Introduction
Sphingolipids are ubiquitous components of
eukaryotic cell membranes, which can be metabolized to ceramide,
sphingosine and their phosphorylated forms, including ceramide
1-phosphate (C1P) and sphingosine 1-phosphate (S1P), which possess
bioactivity and vital biological functions in cell growth and
survival (1). Experimental studies
have denoted S1P as one of the most significant sphingolipid
metabolites (2). S1P plays various
roles in physiological processes, promotes cellular proliferation,
stimulates cell survival and protects cells against apoptosis
through G-protein-coupled receptors (GPCRs) or an intracellular
receptor-independent mechanism (3,4).
Obesity represents a major risk factor of
cardiovascular and endocrine-related diseases, and is defined as an
excessive deposit of adipose tissue and cholesterol. Adipogenesis
is the process whereby preadipocytes undergo differentiation into
mature adipocytes. Adipocytes are derived from mesenchymal stem
cells, which have the potential to differentiate into myoblasts,
chondroblasts, osteoblasts or adipocytes (5). Adipocyte differentiation involves an
elaborate network of transcription factors that regulate the
expression of numerous genes responsible for the phenotype of
mature adipocytes (6). The main
transcription factor that mediates preadipocyte differentiation is
peroxisome proliferator-activated receptor γ (PPARγ), which is
considered the ‘master regulator of adipogenesis’. Other adipogenic
transcription factors include the CCAAT/enhancer binding proteins
(C/EBPα, C/EBPβ and C/EBPγ) (6–8).
The ATP-dependent phosphorylated form of
sphingosine, S1P, may only be produced from sphingosine by
sphingosine kinases, and S1P phosphatase dephosphorylates S1P to
sphingosine (9,10). Acylation of sphingosine and other
long-chain base sphingolipids leads to the formation of ceramide in
the turnover pathway (11). S1P
has been identified in numerous mammalian cells and other
organisms, and harbors distinctive biological functions (13).
Sphingolipids have emerged as multifunctional intra-
and intercellular signal-transducing molecules, in addition to
performing their well-established roles as structural components of
cellular lipid membrane bilayers. S1P belongs to a significant
group of signaling sphingolipids recognized to play a role in a
diverse array of cellular processes, including apoptosis, cell
motility, differentiation and proliferation in a variety of cell
types, including endothelial cells, smooth muscle cells and
macrophages (14,15).
S1P is generated by the phosphorylation of the
sphingosine mediated by sphingosine kinases 1 (Sphk-1) and 2
(Sphk-2) (16). These two isoforms
have been identified in mammals. Sphk enzyme activity is expressed
in various cell types, and is dynamically regulated by various
extracellular stimuli. S1P exerts most of its activity as a ligand
of GPCRs (17). Chemotherapy
induces downregulation of S1P by inhibiting Sphk. Lowering of the
circulating S1P by chemotherapy may switch S1P-mediated adipose
cell stasis to adipogenesis. However, the signal pathways of S1P
and the S1P receptors in the differentiation of preadipocytes in
the context of cellular physiology remain largely unknown.
In the present study, we examined the hypothesis
that S1P regulates adipogenic differentiation and modulates its
functions via S1P receptor 2 (S1P2). We provide evidence
that the adipogenesis of cultured mouse 3T3-L1 preadipocytes is
correlated with the S1P2 receptor, and further
demonstrate that pharmacological inhibition of S1P2
signals completely retrieved S1P-mediated downregulation of the
transcriptional levels of PPARγ, C/EBPα and adiponectin, which are
markers of adipogenic differentiation.
Materials and methods
Reagents
S1P was purchased from Cayman Chemical (Ann Arbor,
MI, USA) and Sigma-Aldrich (St. Louis, MO, USA). S1P was prepared
as a 2-mM solution in 0.3 M NaOH and then further diluted in cell
culture medium. SEW2871, a S1P1 agonist, and W146, a
S1P1 antagonist, were purchased from Cayman Chemical.
The S1P2 JTE-013 antagonist (Cayman Chemical) was
prepared as a 2-mM solution in ethanol and then further diluted in
cell culture medium. Anti-S1P2 goat polyclonal antibody was
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA).
