Introduction
Rheumatoid arthritis (RA) is the most prevalent
chronic inflammatory disease, which is characterized by synovial
proliferation, progressive damage disability and systemic
complications (1). The severity of
the disease is associated with inflammation intensity and joint
damage (2). The current treatment
strategy involves aggressive therapy, which is selected using an
assessment of disease activity in pursuit of clinical remission
(3). Therefore, determination of
disease activity in RA patients has become an important component
for RA management.
Several indices have been used to assess RA disease
activity based on the clinical manifestations, laboratory and
physical measurements. These indices include the disease activity
score for 28 joints (DAS28), rheumatoid arthritis disease activity
index (RADAI) and clinical disease activity index (CDAI) (3). The majority of RA indices rely on
swollen and tender joint counts as well as patient-reported
outcomes, of which DAS28 is widely used to monitor and assess
disease activity in clinical trials and routine patient care
(4,5). However, significant variability
occurs between different observers, which influences results.
Additional laboratory tests are therefore required in order to
augment clinical assessment. Erythrocyte sedimentation rates (ESR)
and C-reactive protein (CRP) are both incorporated into disease
activity assessments in RA patients (6). ESR and CRP are important inflammatory
markers; however, they are reported to be increased with aging and
anemia (7). The presence of
rheumatoid factor (RF) and anti-cyclic citrullinated peptide
antibody (anti-CCP) have been associated with disease severity and
joint erosions, which are included in the criteria for RA (9); however, RF and anti-CCP were reported
to have little correlation with disease activity (10). Therefore, the elucidation of
further surrogate biomarkers for RA disease activity assessment is
critical.
Serum amyloid A (SAA) is an acute phase protein, ~12
kDa in size. SAA is primarily synthesized by hepatocytes and
secreted from certain extrahepatic sites in chronic inflammatory
diseases, including Alzheimer’s disease and RA (10). The circulatory SAA concentration
was reported to be significantly increased during acute phase
responses, including trauma and infection (11). SAA was also shown to exhibit
important immunological functions in the process of inflammation;
for example, SAA may be a chemokine for immune cells, including T
lymphocytes, neutrophils and mast cells. SAA was also found to
stimulate the synthesis of several pro-inflammatory cytokines,
including interleukin-1 (IL-1) and tumor necrosis factor (TNF)
(12). Previous studies have
indicated that SAA positively correlated with disease activity in
ankylosing spondylitis (AS) (13),
juvenile idiopathic arthritis (JIA) (14) and polymyalgia rheumatica (PMR)
(15). The aim of the present
study was to investigate the association between circulating levels
of SAA and disease activity in RA patients.
Materials and methods
Patients and samples
The types of disease and the respective number of
patients that serum samples were obtained from were as follows: RA,
88; osteoarthritis (OA), 54; systemic lupus erythematosus (SLE),
43; and other autoimmune diseases, 30 (Sjogren’s syndrome, 15;
ankylosing spondylitis, 5; systemic scleroderma, 5;
spondyloarthropathy, 2; psoriatic arthritis, 2; and chondritis, 1),
as well as 50 age and gender-matched healthy control subjects (HC)
(Table I). Matched synovial fluid
(SF) and synovial membrane (SM) samples were obtained from 20 RA
and 16 OA patients undergoing arthroscopic biopsies and joint
replacement surgeries. All patients with RA fulfilled the American
College of Rheumatology (ACR; Atlanta, GA, USA) criteria for RA; in
addition, all other patients enrolled in the present study
fulfilled their corresponding diagnostic criteria. The present
study was approved by the Medical Ethics and Human Clinical Trial
Committee of Tianjin Medical University (Tianjin, China).
 | Table IClinical characteristics of patients
in each group. |
Table I
Clinical characteristics of patients
in each group.
| Group | n | Gender (F/M) | Age (years) | Erythrocyte
sedimentation rate (mm/h) | C-reactive protein
(mg/l) |
|---|
| RA | 88 | 65/23 | 58±12 | 49.06±13.79a,b | 41.42±32.83a,b |
| SLE | 43 | 31/12 | 53±17 | 41.80±17.30 | 35.74±31.61 |
| Others | 30 | 25/5 | 51±13 | 45.06±11.43 | 29.82±23.37 |
| OA | 54 | 42/12 | 56±16 | 23.26±18.51 | 28.55±21.05 |
| HC | 50 | 38/12 | 52±8 | 12.50±11.90 | 5.02±3.11 |
Sample preparation
All samples, including clotted serum and SF, were
centrifuged at 1,425.6 g for 10 min, immediately aliquoted and
stored at −80°C. All samples were only allowed to be thawed
once.
Determination of SAA levels in sera and
SF using ELISA
The concentration of SAA in serum and SF was
detected using a sandwich ELISA (human SAA ELISA kit; Xinle Biology
Co., Ltd., Shanghai, China) according to the manufacturer’s
instructions. Optical density (OD) values of each well were
measured using an ELISA plate reader (Multiskan MK3; Thermo
Scientific, Waltham, MA, USA) at 450 nm.
