Introduction
Keloids and hypertrophic scars are two common
proliferative lesions resulting from abnormal wound healing
(1). Unlike hypertrophic scars,
which are limited to the boundaries of the original wound and
degenerate spontaneously, keloids, which are considered to be
dermal benign fibroproliferative tumors, outgrow the original wound
edges and invade the adjacent normal dermis, the regression of
which over time is rare (2).
Despite numerous available treatments, including excision,
treatment for keloids remains ineffective due to their recurrence
(3,4).
At initiation, keloids are defined as granulomatous
lesions characterized by capillary proliferation (5). The areas of angiogenesis in keloids
may represent overproliferation of vascular granulation tissue in
aberrant wound healing, in accordance with the pathology of
keloids, which are characterized by an exuberant healing response
(6). Several studies have
suggested that angiogenesis and vascular factors are important in
keloid progression, due to their prolonged erythematous period and
invasive characteristics. In keloids, endogenous transforming
growth factor-β1 (TGF-β1) and vascular endothelial growth factor
(VEGF) promote angiogenesis (5,7,8). The
levels of VEGF are upregulated and the levels of endostatin are
downregulated in the sera and tissue of patients with keloids
compared with normal controls, which may contribute to the
imbalance in angiogenesis present in keloids (9). Upregulation in the notch signaling
pathway also induces angiogenesis in keloids (10). Despite previous investigation of
the angiogenic activity of keloids in previous studies, the
underlying regulatory mechanism of angiogenesis in keloids remains
to be elucidated.
Our previous study demonstrated that the expression
of periostin is upregulated in keloids compared with hypertrophic
scars and normal skin tissue (11). Periostin is a 90-kDa secreted
extracellular matrix (ECM) protein, which is expressed mainly in
collagen-rich, fibrous connective tissue (12). The upregulation of periostin has
been observed in cutaneous wound healing, cutaneous fibrosis and in
tumor progression. It is also involved in the survival and
differentiation of cells, metastasis and in ECM remodeling
(13,14). Periostin-deficient animal models
demonstrate delayed wound repair (15) and periostin has been described as a
novel pro-angiogenic factor leading to significant enhancement of
angiogenesis in human breast, gastric and ovarian cancer (16,17).
The acquired expression of periostin promotes tumor angiogenesis
through upregulation in the expression of VEGFR2 (18).
Based on the evidence previously mentioned, the
present study hypothesized that periostin is important in
angiogenesis in keloid and, therefore, examined the expression of
periostin in keloids and in normal skin and its association with
blood vessel density. Subsequently, the present study investigated
whether the periostin that is secreted by keloid fibroblasts (KFs)
affects endothelial cell migration and angiogenesis and aimed to
elucuidate the underlying mechanism in vitro. Furthermore,
the association between periostin and other known angiogenic
factors, including VEGF and angiopoietin-1 (Ang-1) were
examined.
Materials and methods
The present study was performed in compliance with
the regulations of the Medical Ethics Committee of Peking
University Third Hospital, Beijing, China.
Skin specimens and immunochemistry
Specimens of keloid tissue (n=15) and normal skin
(n=11) were obtained from the discarded tissues of patients
receiving plastic surgery to remove the keloid, following the
provision of written informed consent. None of the patients (9–84
years old) had received treatment for the keloids prior to surgical
excision.
The tissues were fixed in 4% paraformaldehyde,
embedded in paraffin and mounted on glass slides. These were
deparaffinized and immersed in 0.01 M citrate buffer (pH 6.0;
Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China),
heated in a microwave oven (Midea, Fushan, China), cooled for 30
min to room temperature and then washed in water. Endogenous
peroxidase was inhibited using 3% H2O2. The
slides were then incubated at 4°C overnight with a rabbit antibody
against human periostin (1:100; ab14041; Abcam, Cambridge, MA, USA)
or mouse antibody against human CD31 (1:80; ZM-0044) or CD105
(1:50; ZM-0297) from Zhongshan Golden Bridge Biotechnology Co.,
Ltd. Phosphate-buffered saline (PBS; Zhongshan Golden Bridge
Biotechnology Co., Ltd.) was used as a negative control. The
antibodies were diluted in 1% bovine serum albumin (BSA; Sigma, St.
Louis, MO, USA)/Tris-buffered saline (Zhongshan Golden Bridge
Biotechnology Co., Ltd.). Following washing, the slides were
incubated with biotinylated anti-rabbit or anti-mouse
immunoglobulin (Ig)G (Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) secondary antibody (60 min at 37°C). Following washing with
PBS, the slides were stained using a diaminobenzidine kit
(Zhongshan Golden Bridge Biotechnology Co., Ltd.). Image-Pro Plus
6.0 (Media Cybernetics, Inc., Bethesda, MD, USA) was used for
quantified analysis to calculate the integrated optical density of
each antigen density.