Cell culture and differentiation
The 3T3-L1 cells were obtained from the American
Type Culture Collection (Manassas, VA, USA) and maintained in
Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum
and antibiotics (100 μg·ml−1 gentamycin and 100
μg·ml−1 penicillin-streptomycin). To induce
differentiation, 2-day postconfluent 3T3-L1 cells were incubated in
MDI induction medium (DMEM containing 10% fetal bovine serum, 0.5
mM 3-isobutyl-1-methylxanthine, 1 μm dexamethasone and 1 μg/ml
insulin) for 2 days. In certain experiments, S1P (10 μM), SEW2871
(from 0.1 to 50 μM), W146 (from 0.01 to 10 μM) and JTE-013 (from
0.02 to 2 μM) were added at the time of the induction of
differentiation. Two days after MDI media treatment (day 2), the
cell number was determined by a trypan blue exclusion test (Life
Technologies, Grand Island, NY, USA) and the medium was changed to
insulin medium. The AdipoRed assay and detection of glycerol
release contents were performed on day 7.
Quantification of lipid content
Lipid content was quantified using the commercially
available AdipoRed assay reagent (Lonza, Verviers, Belgium)
according to the manufacturer’s instructions. In brief,
preadipocytes grown in 24-well plates were incubated with MDI
medium or test compounds, including S1p, SEW2871, W146 and JTE-013,
during the adipogenic phase and, on day 7, culture supernatant was
removed and the cells were carefully washed with 500 μl
phosphate-buffered saline (PBS). The wells were then filled with
300 μl PBS, and 30 μl AdipoRed reagent was added and incubated for
10 min at 37°C. Fluorescence was measured with an excitation of 485
nm and emission of 572 nm.
Real-time polymerase chain reaction
(PCR)
Total RNA was extracted from 3T3-L1 cells treated
with S1P using the Easy-spin™ total RNA extraction kit (Intron
Biotechnology, Seoul, Korea). cDNA synthesis was carried out
following the instructions of the Takara PrimeScript™ First Strand
cDNA synthesis kit (Takara Bio, Tokyo, Japan). For real-time PCR, 1
μl gene primers with SYBR-Green (Bio-Rad Laboratories, Hercules,
CA, USA) in 20 μl reaction volume was applied. The sequences of the
primers used for real-time PCR were as follows: S1P1
(forward, 5′GAAACTACACAACGGGAGCAACAG3′ and reverse,
5′AAGCAGGAGCAGAGTGAAGACG3′), S1P2 (forward,
5′AACAGCAAGTTCCACTCAGCAATG3′ and reverse,
5′GGCGGAGAGCGTGATGAAGG3′), PPARγ (forward,
5′CGGAAGCCCTTTGGTGACTTTATG3′ and reverse,
5′GCAGCAGGTTGTCTTGGATGTC3′), C/EBPα (forward,
5′CGGGAACGCAACAACATCGC3′ and reverse, 5′TGTCCAGTTCACGGCTCAGC3′),
adiponectin (forward, 5′TGACGGCAGCACTGGCAAG3′ and reverse,
5′TGATACTGGTCGTAGGTGAAGAGAAC3′), β-actin (forward,
5′TGAGAGGGAAATCGTGCGTGAC3′ and reverse,
5′GCTCGTTGCCAATAGTGATGACC3′). All reactions with iTaq SYBR-Green
Supermix were performed on the CFX96 real-time PCR detection system
(Bio-Rad Laboratories).
Western blot analysis
The 3T3-L1 cells were lysed in a lysis buffer (25 mM
HEPES; pH 7.4, 100 mM NaCl, 1 mM EDTA, 5 mM MgCl2, 0.1
mM dithiothreitol and protease inhibitor mixture). Proteins were
electrophoretically resolved on an 8–15% sodium dodecyl sulfate
gel, and immunoblotting was performed as previously described
(18). Images were captured using
the Fusion FX7 acquisition system (Vilbert Lourmat, Eberhardzell,
Germany). The antibodies used for immunoblotting were PPARγ,
S1P1, S1P2 (Santa Cruz Biotechnology, Inc.)
and β-actin (Sigma-Aldrich).
Statistical evaluation
All data are expressed as the mean ± SEM, and the
data were compared using Student’s t-test, analysis of variance and
Duncan’s multiple range test, with the SAS statistical package (SAS
Institute, Inc., Cary, NC, USA). P<0.05 and P<0.01 were
considered to indicate a statistically significant difference.
