Introduction
Prostate cancer has the second highest
cancer-related mortality rate in males (1). In the early stages of the disease,
the most effective treatment is surgical castration and hormonal
manipulation using gonadotropin-releasing hormone agonists or
androgen receptor antagonists. However, numerous prostate cancer
patients eventually experience recurrence and androgen
independence, which commonly results in accelerated disease
progression and fatality (2,3).
Thus, novel molecular targets for effective prostate cancer
treatment strategies and chemopreventative interventions are
urgently required.
MicroRNAs (miRNAs) are a novel class of endogenous,
small, non-coding, single-stranded RNAs that regulate gene
expression at the post-transcriptional level by targeting the 3′
untranslated region (3′UTR) of target mRNAs (4–7).
miRNAs have been implicated in a wide range of physiological
processes, including cell proliferation, apoptosis and cell
differentiation (8,9). Aberrant miRNA expression has been
demonstrated to be correlated with cell proliferation, invasion,
metastasis and prognosis in various types of cancer, including
prostate cancer (10,11). miR-429, a member of the miR-200
miRNA family, has been shown to be downregulated in gastric
carcinoma and may act as a tumor suppressor by targeting c-myc
(12). Other studies have reported
that miR-429 is upregulated in bladder and endometrial carcinoma
(13,14). In addition, higher expression
levels of miR-429 have been correlated with poor prognosis in
patients with serous ovarian carcinoma (15). These results suggest that miR-429
is correlated with tumorigenesis and may exert different effects in
distinct types of cancer. Whether miR-429 is involved in the
genesis and development of prostate cancer remains to be
elucidated.
In the present study, the expression levels of
miR-429 in two prostate cancer cell lines and six normal prostate
epithelial tissues were analyzed in order to explore the function
of miR-429 in the oncogenesis of prostate cancer.
Materials and methods
Cell culture
The IF11 and IA8 human prostate cancer cell lines
(American Type Culture Collection, Manassas, VA, USA) were cultured
in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin and
100 μg/ml streptomycin (Shanghai Genebase Gen-Tech Co., Ltd.,
Shanghai, China) at 37°C in a 5% CO2 incubator.
Tissue collection
Normal prostate epithelial tissues were obtained
from patients at the Department of Pathology of Tangdu Hospital
(Xi’an, China). All patients provided written informed consent for
the use of the excess pathological specimens for research purposes.
The use of human tissues in the present study was approved by the
Institutional Review Board of The Fourth Military Medical
University (Xi’an, China) and was conducted in accordance with the
International Guidelines for the Use of Human Tissues.
miRNA mimics and inhibitor
The hsa-miR-429 mimics, hsa-miR-429 inhibitor,
negative control miRNA mimics (Mock/mimics NC) and negative control
miRNA inhibitor (inhibitor NC) were chemically synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China).
Cell transfection
Prior to transfection, the logarithmically growing
cells were harvested and seeded in 6-well plates (4×105
cells per well), 24-well plates (1×105 cells per well)
or 96-well plates (1×104 cells per well). Following
overnight proliferation, RNA oligonucleotides (mimics NC/inhibitor
NC or hsa-miR-429 mimics/inhibitor) were transfected into the
adherent cells using Lipofectamine 2000 (Invitrogen) according to
the manufacturer’s instructions.
Luciferase assay
A luciferase reporter assay was conducted using
pMIR-REPORT™ vectors (Guangzhou RiboBio Co., Ltd., Guangzhou,
China). For recombinant vector construction, a full-length 3′UTR of
the p27Kip1 gene was cloned and inserted downstream of
the firefly luciferase gene in the pMIR-REPORT plasmid as follows:
cDNA from the IA8 cells was amplified by polymerase chain reaction
(PCR) using p27Kip1-3′UTR-wild-type (wt) primer for
p27Kip1-3′UTR-wt cloning. The PCR products were then
digested with MluI and SacI (Takara Bio, Inc., Shiga,
Japan), and inserted into the multiple cloning site of the
pMIR-REPORT Luciferase vector (Ambion®; Thermo Fisher
Scientific, Waltham, MA, USA). The recombinant vector was
designated as pMIR-p27Kip1-3′UTR-wt. Using this as a
template, the pMIR-p27Kip1-3′UTR-mutant (mut) plasmid,
which carried the mutated p27Kip1 3′UTR sequence in the
complementary site for the seed region of miRNA-429, was generated
by overlap PCR using p27Kip1-3′UTR-mut-1 and
p27Kip1-3′UTR-mut-2 primers. The primers used in the PCR
are shown in Table I. The cells
were transiently cotransfected with miR-429 mimics or miR-NC mimics
and the
pMIR-p27Kip1-3′UTR-wt/pMIR-p27Kip1-3′UTR-mut
vector. Luciferase activity was measured 48 h after transfection
with a Dual-Luciferase assay kit (Promega Corporation, Madison, WI,
USA) according to the manufacturer’s instructions.