Primary cell culture and treatment
The KFs were isolated from the discarded keloid
tissues obtained from the patients during surgery (n=6). All keloid
tissues were obtained from untreated, primary lesions. The KFs were
maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco,
Grand Island, NY, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone, Logan, UT, USA) containing 100 U/ml penicillin and
100 U/ml streptomycin (Invitrogen Life Technologies, Carlsbad, CA,
USA). The KFs between passages 4 and 8 were used in the subsequent
experiments. Primary human umbilical vein endothelial cells
(HUVECs) were isolated from segments of the human umbilical cord
vein by collagenase digestion and cultured in medium 199 (M199)
supplemented with 10% FBS, as described previously (19). The subsequent experiments were
conducted using cells at passages between 2 and 6.
Recombinant human periostin protein (rhPN;
BioVendor, Brno, Czech Republic) was treated with different
concentrations (10, 50 and 100 ng/ml). For inhibitory studies, the
cells were pretreated with 10 μM U1206 [extracellular
signal-regulated kinase 1/2 (ERK1/2) inhibitor; Cell Signaling
Technology, Beverly, MA, USA)] or 10 μM focal adhesion kinase (FAK)
inhibitor 14 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)
for 1 h at 37°C. For knock down of periostin, the KFs were
transfected with short hairpin RNA (shRNA; Genechem, Shanghai,
China) against periostin, as previously described (20).
Western blot analysis
The KFs were washed twice with ice-cold PBS and then
harvested with lysis buffer (Beyotime, Shanghai, China) containing
phosphatase and protease inhibitors. The protein concentration was
quantified using a Bicinchoninic acid Protein Assay kit (CoWin
Biotech, Beijing, China). The proteins in the lysates were
separated using sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE; Beyotime) and were then transferred onto
a nitrocellulose membrane (Applygen Technologies, Inc., Beijing,
China), which was inhibited with 5% BSA at room temperature for 1
h. This was then incubated at 4°C overnight with the primary
antibodies, anti-protein kinase B (Akt) (ab32038, rabbit monoclonal
to Akt, 1:1,000) or anti-phospho-Akt [ab81283, rabbit monoclonal to
Akt (S473), 1:1,000) (both purchased from Abcam, Cambridge, UK),
anti-ERK1/2 (ab17942, rabbit polyclonal to ERK1 + ERK2, 1:1,000) or
anti-phospho-ERK1/2 [ab76165, rabbit polyclonal to Erk1
(pT202/pY204) + Erk2 (pT185/pY187), 1:1,000] (both purchased from
Epitomics, Cambridge, UK), anti-FAK (ab40794, rabbit monoclonal to
FAK, 1:500) or anti-phospho-FAK [ab4803, rabbit polyclonal to FAK
(phospho Y397), 1:500] (both purchased from Abcam), or anti-β-actin
(TA-09, mouse monoclonal to β-actin; Zhongshan Golden Bridge
Biotechnology, Inc.). This was followed with the corresponding IgG
secondary antibody (1:10,000; LI-COR Biosciences, Lincoln, NE, USA)
for 1 h away from light. All antibodies were anti-human. The
membranes were then scanned using the Odyssey Infrared Imaging
system (LI-COR Biosciences) to detect the expression quantity of
each protein.
Enzyme-linked immunosorbent assay
(ELISA)
The secreted VEGF and Ang-1 were quantified using
ELISA kits (Neobioscience Technology Co., Ltd., Beijing, China and
Abcam, respectively). Briefly, the 96-well microplates were coated
overnight with 100 μl capture antibody in a sealed bag at room
temperature, washed three times with wash buffer, then inhibited
with reagent diluent for 1 h. A 100 μl quantity of all standards
and cell medium samples were added to the 96-well plate for
incubation for 2 h. Following 2 h incubation with the detection
antibody, 20 min incubation with a working dilution of horseradish
peroxidase-conjugated streptavidin and 20 min incubation with the
substrate solution in a light-resistant container, stop solution
was added to each well. The absorbance was calculated at 450 nm,
correcting for plate artifacts at 570 nm and a log-transformed
standard curve.
Fibroblast-conditioned medium
preparation
Cell-conditioned medium was harvested from the KFs
or normal fibroblasts (NFs) 3 days following plating. These were
centrifuged at 2×104 rpm for 5 min to remove cellular
debris and were stored at −20°C prior to the tube formation assay.