Results
S1P1 and S1P2
receptor expression in preadipocytes and differentiated
adipocytes
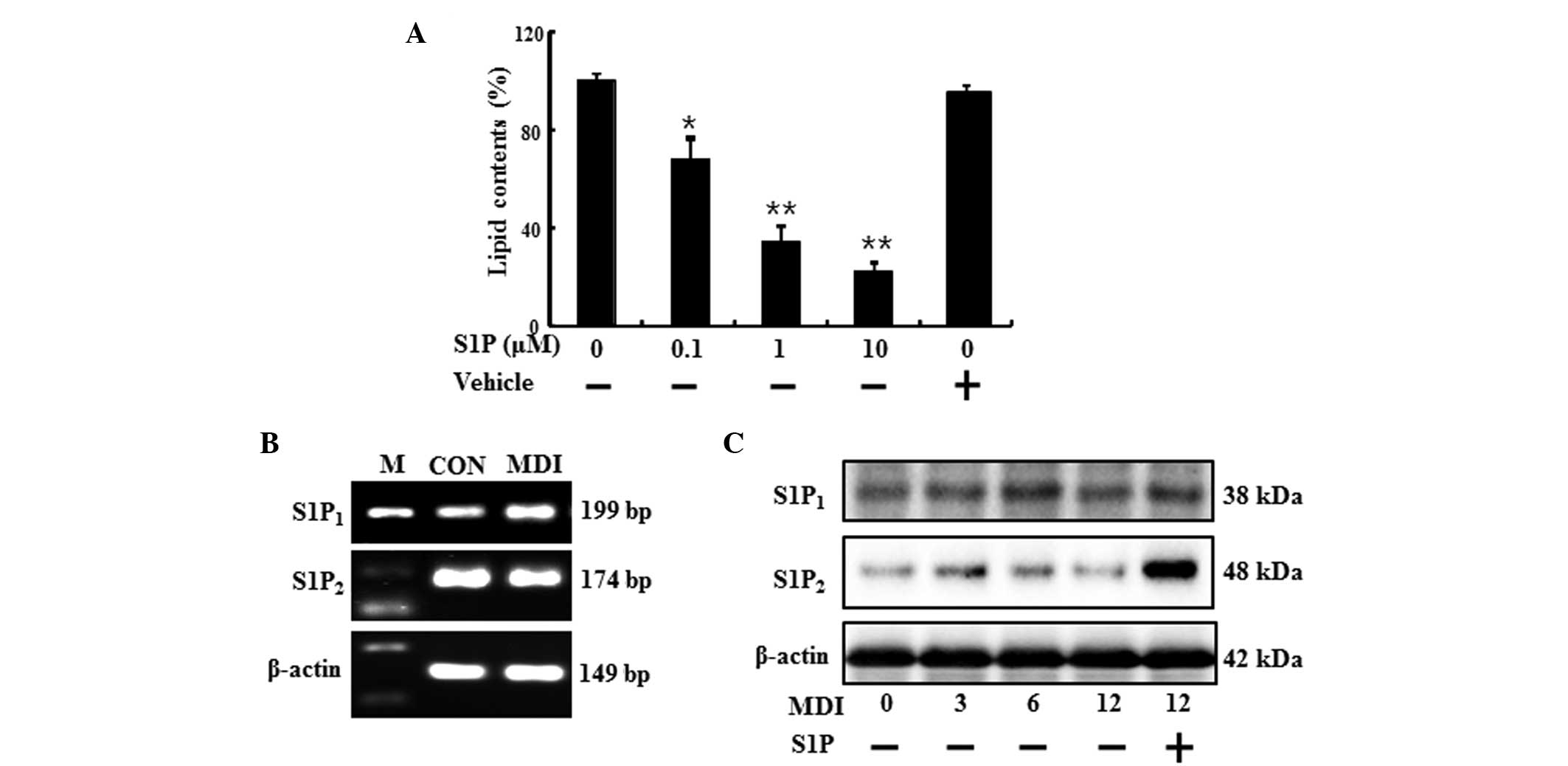
When 3T3-L1 cells were differentiated for over 6
days in the presence of S1P, lipid accumulation was reduced
dose-dependently (Fig. 1A). The
biological action of S1P is largely ascribed to ligation to
specific S1PRs, which evokes distinct biological responses. To
assess which receptor is associated with the S1P anti-adipogenic
effect, the expression pattern of the known S1P-specific receptors
was studied in the 3T3-L1 cells following the addition of MDI
(Fig. 1B and C). As shown in
Fig. 1B, the preadipocytes and
differentiated adipocytes expressed mRNA for S1P1 and
S1P2 receptors, as indicated by the presence of 199- and
174-bp bands, respectively. In addition, the treatment of S1P
increased the protein expression of S1P2 receptor
(Fig. 1C). These results indicated
that preadipocytes and differentiated adipocytes express
S1P1 and S1P2 receptor, which are known to be
associated with cell differentiation, and these receptors may be
correlated with the anti-adipogenic function of S1P.