 | Table IPrimer sequences used in qPCR and
site-directed mutagenesis cloning. |
Table I
Primer sequences used in qPCR and
site-directed mutagenesis cloning.
| Primer | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
|
p27kip1 |
ACCCAAAGACTGATCCGTC |
TTGGGGAACCGTCTGAAAC |
| β-actin |
CAGAAGGAGATTACTGCTCTGGCT |
TACTCCTGCTTGCTGATCCACATC |
|
p27kip1-3′UTR-wt |
GCACGCGTACAGCTCGAATTAATAA |
GCGAGCTCACAATAATTGGCATC |
|
p27kip1-3′UTR-mut-1 |
GCACGCGTACAGCTCGAATTAATAA |
CAATGATTATGAGTTTAAAG |
|
p27kip1-3′UTR-mut-2 |
CTTTAAACTCATAATCATTG |
GCGAGCTCACAATAATTGGCATC |
RNA isolation and quantitative
(q)PCR
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. Total
RNA (2 μg) from each sample was used for cDNA synthesis using RT
primers for miR-429 and U6 small nuclear RNA (Guangzhou Ribobio
Co., Ltd). qPCR was performed using the SYBRII Premix Ex Taq™
(Takara Bio, Inc., Shiga, Japan). The amplification was performed
under the following thermal program: Initial denaturation (95°C, 20
sec), 40 cycles of 95°C for 10 sec, 60°C for 20 secs and 70°C for
10 sec. The qPCR primer sets for miRNA-429 and U6 small nuclear
were purchased from Guangzhou Ribobio Co., Ltd. miR-429 and U6
small nuclear RNA were quantified according to a standard curve and
this was performed in triplicate. U6 small nuclear RNA was used for
normalization. The relative expression levels of miRNA-429 were
calculated using the following equation: Copies miR-429/copies U6.
The quantitative analysis of the change in expression levels was
calculated by qPCR analysis software (Bio-Rad CFX manager 2.0;
Bio-Rad, Hercules, CA, USA).
Western blotting
Total proteins were isolated 24 h after
transfection. The protein concentrations were determined using a
Bio-Rad protein assay kit (Bio-Rad). Equal quantities of total
protein (20 μg) were separated on a 12% SDS-polyacrylamide gel,
then transferred to a polyvinylidene membrane (Millipore,
Billerica, MA, USA). Subsequent to blocking, the membrane was
incubated with mouse monoclonal anti-p27Kip1 antibody
(1:500; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or
rabbit polyclonal anti-β-actin antibody (1:1,000; Bioss
Biotechnology, China) followed by incubation with polyclonal goat
anti-mouse or polyclonal goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.). Signals were determined with a
chemiluminescence detection kit (NEN™ Life Science Products, Inc.,
Boston, MA, USA).
Cell proliferation and colony formation
assays
The cells were seeded in 96-well plates at
~1×104 cells/well and cultured in growth medium. At 0,
24, 48 and 72 h after miR-429 mimic/inhibitor transfection, the
effect of miR-429 overexpression/knockdown on cell viability was
determined by an MTT assay. Each experiment was performed in
triplicate. The absorbance value of each well was measured with a
microplate spectrophotometer (Molecular Devices, Sunnyvale, CA,
USA) at 570 nm. All proliferation assays were repeated as
independent experiments at least three times. For the colony
formation assay, the transfected cells and control cells were
plated on 10-cm plates (500 cells/plate), respectively, cultured
for another 14 days, fixed with 10% formaldehyde for 5 min, stained
with 1.0% crystal violet for 30 sec and counted without
microscopy.
Flow cytometric analysis
The cells were harvested by trypsinization, washed
with ice-cold phosphate-buffered saline, fixed in 75% ice-cold
ethanol and stained with propidium iodide (10 mg/ml; 15 min;
Invitrogen). A total of 2×104 cells were analyzed with a
FACSCalibur Flow Cytometer (Becton-Dickinson, Franklin Lakes, NJ,
USA). The experiments were performed in triplicate.