Western blot analysis of SAA expression
in sera
All serum samples were diluted and denatured at 95°C
for 5 min following the addition of loading buffer (Zhaoran Biology
Co., Ltd., Shanghai, China). Serum proteins were separated using
SDS-PAGE (Zhaoran Biology Co., Ltd.) and subsequently transferred
onto the polyvinylidene difluoride (PVDF; Yongyuan Metal Co., Ltd.,
Suzhou, China) membrane for 1 h at 250 mA. The membrane was then
blocked for 1 h at room temperature in 5% skimmed
milk/tris-buffered saline with Tween 20 (TBST; 20 mM Tris-HCl, pH
7.6; 137 mM NaCl; and 0.05% Tween 20) and incubated with rabbit
anti-human SAA polyclonal antibodies (Abcam, Cambridge, UK) for 1 h
at room temperature (1:2,000 in 5% skimmed milk/TBST). The membrane
was washed with TBST three times for 30 min each and then incubated
with horseradish peroxidase (HRP)-conjugated goat anti-rabbit
immunoglobulin (Ig)G (Bioword Technology, Inc., St. Louis Park, MN,
USA) for 1 h (1:1,000 in 5% skimmed milk/TBST). Following washing,
proteins were detected using a Pierce-enhanced chemiluminescence
system (Solarbio Bioscience and Technology Co., Ltd, Shanghai,
China).
Immunohistochemistry
Synovial tissue sections were fixed in acetone for
10 min, incubated with primary rabbit polyclonal antibodies against
human SAA (1:1,200; Abcam) for 1 h at 37°C. An isotype-matched
mouse monoclonal antibody (1:500; Xinle Biology Co., Ltd.) was used
as the negative control. HRP-conjugated goat anti-rabbit IgG
secondary antibodies (1:1,00) were then added and incubated for 1
h. Color was developed in solutions containing
diaminobenzadine-tetrahydrochloride (Sigma-Aldrich, St. Louis, MO,
USA) and 0.5% H2O2 in phosphate-buffered
saline (pH 7.6; Zhaoran Biology Co., Ltd.). Slides were then
counter-stained with hematoxylin and mounted (Zhaoran Biology Co.,
Ltd.). SAA levels were determined through quantification of the
number of positive-staining cells/high-power field.
Determination of disease activity
DAS28-ESR, a validated scoring system for predicting
disease activity, was employed in order to calculate disease
activity using the following formula: DAS28-ESR=0.56xsqrt(number of
tender joints) + 0.28xsqrt(number of swollen joints) + 0.70xLn(ESR)
+ 0.014xvisual analogue scale (VAS).
VASs are a straight horizontal line of fixed length,
usually 100 mm. The ends are defined as the extreme limits of the
measured parameter (e.g. symptom, pain and health) orientated from
the left (worst) to the right (best). ESR was measured in mm/hr and
VAS was measured in mm. The range of the tests varies from 0 to
10.
Clinical and laboratory measurements
General clinical data, including age, gender,
disease duration, number of swollen joints and number of tender
joints, were collected. Laboratory data were obtained as follows:
ESR was measured using the Westergren method (16); CRP was examined using the
immunonephelometry method (16);
RF-IgA and RF-IgG were measured in serum using rate nephelometry
(Immage® Immunochemistry system; Beckman Coulter, Brea,
CA, USA). The anti-CCP2 antibody was tested using a
second-generation ELISA kit (Shanghai Fuchun-Zhongnan Biotech Co.,
Ltd, Shanghai, China).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were processed using SPSS 11.0 (SPSS Inc., Chicago, IL, USA).
Differences among groups were analyzed using a one-way analysis of
variance (ANOVA). Comparisons between two groups were analyzed
using a Student-Newman-Keuls test or Student’s t-test. Correlations
were determined using the Spearman rank correlation coefficients.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Clinical characters of the
participants
Detailed clinical characteristics of all
participants are shown in Table I.
The data demonstrated no significant differences in gender balance
or average age of patients among the groups (P>0.05). However,
the results showed that ESR and CRP were significantly increased in
the RA group compared to those in the HC and OA groups
(P<0.05).
Increased expression of SAA in patients
with RA
An ELISA assay was performed in order to detect
serum levels of SAA. Data from five groups were analyzed using
one-way ANOVA (Table II). The
results revealed that there was a statistically significant
difference among the five groups (F=5.549; P<0.05). Further
statistical analysis, using the Student-Newman-Keuls test, showed
that serum SAA levels in RA (6.15±3.27 mg/l) were significantly
increased compared to those of the SLE, other autoimmune diseases,
OA and HC groups. However, no significant differences were
identified among the other groups.
 | Table IISerum levels of serum amyloid A in
each group. |
Table II
Serum levels of serum amyloid A in
each group.
| Group | n | Mean ± standard
deviation (mg/l) | F-value | P-value |
|---|
| RA | 88 | 6.15±3.27a,b | | |
| SLE | 43 | 2.54±0.31 | | |
| Others | 30 | 1.55±0.98 | 5.549 | 0.006 |
| OA | 54 | 1.42±0.97 | | |
| HC | 50 | 1.45±0.72 | | |
Further ELISA assays were used to detect levels of
SAA in SF. As shown in Fig. 1A,
SAA levels were significantly increased in the SF of the RA group
(7.63±3.39 mg/l) compared to those of the OA group (1.54±0.48 mg/l;
P<0.05).
