in ionic strength and other factors. Although the apoptotic
rate was not altered in the NHS-cultured RA FLS following SMI
stimulation, it was significantly increased in the RA FLS cultured
in RPS, indicating that SMI does have the ability to restore RA FLS
apoptosis. In addition, in the RPS group, the baseline apoptotic
rate of the RA FLS was lower than that of the cells cultured in NHS
and FBS in the absence of SMI. This is consistent with the
phenomenon that RA FLS apoptosis is rare in vivo. The
apoptotic rate increased following SMI stimulation, indicating that
SMI has the ability to promote apoptosis of RA FLS in vitro.
Therefore, it is feasible to culture primary cells with FBS in
vitro if patient serum is not available, whereas the use of NHS
in the culture medium would produce an opposite result since NHS
can promote apoptosis and inhibit drug activity. The finding that
SMI was capable of increasing apoptotic rate in the RA FLS cultured
with RPS, but not the RA FLS cultured with NHS, indicates that the
ability of SMI to restore RA FLS apoptosis may be associated with
the experimental environment. This function of SMI may due to
improved blood environment (7,8).
Finally, SMI restored the Bax mRNA expression levels in the RA FLS
cultured with RPS. These findings, together with the previous
results that SMI inhibited NF-κB activation, show that SMI has the
ability to restore the apoptosis of RA FLS through a
NF-κB-dependent pathway.
The findings of the present study demonstrate that
SMI inhibited RA FLS proliferation and induced RA FLS apoptosis,
providing a novel mechanism for the inhibitory effects of SMI on
RA. However, it remains unknown which active component of SMI is
responsible for inducing apoptosis of RA FLS. SMI consists of
tanshinol/danshensu, salvianolic acid, tanshinone,
dihydrotanshinone, ursolic acid, and cryptotanshinone. Numerous
studies have demonstrated that salvianolic acid (18, 37), and tanshinone (38,39)
have the ability to induce tumor cell apoptosis. Although there has
been no previous evidence suggesting that salvianolic acid and
tanshinone are capable of facilitating RA FLS apoptosis, it has
been noted that RA FLS have a tumor-like phenotype (19). Therefore, salvianolic acid and
tanshinone may be the possible substances that induce apoptosis of
RA FLS. Furthermore, the target in RA FLS which may interact with
the active components of SMI, remains to be elucidated. Previous
studies have determined that epidermal growth factor receptor
(EGFR) (40) and matrix
metalloproteinase-9 (MMP-9) (41)
are capable of interacting with salvianolic acid B. Yamane et
al (42) found that there was
no significant difference in the expression levels of EGFR in RA
FLS, as compared with normal and osteoarthritis (OA) FLS; however
amphiregulin, a member of the EGF family that can connect with
EGFR, was significantly increased in RA FLS. MMPs are a family of
zinc neutral endopeptidases, which can hydrolyze FasL into soluble
FasL (43). Previous research has
shown that MMP-9 is significantly increased in the joint effusion
from patients with RA, as compared with those from patients with OA
(44). In addition, in the present study, SMI could not induce
apoptosis in the RA FLS cultured with NHS. It remains unknown
whether there are cytokines that may inhibit SMI-induced apoptosis
of RA FLS, which are present in NHS.
In conclusion, the findings of the present study
demonstrate that SMI exerts anti-proliferative effects against RA
FLS by promoting apoptosis through the induction of apoptotic
signaling pathways and restoring normal apoptotic function. These
results indicate that SMI may have potential therapeutic
implications in the treatment of RA.
Acknowledgements
The present study was supported by the Department of
Health of Sichuan (nos. 060085, 090141 and 100151) and the Key
Laboratory Open Fund of the North Sichuan Medical College (no. KFJJ
09-03).
References
|
1
|
Müller-Ladner U, Pap T, Gay RE, Neidhart M
and Gay S: Mechanisms of disease: the molecular and cellular basis
of joint destruction in rheumatoid arthritis. Nat Clin Pract
Rheumatol. 1:102–110. 2005. View Article : Google Scholar
|
|
2
|
Smith MD, Barg E, Weedon H, et al:
Microarchitecture and protective mechanisms in synovial tissue from
clinically and arthroscopically normal knee joints. Ann Rheum Dis.