Prior to performing the assays, the conditioned medium and M199 at
10% were mixed at a 1:1 ratio.
Cell migration assay
The KFs that were incubated with DMEM with 0.1% FBS
were seeded at 5×104/ml onto Transwell inserts (Millipore,
Billerica, MA, USA) with an 8 μm pore-size membrane above 24-well
plates and 600 μl DMEM with 10% FBS was added to each well.
Following incubation for 24 h, the inserts were fixed with methanol
for 15 min and washed with PBS prior to staining with 0.5% crystal
violet solution (Sigma) for 30 min. The number of migrated cells
was counted using phase-contrast microscopy (magnification, ×200).
Six randomly selected fields were counted per insert.
Tube formation assay
The 96-well plates were coated with 100 μl
growth-factor-reduced Matrigel™ (BD Biosciences, San Jose, CA,
USA), which was allowed to polymerize for 1 h at 37°C. The HUVECs
(2×104 cells/well), which were treated with different
conditioned medium were seeded onto the Matrigel™. Tube formation
and were assessed following 24 h incubation under an inverted phase
contrast microscope (Olympus, Tokyo, Japan). The mean total length
of the tube formed was determined using Image-Pro Plus software
(Media Cybernetics LP, Silver Spring, MD, USA) in 6 randomly
selected fields/well.