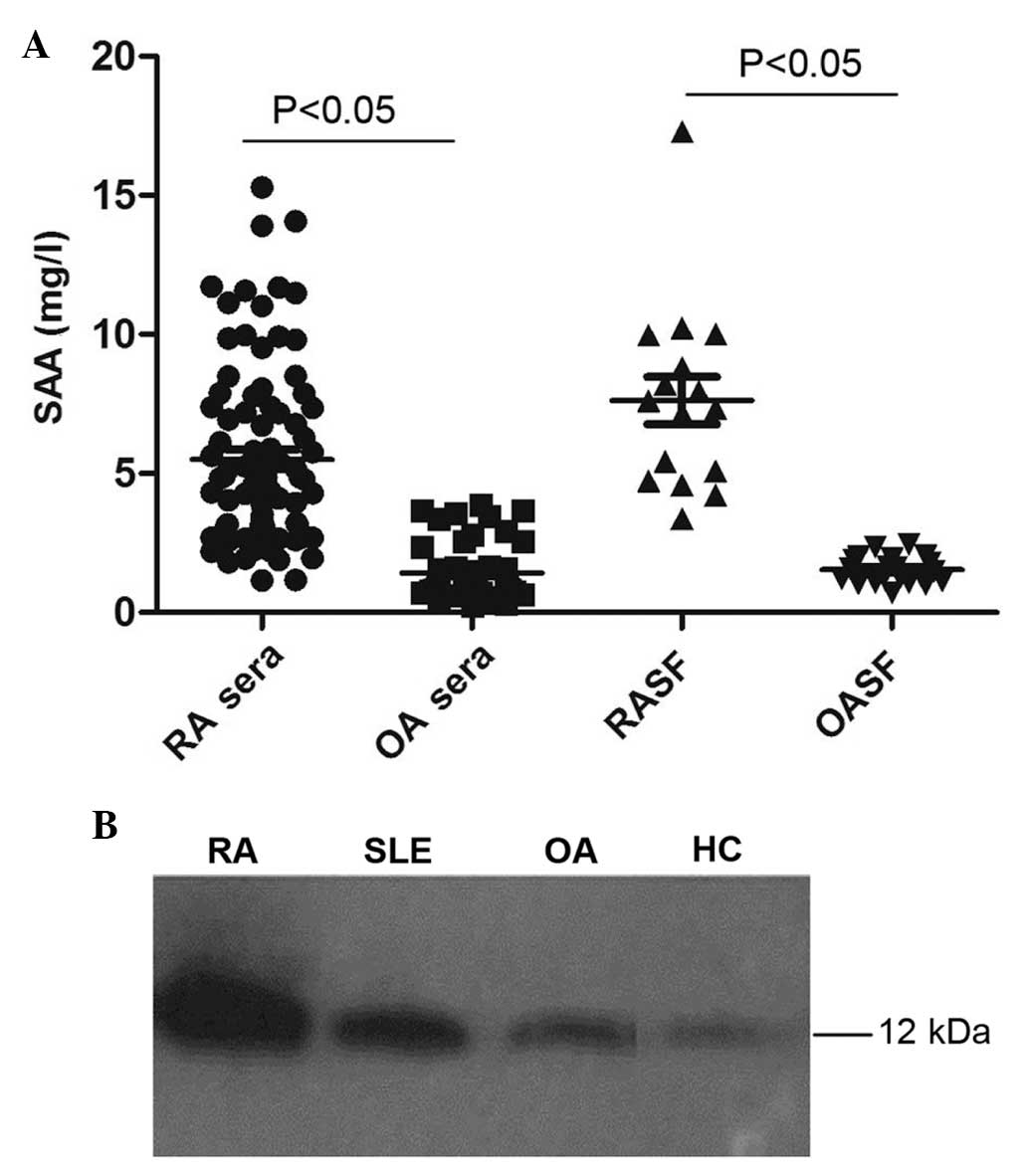 | Figure 1Increased levels of SAA in the serum
and synovial fluid of RA patients. (A) ELISA analysis of serum SAA
in RA and OA patients (n=88 and 54, respectively), as well as SF
SAA levels in RA and OA patients (n=16 and 20, respectively). (B)
Representative western blot analysis of SAA expression in the serum
of RA, SLE, OA and HC patients. SAA, serum amyloid A; SF, synovial
fluid; RA, rheumatoid arthritis; OA, osteoarthritis; SLE, systemic
lupus erythematosus; HC, healthy control. |
Western blot analysis of serum SAA levels revealed
that SAA protein expression levels were markedly increased in RA
patients compared with those of individuals in the SLE, OA and HC
groups (Fig. 1B).