Statistical analysis
All experiments were repeated at least three times
and data are expressed as the mean ± standard deviation. The
statistical significance between groups was determined using
Student’s t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
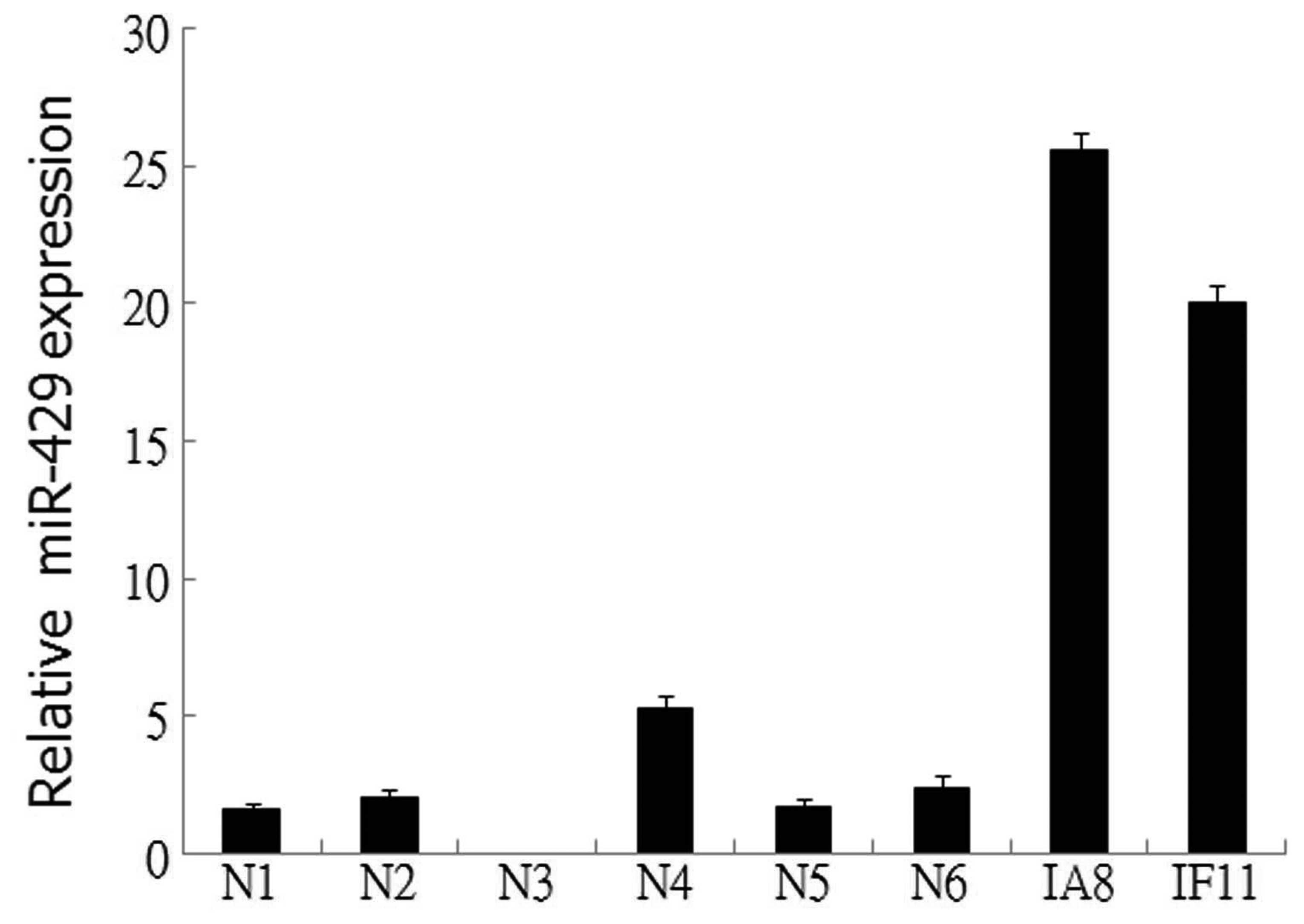
miR-429 is upregulated in prostate cancer
cell lines
The expression levels of miR-429 were examined in
two prostate cancer cell lines and six normal prostate epithelial
tissues by qPCR. The results revealed that miR-429 expression was
upregulated in the IF11 and IA8 prostate cancer cell lines,
compared with the normal prostate epithelial tissue (Fig. 1), indicating a potential role for
miR-429 in the tumorigenesis of prostate cancer.
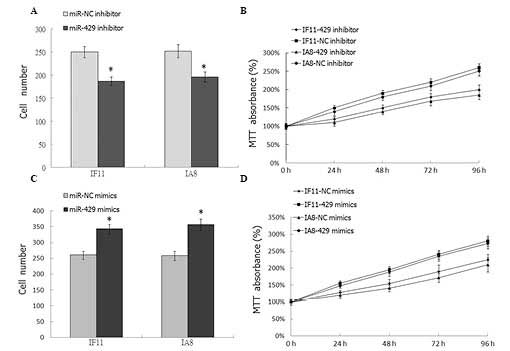
Downregulation of miR-429 inhibits the
proliferation of prostate cancer cells
In order to investigate the effect of miR-429 on
prostate cancer cell proliferation, the has-miR-429 inhibitor was
transfected into the IF11 and IA8 prostate cancer cells. Colony
formation assays and MTT were employed to analyze cell
proliferation. As shown in Fig. 2A and
B, downregulation of miR-429 in IF11 and IA8 cells
significantly inhibited cell proliferation (P<0.05), compared
with the NC transfection. To further demonstrate the effect of
miR-429 on cell proliferation, has-miR-429 mimics was transfected
into IF11 and IA8 prostate cancer cells; compared with the mock
group, the proliferation of tumor cells in the miR-429 mimics
transfected group was significantly increased (P<0.05; Fig. 2C and D).