S1P1 receptor does not affect
adipogenic differentiation
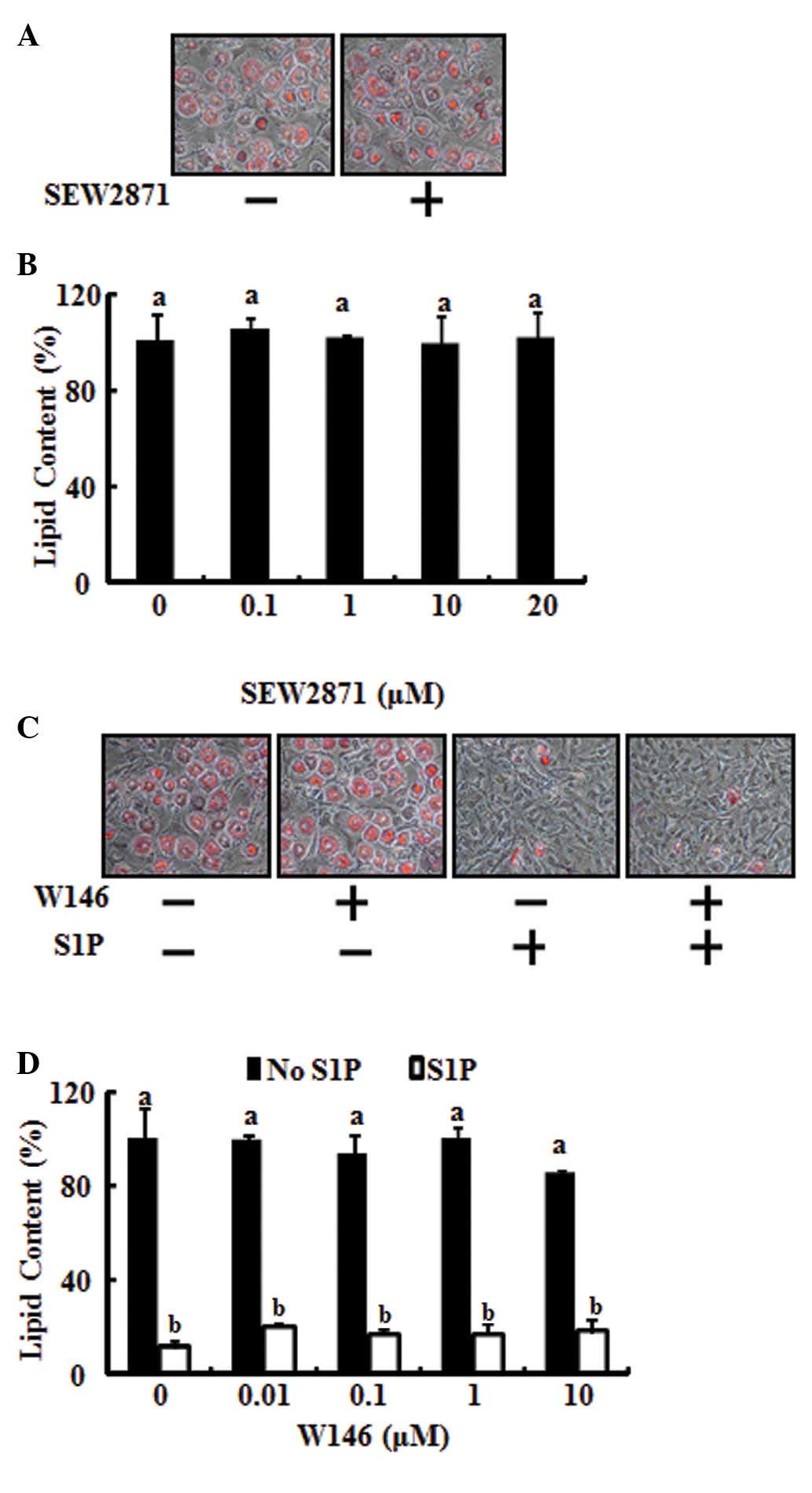
To test the involvement of the S1P1
receptor in anti-adipogenic differentiation, 3T3-L1 preadipocytes
were stimulated with SEW2871, a S1P1 selective agonist,
to specifically activate the S1P1 receptor. As shown in
Fig. 2A and B, SEW2871 had no
effect on the adipocyte differentiation inhibitory action of S1P.
In addition, the inhibitory action of S1P on lipid accumulation was
not restored by blocking the S1P1 receptor using W146, a
selective S1P1 antagonist (Fig. 2C and D). These results demonstrate
that the anti-adipogenic action of S1P does not occur through the
S1P1 receptor.