Immunohistochemical analysis of SAA
expression in the SM
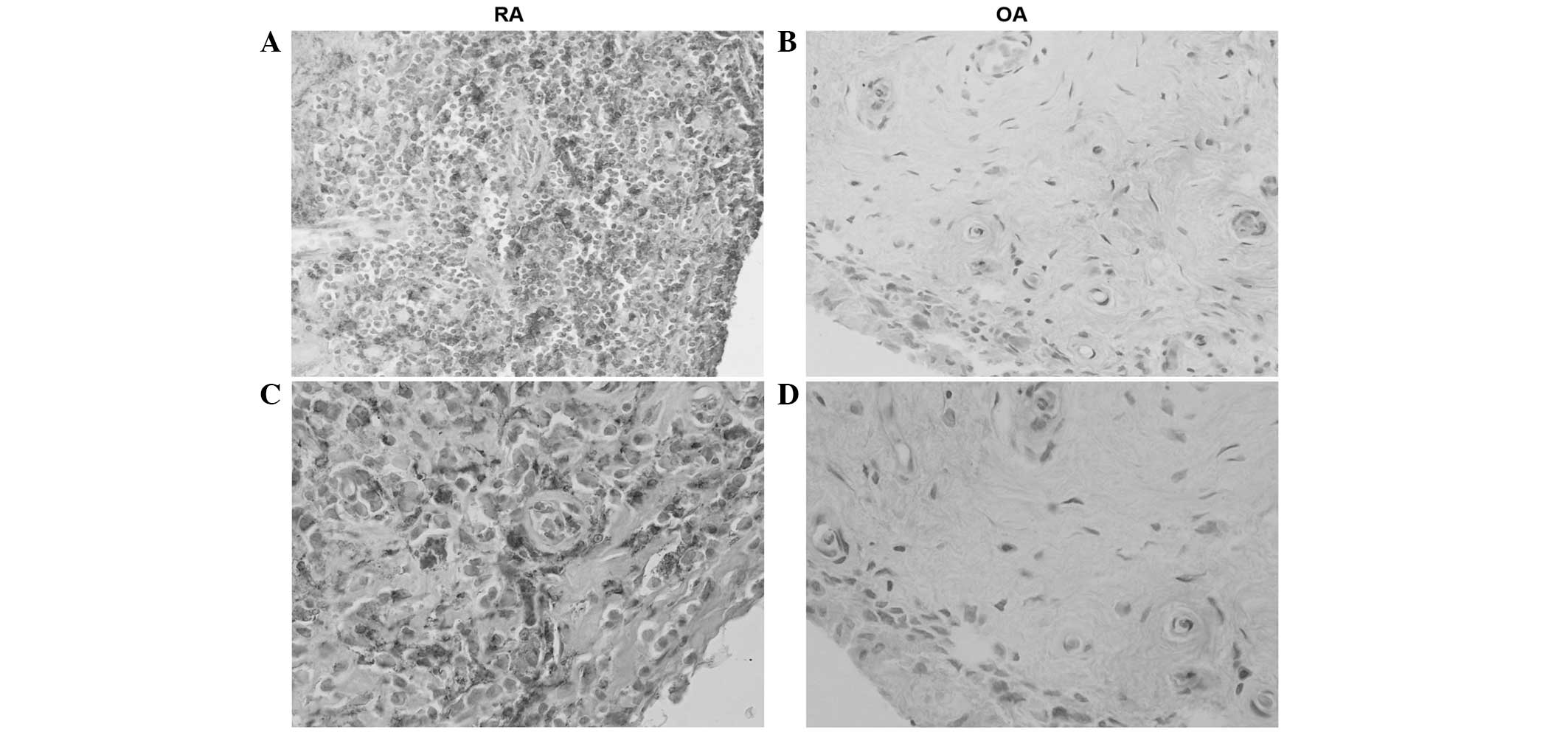
Hematoxylin staining revealed high levels of SAA in
all tissue samples from RA patients. The histological distribution
of SAA was located in synovial lining and sublining layers; of
note, in fibroblast-like synovial cells (FLSs), inflammatory cells,
vascular endothelial cells and perivascular areas. However,
staining for SAA in tissue samples from patients in the OA group
was markedly weaker in perivascular areas and FLSs (Fig. 2).