Statistical analysis
Statistical analysis involved use of SPSS 12.0 (SPSS
Inc., Chicago, IL, USA). Quantitative data are expressed as the
mean ± standard deviation (SD) from at least three independent
experiments. Pearson’s correlation test was used for correlative
analysis. One-way ANOVA followed by Scheffe’s post-hoc test were
used to compare multiple groups. P<0.05 indicated a
statistically significant difference.
Results
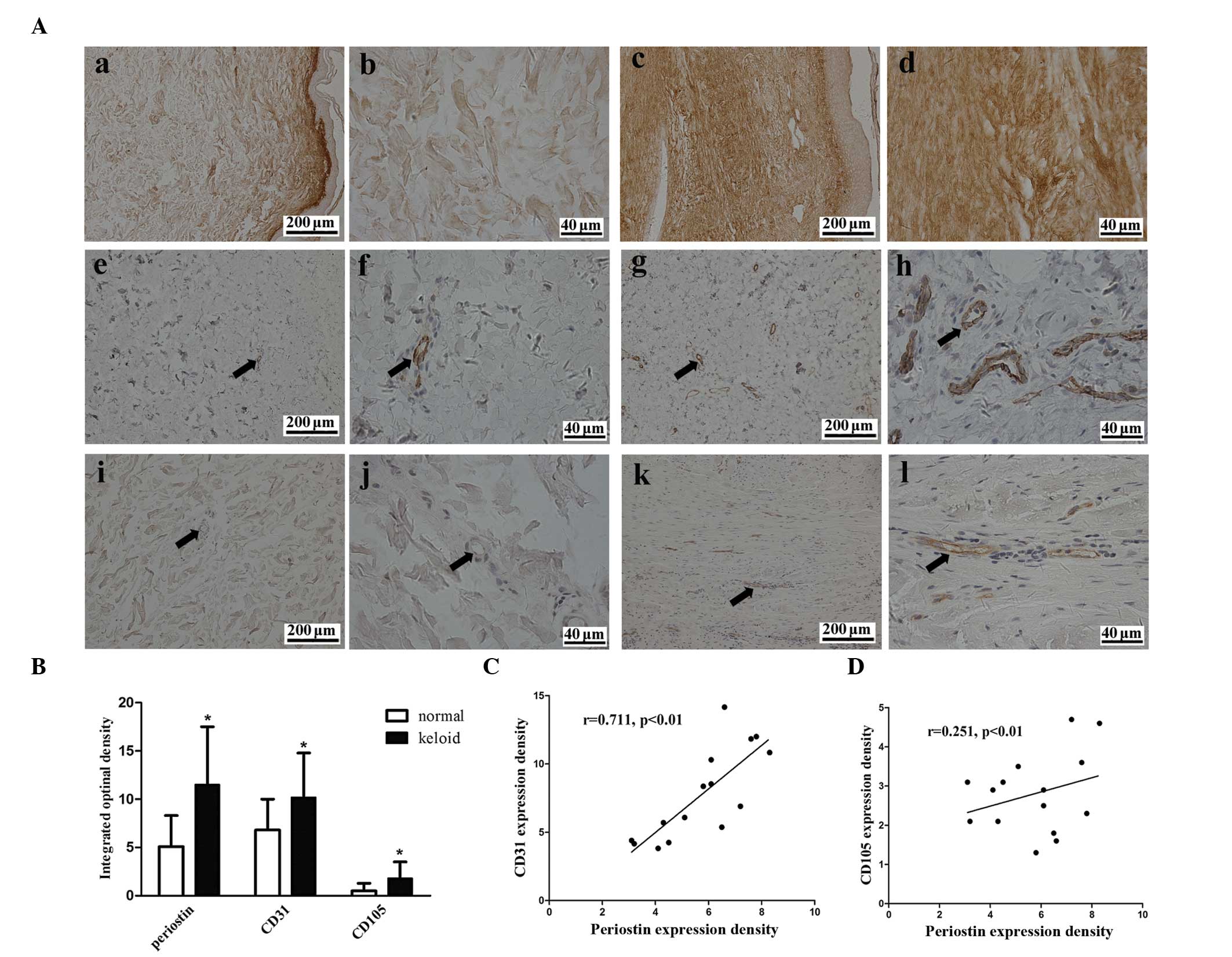
Periostin level is increased and
correlated with blood vessel density in human keloid tissue
The expression of periostin and the vascular
endothelial-cell markers CD31 and CD105 were examined using
immunohistochemistry to examine whether the expression of periostin
and the density of blood vessels differed between the keloid and
the normal skin tissue. Periostin was expressed in the epidermis
and dermis of the two tissues (Fig.
1A). The protein level of periostin was 2.3-fold higher in the
keloid tissue compared with the normal skin (Fig. 1B). Blood vessel density, which was
assessed by CD31 and CD105 staining, was 1.6- and 3.6-fold higher
in the keloidtissue compared to the normal tissue, respectively
(Fig. 1B). A marked positive
correlation was observed between the level of periostin protein and