Adipogenic differentiation is regulated
via activation of the S1P2 receptor and blockade of the
S1P2 receptor using S1P2 antagonist
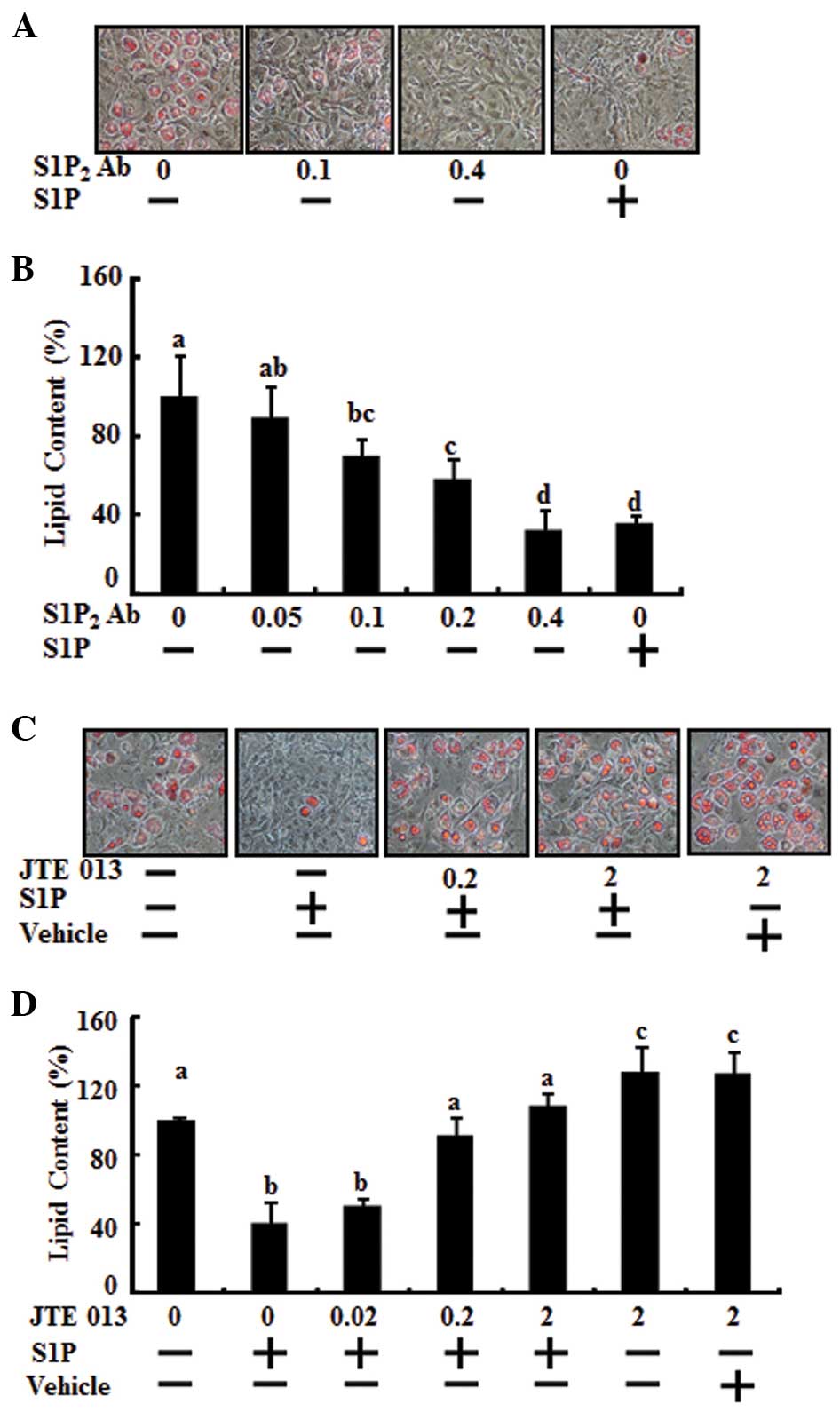
To determine whether the S1P2 receptor
was involved in the anti-adipogenic activity of S1P, adipocyte
differentiation was analyzed in mouse 3T3-L1 preadipocytes. Day 0
postconfluent 3T3-L1 preadipocytes were induced to differentiate
into adipocytes using the standard differentiation protocol and
co-treatment with S1P2 antibody, dose-dependently
(0.05–0.4 μg), or S1P (10 μM). The effect of S1P2
antibody was significant at 0.1 μg and was maximal at 0.4 μg,
having the same anti-adipogenic effect of 10 μM S1P (Fig. 3A and B). Differentiation of the
preadipocytes was induced by MDI treatment and co-treatment with
JTE013 as a S1P2 antagonist at various doses (from 0.02
μM to 2 μM) and/or 10 μM S1P during the adipogenesis period. When
JTE013 was added to the cultures, the reduction of lipid
accumulation by S1P, compared with the MDI control, was restored as
treatment with JTE013 increased (Fig.