Serum SAA levels correlate with RA
disease activity, ESR and CRP
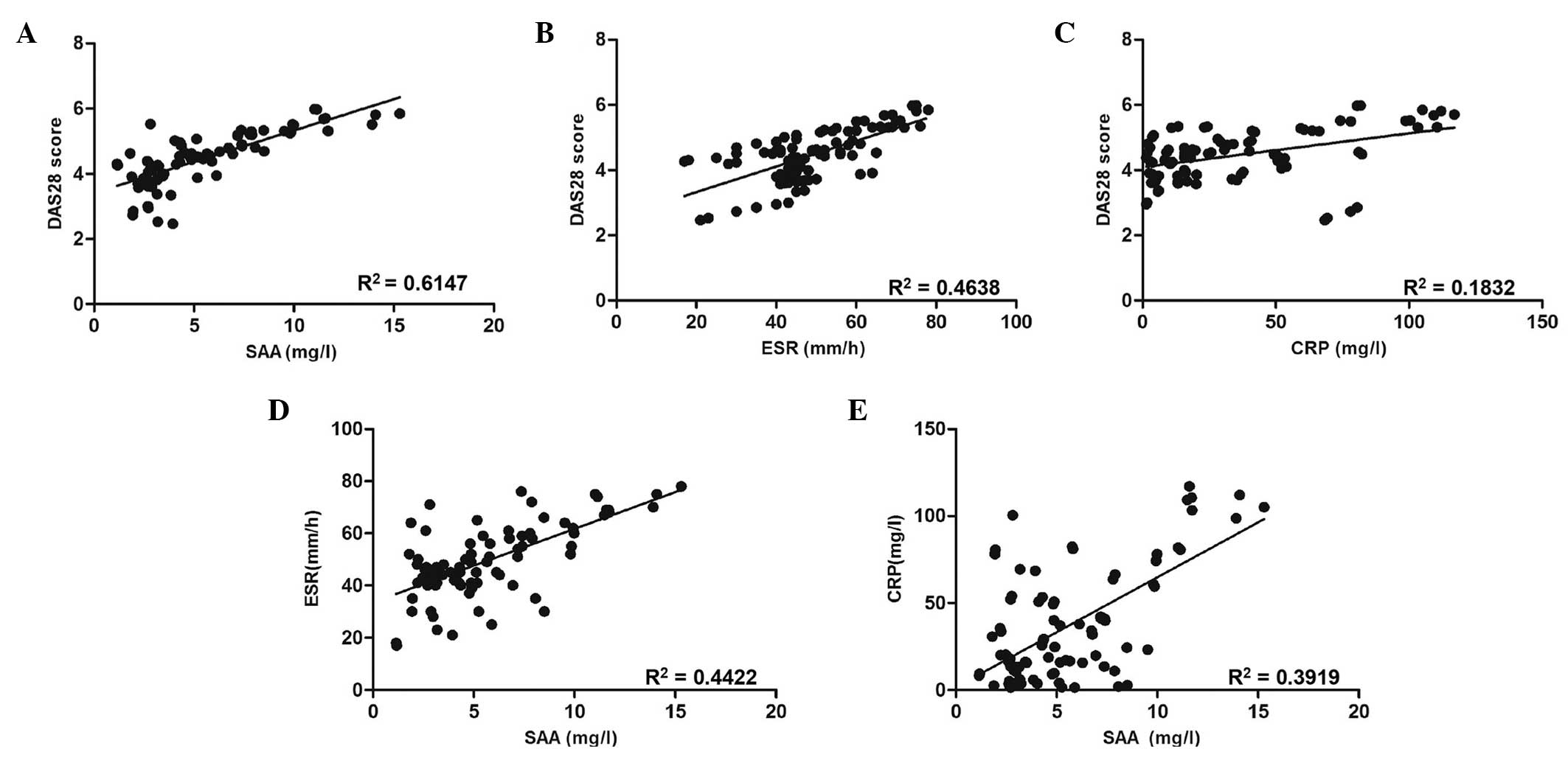
Spearman’s rank correlation analyses were performed
to assess correlations between the levels of SAA in RA patients’
sera and DAS28 (Fig. 3A). A
significant correlation was observed between SAA levels in sera and
DAS28 in RA patients (R2=0.6147; P<0.001), therefore
indicating that serum levels of SAA in RA patients were positively
correlated with disease activity. In addition, the correlation of
serum SAA levels with other serologic biomarkers was assessed. As
shown in Fig. 3B and C, ESR and
CRP levels were demonstrated to be positively correlated with DAS28
(R2=0.4638, P<0.001; and R2=0.1832,
P=0.004, respectively). Furthermore, SAA levels were found to be
positively correlated with ESR (R2=0.4422; P<0.001)
and CRP (R2=0.3919; P<0.001) (Fig. 3D and E).