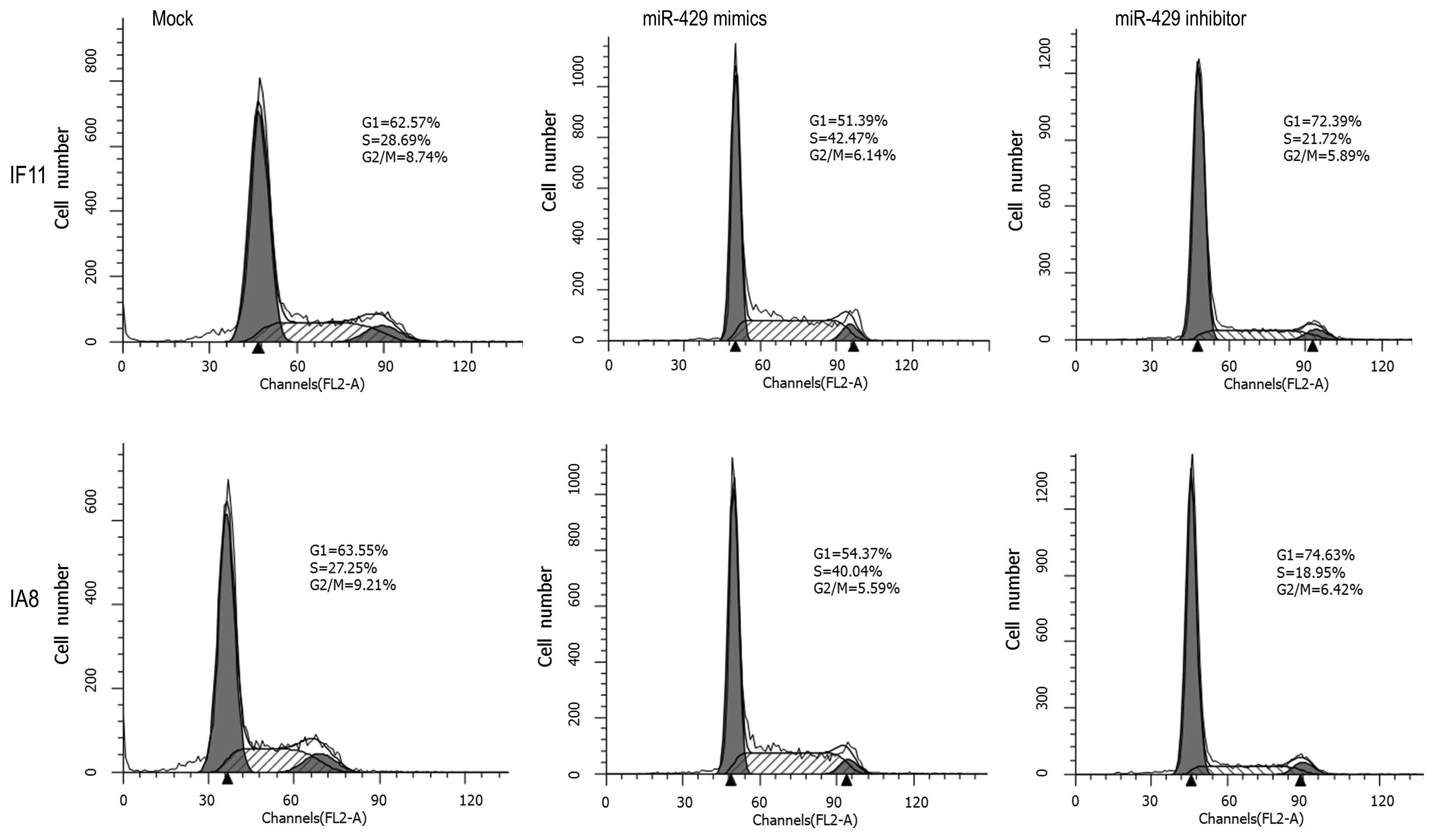
Downregulation of miR-429 arrests IF11
and IA8 cell division in the G1 phase
An important characteristic of tumor cells is the
increased proliferative capability, which commonly results from
impaired regulation of the cell cycle. Thus, the effect of miR-429
on the prostate cancer cell cycle was investigated using flow
cytometry. As shown in Fig. 3,
miR-429 mimic-transfected IF11 and IA8 cells exhibited lower
percentages of cells in the G1 phase (IF11, 51.39%; IA8, 54.37%)
and increased percentages of cells in the S (IF11, 42.47%; IA8,
40.04%) and G2/M phases (IF11, 6.14%; IA8, 5.59%), compared with
the mock control group cells (G1: IF11, 62.57%; IA8, 63.55%; S:
IF11, 28.69%; IA8, 27.25%; and G2/M: IF11, 8.74%; IA8, 9.21%).