3C and D). The effect of JTE013 was significant at 0.2 μM and
was maximal at 2 μM. At 0.2 μM JTE013, lipid accumulation returned
to the triglyceride level of the MDI control. Moreover, 2 μM of
JTE013 increased lipid accumulation compared with MDI induction
only. A single treatment of JTE013 alone enhanced adipocyte
differentiation (Fig. 3C and
D).
S1P induces downregulation of PPARγ,
C/EBPα and adiponectin expression
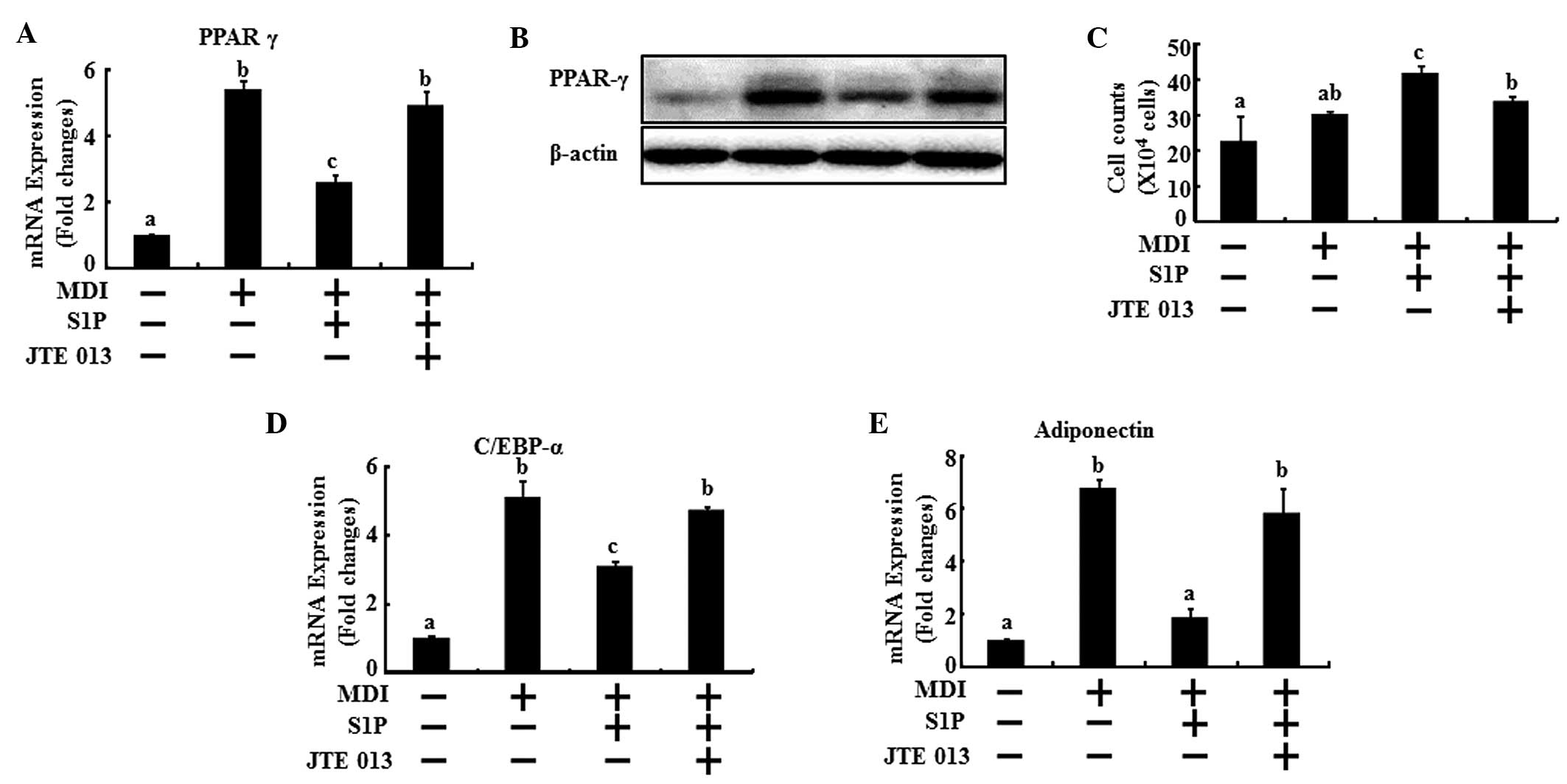
Next, the mRNA levels of the key genes of
differentiation were examined to evaluate the effects of S1P on the
regulation of the main transcriptional factors in adipogenesis via
the S1P2 receptor. The 3T3-L1 cells underwent
differentiation with MDI treatment and were co-treated with 10 μM
S1P and/or 0.2 μM JTE013. After two days, the cells were collected
for real-time PCR. A reduction in the mRNA levels of PPARγ, C/EBPα
and adiponectin was observed with the addition of S1P; however, the
S1P2 antagonist effectively restored the mRNA levels of
PPARγ, C/EBPα and adiponectin to the level of the MDI control
(Fig. 4A, D and E). In addition,
the protein expression of PPARγ showed the same results as the mRNA
levels (Fig. 4B). In addition, the
anti-mitotic activity of C/EBPα induces 3T3-L1 cell arrest
(19). Following the initial phase
of DNA replication, 3T3-L1 cells are arrested in the G1 phase of
the cell cycle. The arrest coincides with the induction of C/EBPα
expression (20). These previous
studies supported our results that S1P treatment increases the
number of cells 48 h after MDI induction, which coincides with a
lowering of C/EBPα expression. However, in the presence of JTE013,
the numbers of cells were lowered and the C/EBPα expression was
reversed similar to MDI induction in the controls (Fig. 4C and 4D). It was observed that cell
numbers of the MDI induction were doubled in the control and those
of the S1P treatment were similar to the control. There were no
significant quantitative differences between the control and MDI
induction. MDI induction and S1P co-treatment increased cell
numbers effectively compared with MDI treatment alone (Fig. 4C). These results demonstrate that
the S1P2 receptor plays a dominant role in the
anti-differentiating action of S1P.
Discussion
We have shown that treatment with exogenous S1P
effectively inhibits adipogenesis by downregulating the expression
of adipocyte-specific differentiation markers. Neither the
S1P1 selective agonist (SEW2871) nor the antagonist
(W146) affected the adipocyte differentiation function of S1P. By
contrast, abrogation of the S1P2 receptor recovered the
impaired differentiation caused by S1P as well as enhancing
differentiation. These anti-adipogenic effects of S1P via the
S1P2 receptor occurred through the regulation of MAPK
pathways and the master of adipogenic transcriptional factors.