Association between SAA and
autoantibodies in RA
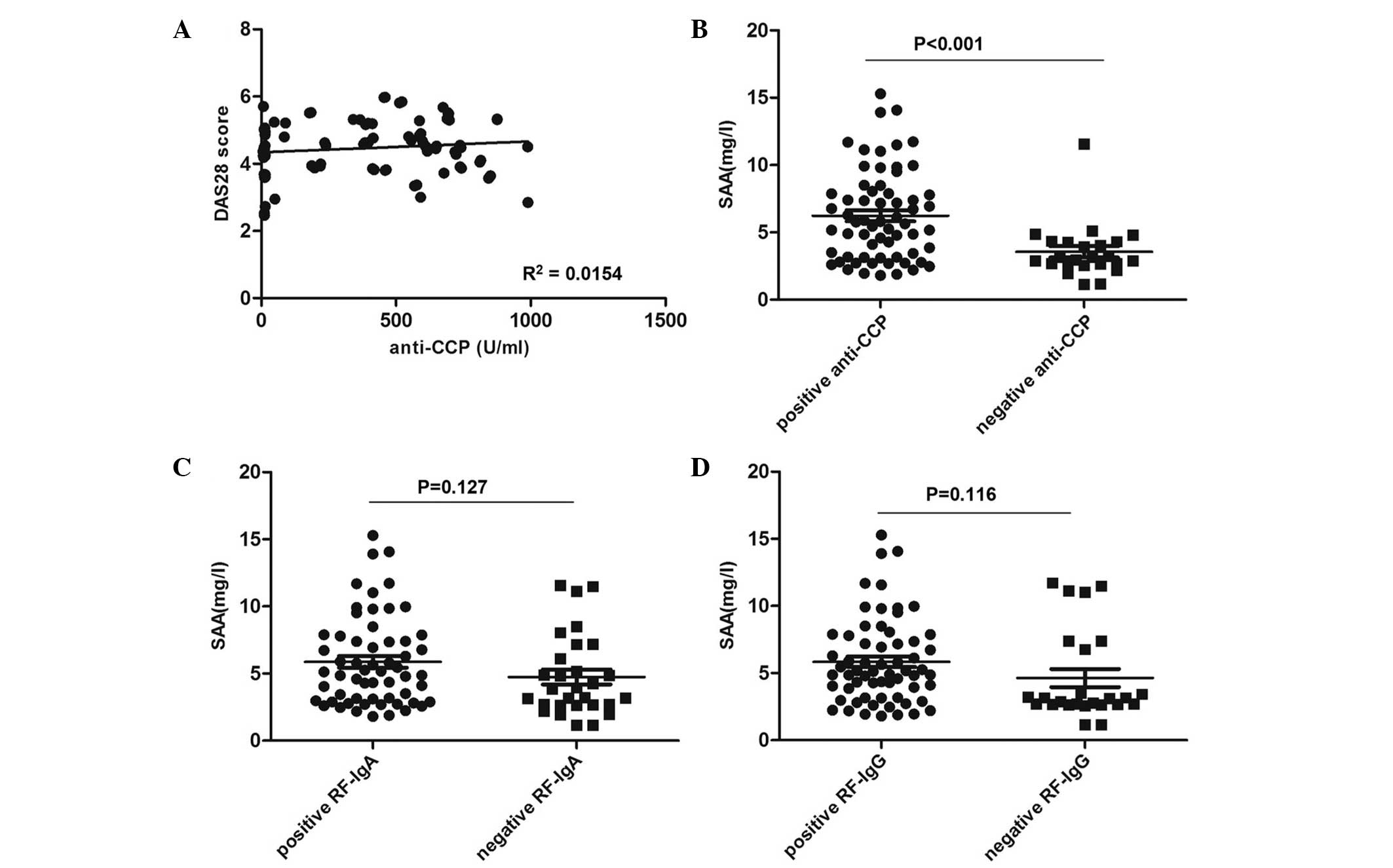
As shown in Fig.
4A, no significant correlation was identified between serum
anti-CCP levels and DAS28 (R2=0.0154; P=0.250). However,
RA patients positive for anti-CCP showed significantly increased
levels of SAA compared to those of patients negative for anti-CCP
(6.88±3.36 and 4.20±2.02 mg/l, respectively; P<0.001) (Fig. 4B). In addition, RA patients
positive for RF-IgA demonstrated a non-significant increase in SAA
levels compared to those of IgA-negative patients (6.53±3.33 and
5.41±3.00 mg/l, respectively; P=0.127) (Fig. 4C). Identical conclusions were drawn
from the results for RF-IgG-positive and -negative groups
(6.50±3.17 and 5.29±3.37 mg/l; P=0.116) (Fig. 4D).
Discussion
The aim of the present study was to investigate the
association between circulating levels of SAA and disease activity
in RA patients. The results showed that SAA levels in RA patients
were significantly increased compared to those of the control and
other disease groups. In addition, the results of the present study
demonstrated a significant correlation between SAA levels and DAS28
as well as other validated disease activity measures; this
therefore indicated that SAA was a useful indicator of RA disease
activity.
Previous studies have reported that serum SAA levels
were elevated in RA patients compared with those of SLE patients
(17–19). The results of the present study
were consistent with these findings, demonstrating that serum SAA
levels were significantly increased not only compared with the SLE
group but also with patients with other autoimmune diseases OA and
HC; therefore, this implied its potential value for monitoring RA
disease activity. The results of the present study were also
consistent with a study by de Seny et al (20), which showed significantly elevated
plasma SAA levels in RA. SAA is produced via the signaling pathways
of pro-inflammatory cytokines, including interleukin (IL)-6 and
IL-8 (12). During acute phase
responses, including inflammation and infection, SAA concentrations
may be elevated as much as 1,000 fold (7). A study by Metawi et al
(21) showed that IL-17, secreted
by Th17 cells, acted as an indicator of RA disease activity.
Another study confirmed that SAA induced the production of CCL20,
which has a role in the recruitment of Th17 cells to the
inflammatory sites; moreover, the expression of CCL20 was reported
to be upregulated by IL-17 (22).
These results also suggested the possible role of SAA in disease
activity of RA. In the present study, western blot analysis was
used in order to determine the expression of SAA in sera, the
result of which was consistent with that of the ELISA assays
performed. Of note, SAA monomers were located in sera, whereas
other forms, including dimers or combined SAA, were not
observed.