However, the miR-429 inhibitor-transfected IF11 and IA8 cells
exhibited higher percentages of cells in the G1 phase (IF11,
72.39%; IA8, 74.63%) and reduced percentages of cells in the S
(IF11, 21.72%; IA8, 18.95%) and G2/M phases (IF11, 5.89%; IA8,
6.42%), compared with the mock control groups. These results
indicated that downregulation of miR-429 mainly arrested IF11 and
IA8 cells in the G1 phase, which may result in cell proliferation
inhibition.
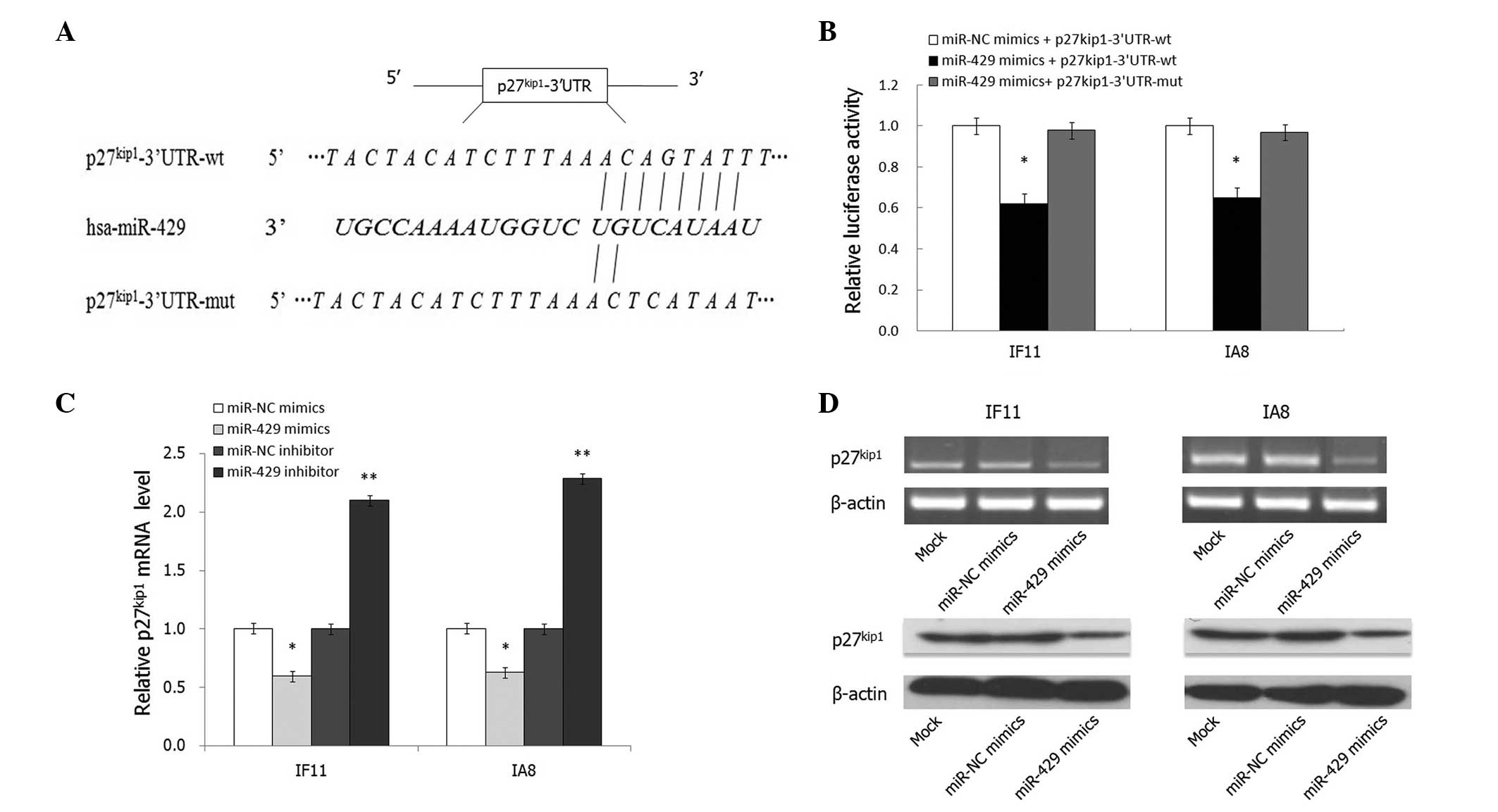
p27Kip1 is a direct target of
miR-429
miRNAs usually exert effects by binding to the 3′UTR
of target genes; thus, the targets of miR-429 mimics were
investigated to elucidate the underlying mechanism of this effect
in IF11 and IA8 prostate cancer cells. Bioinformatic analysis using
the miRanda algorithm (http://www.microrna.org) and target scan (http://www.targetscan.org/) indicated that the 3′UTR
of p27Kip1 contains a predicted binding site for miR-429
(Fig. 4A). To verify whether
p27Kip1 is a direct target of miR-429, a Dual-luciferase
reporter system using pMIR-REPORT™ luciferase vectors containing wt
or mutant p27Kip1 3′UTR was employed (Fig. 4A). Cotransfection with miR-429
significantly suppressed the luciferase activity of the reporter
containing wt 3′UTR but did not suppress the mutant reporter
(P<0.05; Fig. 4B). Consistent
with these results, transfection with miR-429 mimics significantly
reduced the endogenous p27Kip1 mRNA and protein
expression levels in IF11 and IA8 cells (P<0.05; Fig. 4C and D). In conclusion, these data
suggest that p27Kip1 is a direct target gene of
miR-429.