There is a previous relevant study concerning Sphk-1
and adipogenesis, which reveals that Sphk-1 is induced and S1P
content increased in adipogenesis, and that its downregulation
(using Sphk-1 inhibitors and siRNA) blocks differentiation
(21). In addition, S1P content
increased in adipogenesis. However, there was no information as to
whether direct treatment of S1P has a pro-adipogenic effect. In
addition, there is no information on the adipogenic effect of
activating Sphk-1 and whether or not the activation of Sphk-1
promotes adipogenesis or increases S1P content. Therefore, we
examined the direct effect of S1P and our results revealed that the
direct effect of S1P exerted an anti-adipogenic effect via
S1P2.
Numerous signaling pathways that are activated in
response to the stimulation of cells by S1P are initiated by
activation of S1P-specific receptors. The results of this study
revealed that the anti-adipogenic action of S1P was not associated
with S1P1 (Fig. 2).
Neither the S1P1 selective agonist (SEW2871) nor the
S1P1 antagonist (W146) affected the inhibition of
adipocyte differentiation by S1P. The anti-adipogenic effect of S1P
occurred via S1P2. In addition, the S1P2
selective antagonist significantly reversed the inhibition of S1P
during adipocyte differentiation (Fig.
3). A single treatment of JTE013 alone enhanced adipocyte
differentiation. The adipogenesis process is tightly controlled by
PPARγ and C/EBPα. PPARγ is the master regulator of adipogenesis.
C/EBPα induces numerous adipocyte genes directly (22). As a result, as noted in Fig. 4, S1P lowered PPARγ and C/EBPα
expression; however, S1P lost its ability to impair PPARγ and
C/EBPα expression in the abrogation of S1P2 using JTE013
treatment (Fig. 4). These previous
studies supported our results that S1P treatment increases the
number of cells 48 h after MDI induction, which coincides with a
lowering of C/EBPα expression. However, in the presence of JTE013,
the numbers of cells were lowered and C/EBPα expression was
reversed similar to MDI induction in the controls (Fig. 4).
In conclusion, the results of this study
demonstrated that exposure of preadipocytes to S1P inhibited their
differentiation into adipocytes, as confirmed by a reduction in
triglyceride accumulation and a reduction in the expression of
adipocyte-specific genes. Therefore, S1P functioned as an
anti-adipogenic compound and S1P2 was shown to be
responsible for the anti-adipogenic activity of S1P. This study
identifies the activation of S1P2 receptor as a possible
mechanism of the anti-adipogenic differentiating action of S1P and
suggests that S1P2 activation may be a therapeutic
target for anti-obesity.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea (NRF), funded by the Korean
government (2012R1A1B3000463).