Furthermore, the results of the present study showed
that SAA levels were significantly increased in the synovial fluid
and synovial membrane of RA patients compared to those of OA
patients; these results were in accordance with previous studies
(20,23). Radiographic data revealed that RA
patients enrolled in the present study showed signs of cartilage
destruction, coarse and erosion (data not shown). It is
well-established that SAA in synovial fluid is primarily produced
by local joint synoviocytes; in addition, it was reported that SAA
messenger RNA was upregulated in RA synovium (17). Okamoto et al (24) demonstrated that SAA activated
nuclear factor-κB via binding to the advanced glycation end-product
(RAGE) receptor in rheumatoid synovial fibroblasts, which
subsequently promoted the expression of pro-inflammatory cytokines
IL-6 and IL-8. These results therefore suggested that SAA may have
a pathophysiological role in the pathogenesis of RA.
In the present study, a significant correlation was
identified between SAA and established disease activity measures.
DAS28 is the most commonly used index for assessing disease
activity levels in RA patients (25). The results of the present study
indicated that SAA correlated with DAS28 (R2=0.6147) in
RA. Connolly et al (26)
reported that SAA levels correlated with RA disease activity, as
measured using swollen joint counts (R2=0.26; P=0.048).
In the present study, RA disease activity was calculated using
DAS28, which included swollen joint counts, tender joint counts,
ESR and VAS. In addition, the patients recruited to the present
study were in the stage of acute inflammation with no therapy; by
contrast. The patients recruited by Connolly et al were at
0–3 months into anti-tumor necrosis factor a (anti-TNFa) therapy.
The results of the present study demonstrated a significant
positive correlation between SAA and ESR, CRP as well as disease
activity in RA patients. ESR and CRP are both effective indicators
of disease activity. It was previously reported that SAA and CRP
were primarily produced in the liver; however, serum levels of SAA
depend on a greater amount of pro-inflammatory cytokines compared
with CRP (27), this therefore
suggested that SAA may be a more sensitive biomarker than CRP for
reflecting disease activity.
Autoantibodies were suggested to be important for RA
diagnosis and prognosis. Anti-CCP is highly sensitive and specific
for RA diagnosis, which has been associated with the presence of
severe bone erosion (28). In the
present study, an association was identified between elevated SAA
levels and anti-CCP-positive patients, indicating that SAA may be
associated with bone erosion. Increased levels of SAA in RF-IgA and
RF-IgG-positive groups were observed; however, these results were
not significant compared to those of the negative group. These
results may be explained by the low specificity of RF, since RF can
also be detected in other infectious diseases, autoimmune diseases
and even healthy people.
In conclusion, the results of the present study
demonstrated that increased circulating and local SAA levels were
significantly correlated with the degree of RA disease activity.
These results provided further evidence for the pathological role
of SAA in RA, which may be a useful biomarker in the assessment of
disease severity and response to therapy. Further studies are
required prior to the use of SAA as an indicator of RA disease
activity in a clinical environment, for which a larger scale of
samples is recommended.
Acknowledgements
The present study was supported by a grant from the
Specialized Research Fund for the Doctoral Program of Higher
education funded by the Ministry of Education (no. 20101202110008)
and the Natural Science Foundation of Tianjin (no. 14JCYBJC25600).
The authors would like to thank all subjects for their
participation in the present study.
References
|
1
|
Scott DL, Wolfe F and Huizinga TW:
Rheumatoid arthritis. Lancet. 376:1094–1108. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McInnes IB and Schett G: The pathogenesis
of rheumatoid arthritis. N Engl J Med. 365:2205–2219. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Emery P: Evidence supporting the benefit
of early intervention in rheumatoid arthritis. J Rheumatol. 66:3–8.
2002.
|
|
4
|
Fransen J and van Riel PL: The disease
activity score and the EULAR response criteria. Clin Exp Rheumatol.
23(5 Suppl 39): S93–S99. 2005.PubMed/NCBI
|
|
5
|
Aletaha D, Landewe R, Karonitsch T, et al:
EULAR, ACR: Reporting disease activity in clinical trials of
patients with rheumatoid arthritis: EULAR/ACR collaborative
recommendations. Arthritis Rheum. 59:1371–1377. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsui T, Kuga Y, Kaneko A, et al: Disease
Activity Score 28 (DAS28) using C-reactive protein underestimates
disease activity and overestimates EULAR response criteria compared
with DAS28 using erythrocyte sedimentation rate in a large
observational cohort of rheumatoid arthritis patients in Japan. Ann
Rheum Dis. 66:1221–1226. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Da Mota LM, dos Santos Neto LL, Burlingame
R, et al: Laboratory characteristics of a cohort of patients with
early rheumatoid arthritis. Rev Bras Rheumatol. 50:375–388.