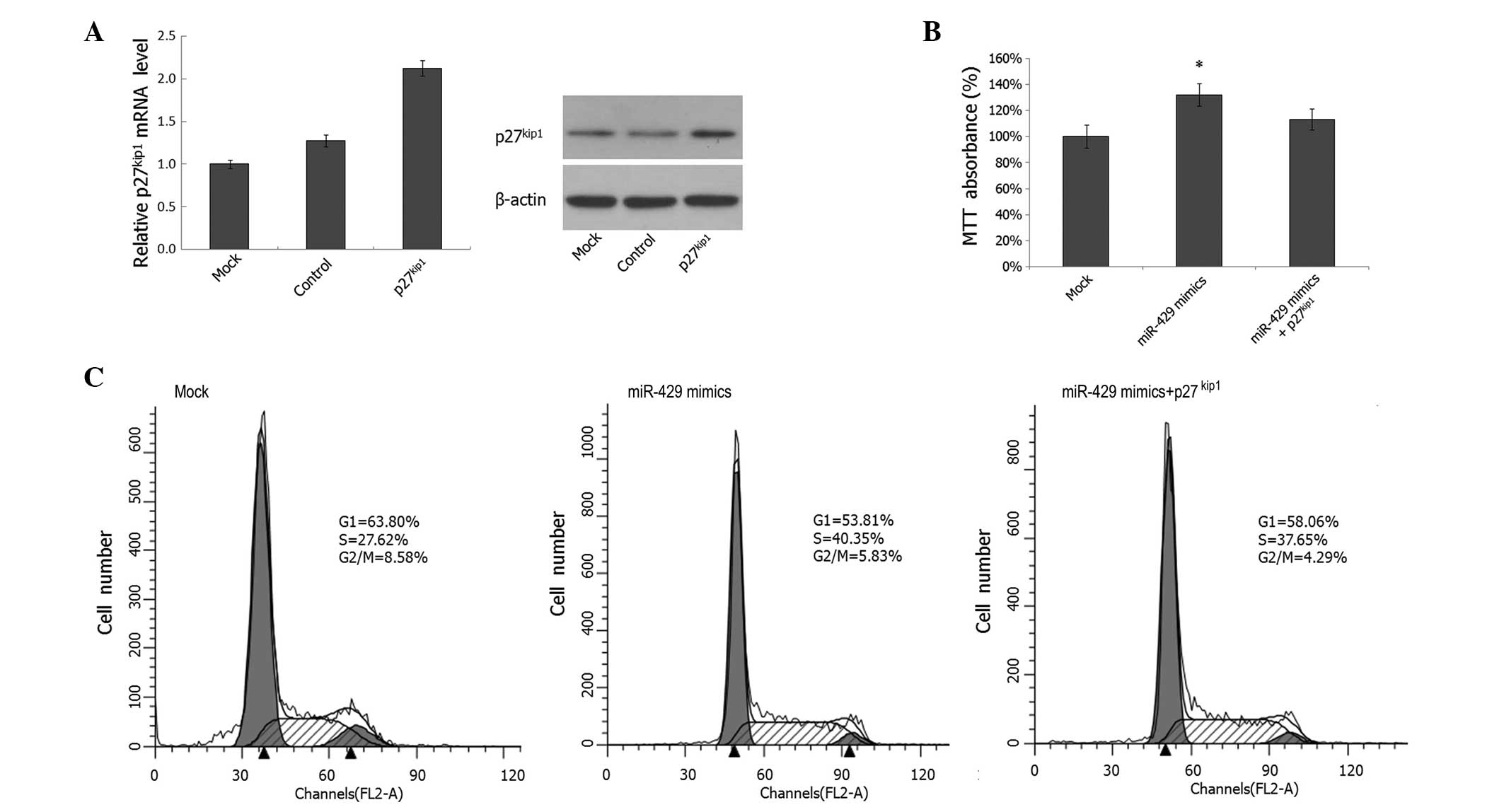
Overexpression of p27Kip1
partially rescues the proliferation-promoting effect of miR-429 on
prostate cancer cells
To further confirm whether miR-429 mediates
tumorigenic effects through p27Kip1 in prostate cancer
cells, IA8 cells were cotransfected with miR-429 mimics and the
p27kip1 expression plasmid; the increased
p27Kip1 expression levels were confirmed by PCR and
western blotting, as shown in Fig.