Abbreviations:
|
S1P
|
sphingosine 1-phosphate
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
C/EBPα
|
CCAAT/enhancer binding protein α
|
References
|
1
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olivera A and Spiegel S:
Sphingosine-1-phosphate as second messenger in cell proliferation
induced by PDGF and FCS mitogens. Nature. 365:557–560. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Spiegel S and Milstien S:
Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol
Cell Biol. 4:397–407. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goparaju SK, Jolly PS, Watterson KR, et
al: The S1P2 receptor negatively regulates platelet-derived growth
factor-induced motility and proliferation. Mol Cell Biol.
25:4237–4249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rayalam S, Della-Fera MA and Baile CA:
Phytochemicals and regulation of the adipocyte life cycle. J Nutr
Biochem. 19:717–726. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee H, Lee YJ, Choi H, Ko EH and Kim JW:
Reactive oxygen species facilitate adipocyte differentiation by
accelerating mitotic clonal expansion. J Biol Chem.
284:10601–10609. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nerurkar PV, Lee YK and Nerurkar VR:
Momordica charantia (bitter melon) inhibits primary human adipocyte
differentiation by modulating adipogenic genes. BMC Complement
Altern Med. 10:342010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yanagiya T, Tanabe A and Hotta K:
Gap-junctional communication is required for mitotic clonal
expansion during adipogenesis. Obesity (Silver Spring). 15:572–582.
2007. View Article : Google Scholar
|
|
9
|
Hait NC, Oskeritzian CA, Paugh SW,
Milstien S and Spiegel S: Sphingosine kinases, sphingosine
1-phosphate, apoptosis and diseases. Biochim Biophys Acta.
1758:2016–2026. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hannun YA, Luberto C and Argraves KM:
Enzymes of sphingolipid metabolism: from modular to integrative
signaling. Biochemistry. 40:4893–4903. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith ER and Merrill AH Jr: Differential
roles of de novo sphingolipid biosynthesis and turnover in the
‘burst’ of free sphingosine and sphinganine, and their 1-phosphates
and N-acyl-derivatives, that occurs upon changing the medium of
cells in culture. J Biol Chem. 270:18749–18758. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cremesti AE, Goni FM and Kolesnick R: Role
of sphingomyelinase and ceramide in modulating rafts: do
biophysical properties determine biologic outcome? FEBS Lett.
531:47–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spiegel S and Milstien S: Sphingosine
1-phosphate, a key cell signaling molecule. J Biol Chem.
277:25851–25854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Johnstone ED, Chan G, Sibley CP, Davidge
ST, Lowen B and Guilbert LJ: Sphingosine-1-phosphate inhibition of
placental trophoblast differentiation through a G(i)-coupled
receptor response. J Lipid Res. 46:1833–1839. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goetzl EJ, Wang W, McGiffert C, Liao JJ
and Huang MC: Sphingosine 1-phosphate as an intracellular messenger
and extracellular mediator in immunity. Acta Paediatr Suppl.
96:49–52. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He X, H’Ng SC, Leong DT, Hutmacher DW and
Melendez AJ: Sphingosine-1-phosphate mediates proliferation
maintaining the multipotency of human adult bone marrow and adipose
tissue-derived stem cells. J Mol Cell Biol. 2:199–208. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schuppel M, Kurschner U, Kleuser U,
Schafer-Korting M and Kleuser B: Sphingosine 1-phosphate restrains
insulin-mediated keratinocyte proliferation via inhibition of Akt
through the S1P2 receptor subtype. J Invest Dermatol.
128:1747–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moon MH, Jeong JK, Seo JS, et al:
Bisphosphonate enhances TRAIL sensitivity to human osteosarcoma
cells via death receptor 5 upregulation. Exp Mol Med. 43:138–145.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ntambi J and Young-Cheul K: Adipocyte
differentiation and gene expression. J Nutr. 130:3122S–3126S.
2000.
|
|
20
|
Reichert M and Eick D: Analysis of cell
cycle arrest in adipocyte differentiation. Oncogene. 18:459–466.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hashimoto T, Igarashi J and Kosaka H:
Sphingosine kinase is induced in mouse 3T3-L1 cells and promotes
adipogenesis. J Lipid Res. 50:602–610. 2009. View Article : Google Scholar :
|
|
22
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006. View
Article : Google Scholar : PubMed/NCBI
|