2010.
|
|
8
|
Aletaha D, Neogi T, Silman AJ, et al: 2010
Rheumatoid arthritis classification criteria: an American College
of Rheumatology/European League Against Rheumatism collaborative
initiative. Arthritis Rheum. 62:2569–2581. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papadopoulos NG, Tsiaousis GZ,
Pavlitou-Tsiontsi A, Giannakou A and Galanopoulou VK: Does the
presence of anti-CCP autoantibodies and their serum levels
influence the severity and activity in rheumatoid arthritis
patients? Clin Rev Allergy Immunol. 34:11–15. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mullan RH, Bresnihan B, Golden-Mason L, et
al: Acute-phase serum amyloid A stimulation of angiogenesis,
leukocyte recruitment, and matrix degradation in rheumatoid
arthritis through an NF-κB-dependent signal transduction pathway.
Arthritis Rheum. 54:105–114. 2006. View Article : Google Scholar
|
|
11
|
Dong Z, Wu T, Qin W, et al: Serum amyloid
A directly accelerates the progression of atherosclerosis in
apolipoprotein E-deficient mice. Mol Med. 17:1357–1364. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eklund KK, Niemi K and Kovanen PT: Immune
functions of serum amyloid A. Crit Rev Immunol. 32:335–348. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jung SY, Park MC, Park YB and Lee SK:
Serum amyloid a as a useful indicator of disease activity in
patients with ankylosing spondylitis. Yonsei Med J. 48:218–224.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cantarini L, Giani T, Fioravanti A, et al:
Serum amyloid A circulating levels and disease activity in patients
with juvenile idiopathic arthritis. Yonsei Med J. 53:1045–1048.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimojima Y, Matsuda M, Gono T, Ishii W
and Ikeda S: Serum amyloid A as a potent therapeutic marker in a
refractory patient with polymyalgia rheumatica. Intern Med.
44:1009–1012. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrari R, Tanni SE, Caram LM, Corrêa C,
Corrêa CR, et al: Three-year follow-up of interleukin 6 and
C-reactive protein in chronic obstructive pulmonary disease. Respir
Res. 20:14–24. 2013.
|
|
17
|
O’Hara R, Murphy EP, Whitehead AS,
FitzGerald O and Bresnihan B: Acute-phase serum amyloid A
production by rheumatoid arthritis synovial tissue. Arthritis Res.
2:142–144. 2000. View
Article : Google Scholar
|
|
18
|
Momohara S, Okamoto H and Yamanaka H:
Chondrocyte of rheumatoid arthritis serve as a source of
intra-articular acute-phase serum amyloid A protein. Clin Chim
Acta. 398:155–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vallon R, Freuler F, Desta-Tsedu N, et al:
Serum amyloid A (apoSAA) expression is up-regulated in rheumatoid
arthritis and induces transcription of matrix metalloproteinases. J
Immunol. 66:2801–2807. 2001. View Article : Google Scholar
|
|
20
|
de Seny D, Cobraiville G, Charlier E, et
al: Acute-phase serum amyloid a in osteoarthritis: regulatory
mechanism and proinflammatory properties. PloS One. 8:e667692013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Metawi SA, Abbas D, Kamal MM and Ibrahim
MK: Serum and synovial fluid levels of interleukin-17 in
correlation with disease activity in patients with RA. Clin
Rheumatol. 30:1201–1207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Migita K, Koga T, Torigoshi T, et al:
Serum amyloid A protein stimulates CCL20 production in rheumatoid
synoviocytes. Rheumatology (Oxford). 48:741–747. 2009. View Article : Google Scholar
|
|
23
|
Mullan RH, Bresnihan B, Golden-Mason L, et
al: Acute-phase serum amyloid A stimulation of angiogenesis,
leukocyte recruitment, and matrix degradation in rheumatoid
arthritis through an NF-κB-dependent signal transduction pathway.
Arthritis Rheum. 54:105–114. 2006. View Article : Google Scholar
|
|
24
|
Okamoto H, Katagiri Y, Kiire A, Momohara S
and Kamatani N: Serum amyloid A activates nuclear factor-kappa B in
rheumatoid synovial fibroblasts through binding to receptor of
advanced glycation end-products. J Rheumatol. 35:752–756.
2008.PubMed/NCBI
|
|
25
|
Farheen K and Agarwal SK: Assessment of
disease activity and treatment outcomes in rheumatoid arthritis. J
Manag Care Pharm. 17(9 Suppl B): S09–S13. 2011.PubMed/NCBI
|
|
26
|
Connolly M, Mullan RH, McCormick J, et al:
Acute-phase serum amyloid A regulates tumor necrosis factor α and
matrix turnover and predicts disease progression in patients with
inflammatory arthritis before and after biologic therapy. Arthritis
Rheum. 64:1035–1045. 2012. View Article : Google Scholar
|
|
27
|
Thorn CF, Lu ZY and Whitehead AS:
Regulation of the human acute phase serum amyloid A genes by tumour
necrosis factor-alpha, interleukin-6 and glucocorticoids in hepatic
and epithelial cell lines. Scand J Immunol. 59:152–158. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Agrawal S, Misra R and Aggarwal A:
Autoantibodies in rheumatoid arthritis: association with severity
of disease in established RA. Clin Rheumatol. 26:201–204. 2007.
View Article : Google Scholar
|