5A. MTT analysis revealed that cotransfection of the
p27Kip1 expression plasmid partially reversed the effect
of miR-429 on cell proliferation (Fig.
5B). Furthermore, cotransfection of the p27Kip1
expression plasmid also partially abrogated the effect of the
miR-429 mimics on G1 phase arrest (Fig. 5C). These findings demonstrate that
the cell cycle arrest effect of miR-429 is achieved, at least in
part, by the direct downregulation of p27Kip1
expression. Consequently, cell cycle arrest results in
proliferation inhibition in prostate cancer cells.
Discussion
miRNAs are endogenous small non-coding RNA molecules
that regulate gene expression at the posttranscriptional level by
targeting the 3′UTR (4–7). Aberrant expression of miRNAs is
involved in the development of cancer (16). Notably, certain miRNAs may be
either down- or upregulated in different types of cancer. miR-429
is a member of the miR-200 family and is located on chromosome
1p36. miR-429 has been demonstrated to be downregulated in
particular types of cancer and may function as a tumor suppressor
(12,17–19).
However, certain studies have shown that miR-429 may act as
oncogene in other types of cancer (13–15,20).
The discrepancies in the function of miR-429 in different types of
cancer may reflect the differences of cellular types or differences
in the targeted genes.
Prostate cancer, the second leading cause of
fatalities in males, continues to be a problem (1). Several studies have observed that
certain miRNAs regulate the expression of cancer-related genes in
prostate cancer, affecting the phenotype of these cells (8,10,21,22).
However, the relevance of miRNAs in the development, progression
and prognosis of prostate cancer is not fully understood. In the
present study, miR-429 was, to the best of our knowledge, found for
the first time to be upregulated in the IF11 and IA8 human prostate
cancer cell lines, compared with normal prostate epithelial
tissues. Downregulation of miR-429 arrested the prostate cancer
cell cycle in the G1 phase. The progression of the cell cycle in
eukaryotes is governed by complex-containing cyclins and
cyclin-dependent kinase (CDK). Deregulation of G0/G1 phase cell
cycle regulators is hypothesized to promote the aberrant
proliferation of cancer cells (9,23,24).
The data from the present study indicate that miR-429 regulated the
proliferation of prostate cancer cells through targeting the
progression of the cell cycle.
Since miRNAs exert effects by regulating the
expression of other target genes, several algorithms were employed
to determine these genes, and p27Kip1 was identified as
a potential target of miR-429. As shown in the luciferase assay and
western blot analysis in the present study, the expression of the
potential target gene p27Kip1 was directly regulated by
miR-429 in prostate cancer cells. Upregulation of
p27Kip1 by transfection with an exogenous expression
vector partially reversed the effects exerted by miR-429 on the
cell cycle and cell proliferation. The cyclin/CDK inhibitor
p27Kip1 has been established as important in the
regulation of cell cycle progression. Studies have shown that
p27Kip1 interacts with cyclin E or cyclin A/CDK2 or
other cyclin/CDK binary complexes to inhibit the respective kinase
activities. Thus, p27Kip1 blocks cell cycle progression
by inhibiting the activity of cyclin E/CDK2 complexes that normally
promote G1/S phase progression (25,26).
The results obtained in the present study indicate that miR-429
regulates cell cycle progression primarily through targeting the
p27Kip1 cyclin/CDK inhibitor.
In conclusion, to the best of our knowledge, this is
the first study documenting that miR-429 is overexpressed in IF11
and IA8 prostate cancer cells. Overexpression of miR-429 was
associated with cell cycle progression and cell proliferation in
prostate cancer. Additional results demonstrated that the effect of
miR-429 in prostate cancer cells was executed through targeting the
p27Kip1 CDK inhibitor; p27Kip1 was
demonstrated to be directly regulated by miR-429 in the cells.
Acknowledgements
The authors would like to thank all members of the
Department of Clinical Laboratory, Tangdu Hospital, the Fourth
Military Medical University (Xi’an, China) for assistance. This
study was supported by the National Natural Science Foundation of
China (grant no. 31100546).