62:303–307. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartok B and Firestein GS: Fibroblast-like
synoviocytes: key effector cells in rheumatoid arthritis. Immunol
Rev. 233:233–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsumoto S, Müller-Ladner U, Gay RE,
Nishioka K and Gay S: Ultrastructural demonstration of apoptosis,
Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J
Rheumatol. 23:1345–1352. 1996.PubMed/NCBI
|
|
5
|
Perlman H, Georganas C, Pagliari LJ, et
al: Bcl-2 expression in synovial fibroblasts is essential for
maintaining mitochondrial homeostasis and cell viability. J
Immunol. 164:5227–5235. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kammouni W, Wong K, Ma G, et al:
Regulation of apoptosis in fibroblast-like synoviocytes by the
hypoxia-induced Bcl-2 family member Bcl-2/adenovirus E1B 19-kd
protein-interacting protein 3. Arthritis Rheum. 56:2854–2863. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Imamura F, Aono H, Hasunuma T, et al:
Monoclonal expansion of synoviocytes in rheumatoid arthritis.
Arthritis Rheum. 41:1979–1986. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakajima T, Aono H, Hasunuma T, et al:
Apoptosis and functional Fas antigen in rheumatoid arthritis
synoviocytes. Arthritis Rheum. 38:485–491. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han Z, Boyle DL, Green DR and Firestein
GS: Dominant-negative p53 mutations in rheumatoid arthritis.
Arthritis Rheum. 42:1088–1092. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hashiramoto A, Sano H, Maekawa T, et al:
C-myc antisense oligeodeoxynucleotides can induce apoptosis and
down-regulate Fas expression in rheumatoid synoviocytes. Arthritis
Rheum. 42:954–962. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang X, Morris-Natschke SL and Lee KH: New
developments in the chemistry and biology of the bioactive
constituents of Tanshen. Med Res Rev. 27:133–148. 2007. View Article : Google Scholar
|
|
12
|
Tsai TH: Analytical approaches for
traditional Chinese medicines exhibiting antineoplastic activity. J
Chromatogr B Biomed Sci Appl. 764:27–48. 2001. View Article : Google Scholar
|
|
13
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics, and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu P, Luo GA, Zhao Z and Jiang ZH: Quality
assessment of radix Salviae miltiorrhizae. Chem Pharm Bull (Tokyo).
53:481–486. 2005. View Article : Google Scholar
|
|
15
|
Ling Z, Xiping Z, Fengmei Q, Ping Y and
Qihui C: Protective effects of Salvia miltiorrhizae on multiple
organs of rats with obstructive jaundice. Mediators Inflamm.
2009:6029352009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Shen HM and Ong CN: Salvia
miltiorrhiza inhibits cell growth and induces apoptosis in human
hepatoma HepG(2) cells. Cancer Lett. 153:85–93. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hao Y, Xie T, Korotcov A, et al:
Salvianolic acid B inhibits growth of head and neck squamous cell
carcinoma in vitro and in vivo via cyclooxygenase-2 and apoptotic
pathways. Internat J Cancer. 124:2200–2209. 2009. View Article : Google Scholar
|
|
18
|
Zhao Y, Hao Y, Ji H, et al: Combination
effects of salvianolic acid B with low-dose celecoxib on inhibition
of head and neck squamous cell carcinoma growth in vitro and in
vivo. Cancer Prev Res (Phila). 3:787–796. 2010. View Article : Google Scholar
|
|
19
|
Fassbender HG and Simmling-Annefeld M: The
potential aggressiveness of synovial tissue in rheumatoid
arthritis. J Pathol. 139:399–406. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim HJ, Hawke N and Baldwin AS: NF-kappaB
and IKK as therapeutic targets in cancer. Cell Death Differ.
13:738–747. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puppo F, Contini P, Ghio M and Indiveri F:
Soluble HLA class I molecules/CD8 ligation trigger apoptosis of
CD8+ cells by Fas/Fas-ligand interaction. Scientific World Journal.