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Marzo AM, DeWeese TL, Platz EA, et al:
Pathological and molecular mechanisms of prostate carcinogenesis:
implications for diagnosis, detection, prevention, and treatment. J
Cell Biochem. 91:459–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Isaacs WB, Bova GS, Morton RA, et al:
Molecular biology of prostate cancer progression. Cancer Surv.
23:19–32. 1995.PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rigoutsos I: New tricks for animal
microRNAS: targeting of amino acid coding regions at conserved and
nonconserved sites. Cancer Res. 69:3245–3248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iorio MV and Croce CM: MicroRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hirata H, Ueno K, Shahryari V, et al:
MicroRNA-182–5p promotes cell invasion and proliferation by down
regulating FOXF2, RECK and MTSS1 genes in human prostate cancer.
PLoS One. 8:e555022013. View Article : Google Scholar
|
|
9
|
Wang H, Lu YT, Luo L, et al: MicroRNA-195
inhibits the proliferation of human glioma cells by directly
targeting cyclin D1 and cyclin E1. PLoS One. 8:e549322013.
View Article : Google Scholar
|
|
10
|
Musiyenko A, Bitko V and Barik S: Ectopic
expression of miR-126*, an intronic product of the
vascular endothelial EGF-like 7 gene, regulates prostein
translation and invasiveness of prostate cancer LNCaP cells. J Mol
Med (Berl). 86:313–322. 2008. View Article : Google Scholar
|
|
11
|
Vrba L, Jensen TJ, Garbe JC, et al: Role
for DNA methylation in the regulation of miR-200c and miR-141
expression in normal and cancer cells. PLoS One. 5:e86972010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun TW, Wang C, Xing J and Wu D: miR-429
modulates the expression of c-myc in human gastric carcinoma cells.
Eur J Cancer. 47:2552–2559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Han Y, Chen J, Zhao X, et al: MicroRNA
expression signatures of bladder cancer revealed by deep
sequencing. PLoS One. 6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Snowdon J, Zhang X, Childs T, Tron VA and
Feilotter H: The microRNA-200 family is upregulated in endometrial
carcinoma. PLoS One. 6:e228282011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam EJ, Yoon H, Kim SW, et al: MicroRNA
expression profiles in serous ovarian carcinoma. Clin Cancer Res.
14:2690–2695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Heneghan HM, Miller N and Kerin MJ: MiRNAs
as biomarkers and therapeutic targets in cancer. Curr Opin
Pharmacol. 10:543–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gregory PA, Bert AG, Paterson EL, et al:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu W, Xu H, Zhu D, et al: miR-200bc/429
cluster modulates multidrug resistance of human cancer cell lines
by targeting BCL2 and XIAP. Cancer Chemother Pharmacol. 69:723–731.
2012. View Article : Google Scholar
|
|
19
|
Uhlmann S, Zhang JD, Schwäger A, et al:
miR-200bc/429 cluster targets PLCγ1 and differentially regulates
proliferation and EGF-driven invasion than miR-200a/141 in breast
cancer. Oncogene. 29:4297–4306. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JW, Park YA, Choi JJ, et al: The
expression of the miRNA-200 family in endometrial endometrioid
carcinoma. Gynecol Oncol. 120:56–62. 2011. View Article : Google Scholar
|
|
21
|
Wu Z, Sun H, Zeng W, et al: Upregulation
of MicroRNA-370 induces proliferation in human prostate cancer
cells by downregulating the transcription factor FOXO1. PLoS One.
7:e458252012. View Article : Google Scholar
|
|
22
|
Ishteiwy RA, Ward TM, Dykxhoorn DM and
Burnstein KL: The microRNA-23b/-27b cluster suppresses the
metastatic phenotype of castration-resistant prostate cancer cells.
PLoS One. 7:e521062012. View Article : Google Scholar
|
|
23
|
Davidovic L, Durand N, Khalfallah O, et
al: A novel role for the RNA-binding protein FXR1P in myoblasts
cell-cycle progression by modulating p21/Cdkn1a/Cip1/Waf1 mRNA
stability. PLoS Genet. 9:e10033672013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai L, Liu YQ, Liu JY, et al: A novel
cyclinE/cyclinA-CDK inhibitor targets p27(Kip1) degradation, cell
cycle progression and cell survival: implications in cancer
therapy. Cancer Lett. 333:103–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lacy ER, Wang Y, Post J, et al: Molecular
basis for the specificity of p27 toward cyclin-dependent kinases
that regulate cell division. J Mol Biol. 349:764–773. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slingerland J and Pagano M: Regulation of
the cdk inhibitor p27 and its deregulation in cancer. J Cell
Physiol. 183:10–17. 2000. View Article : Google Scholar : PubMed/NCBI
|