2:421–423. 2002. View Article : Google Scholar
|
|
22
|
Kloesch B, Becker T, Dietersdorfer E,
Kiener H and Steiner G: Anti-inflammatory and apoptotic effects of
the polyphenol curcumin on human fibroblast-like synoviocytes. Int
Immunopharmacol. 15:400–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miagkov AV, Kovalenko DV, Brown CE, et al:
NF-kappaB activation provides the potential link between
inflammation and hyperplasia in the arthritic joint. Proc Natl Acad
Sci USA. 95:13859–13864. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang XY, Chen FH, Li J, et al: Mechanism
of fibroblast-like synoviocyte apoptosis induced by recombinant
human endostatin in rats with adjuvant arthritis. Anat Rec
(Hoboken). 291:1029–1037. 2008. View
Article : Google Scholar
|
|
25
|
Phillips R, Sarang M and Gibson N:
Semiquantitative measurement of gene-expression by rt-PCR - a
cautionary tale. Int J Oncol. 3:1097–1102. 1993.PubMed/NCBI
|
|
26
|
Lekanne Deprez RH, Fijnvandraat AC,
Ruijter JM and Moorman AF: Sensitivity and accuracy of quantitative
real-time polymerase chain reaction using SYBR green I depends on
cDNA synthesis conditions. Anal Biochem. 307:63–69. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C (T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar
|
|
28
|
Vince JE, Wong WW, Khan N, et al: IAP
antagonists target cIAP1 to induce TNFalpha-dependent apoptosis.
Cell. 131:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Varfolomeev E, Blankenship JW, Wayson SM,
et al: IAP antagonists induce autoubiquitination of c-IAPs,
NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell.
131:669–681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Petersen SL, Wang L, Yalcin-Chin A, et al:
Autocrine TNFalpha signaling renders human cancer cells susceptible
to Smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Müller-Ladner U, Kriegsmann J, Franklin
BN, et al: Synovial fibroblasts of patients with rheumatoid
arthritis attach to and invade normal human cartilage when
engrafted into SCID mice. Am J Pathol. 149:1607–1615.
1996.PubMed/NCBI
|
|
32
|
Karouzakis E, Neidhart M, Gay RE and Gay
S: Molecular and cellular basis of rheumatoid joint destruction.
Immunol Lett. 106:8–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pap T, Müller-Ladner U, Gay RE and Gay S:
Fibroblast biology. Role of synovial fibroblasts in the
pathogenesis of rheumatoid arthritis. Arthritis Res. 2:361–367.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Okamoto K, Asahara H, Kobayashi T, et al:
Induction of apoptosis in the rheumatoid synovium by Fas ligand
gene transfer. Gene Ther. 5:331–338. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Jiang M and Zheng D: Rheumatoid
arthritis synoviocyte hyperplasia and expression of fas and bcl-2
genes. Zhonghua Yi Xue Za Zhi. 78:175–178. 1998.(In Chinese).
|
|
36
|
Guo L, Lei CK and Shan M: Study of growth
inhibitory effect and apoptosis induced by different matches of
Tanshinone IIA and Salvianolic Acid B on Acute Promyelocytic
Leukemia cells (HL-60). Zhong Yao Cai. 31:1512–1514. 2008.(In
Chinese).
|
|
37
|
Tang C, Xue HL, Huang HB and Wang XG:
Tanshinone IIA inhibits constitutive STAT3 activation, suppresses
proliferation, and induces apoptosis in rat C6 glioma cells.
Neurosci Lett. 470:126–129. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hu H, Zhang Y, Huang F and Deng H:
Inhibition of proliferation and induction of apoptosis by
tanshinone II A in NCI-H460 cell. Zhong Yao Cai. 28:301–304.
2005.(In Chinese). PubMed/NCBI
|
|
39
|
Feng LX, Jing CJ, Tang KL, et al:
Clarifying the signal network of salvianolic acid B using proteomic
assay and bioinformatic analysis. Proteomics. 11:1473–1485. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jiang BH, Chen J, Xu L, et al: Salvianolic
acid B functioned as a competitive inhibitor of matrix
metalloproteinase-9 and efficiently prevented cardiac remodeling.
BMC Pharmaco. 10:102010. View Article : Google Scholar
|
|
41
|
Yamane S, Ishida S, Hanamoto Y, et al:
Proinflammatory role of amphiregulin, an epidermal growth factor
family member whose expression is augmented in rheumatoid arthritis
patients. J Inflam (Lond). 5:52008. View Article : Google Scholar
|
|
42
|
Tanaka M, Itai T, Adachi M and Nagata S:
Downregulation of Fas ligand by shedding. Nat Med. 4:31–36. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim KS, Choi HM, Lee YA, et al: Expression
levels and association of gelatinases MMP-2 and MMP-9 and
collagenases MMP-1 and MMP-13 with VEGF in synovial fluid of
patients with arthritis. Rheumatol Int. 31:543–547. 2011.
View Article : Google Scholar
|