the blood vessel density in the CD31 staining (r=0.711, P<0.01)
and a weak correlation was observed following CD105 staining
(r=0.251, P<0.01) in keloid tissue (Fig. 1C and D).
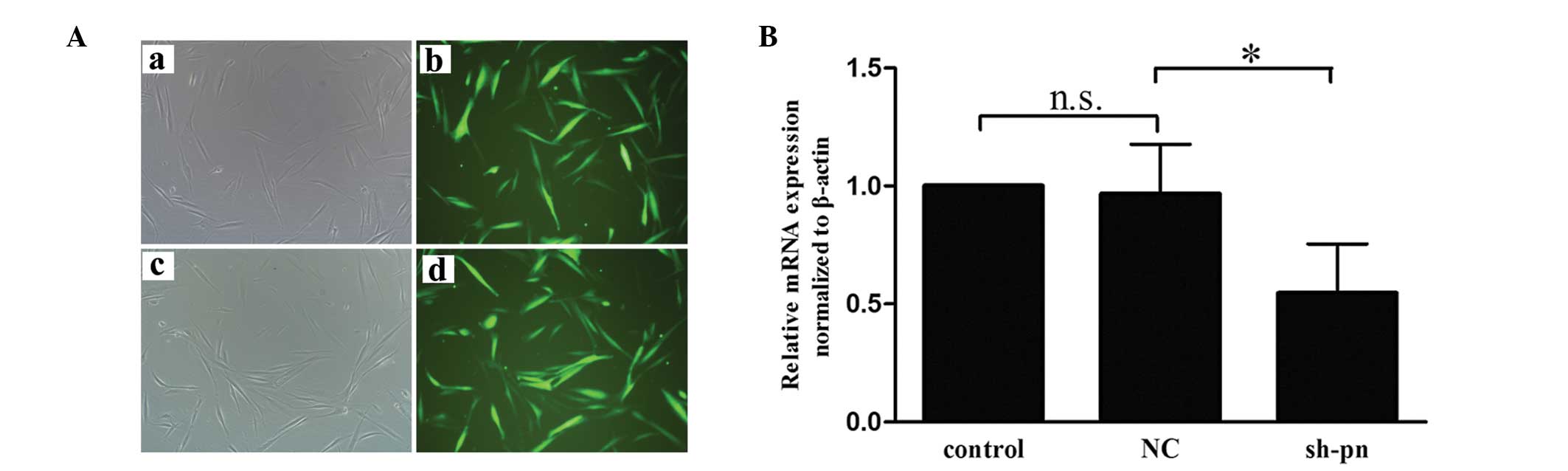
Upregulated periostin in conditioned
medium from KFs increases HUVEC migration and tube formation
To determine whether the periostin secreted by
fibroblasts affected angiogenesis, the present study examined the
migration and tube formation of HUVECs using conditioned medium
from the KFs and NFs. Firstly, the KFs were transfected with either
shRNA against periostin or control shRNA. Fluorescence microscopy
and reverse transcription quantitative polymerase chain reaction
(RT-qPCR) analyses were performed to ensure the efficiency of
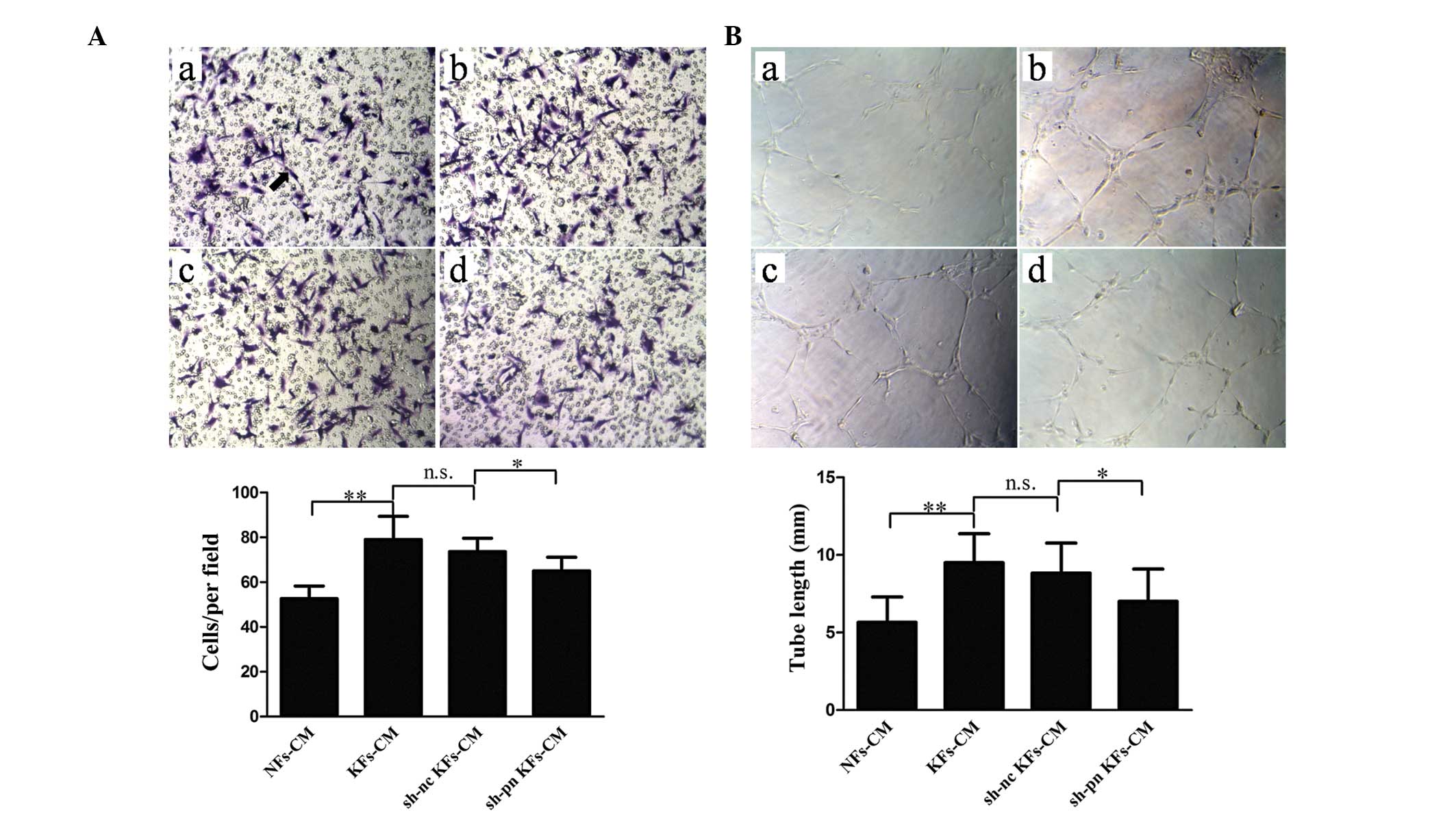
transfection prior to each experiment (Fig. 2). Use of the KF-conditioned medium
significantly increased the number of migrating cells compared with
the NF-conditioned medium (Fig.
3A). In addition, KF-conditioned medium promoted tube formation
when compared with the NF-conditioned medium (Fig. 3B). Notably, the conditioned medium
from the KFs with shRNA knock down of periostin significantly
inhibited migration and tube formation compared with the controls.
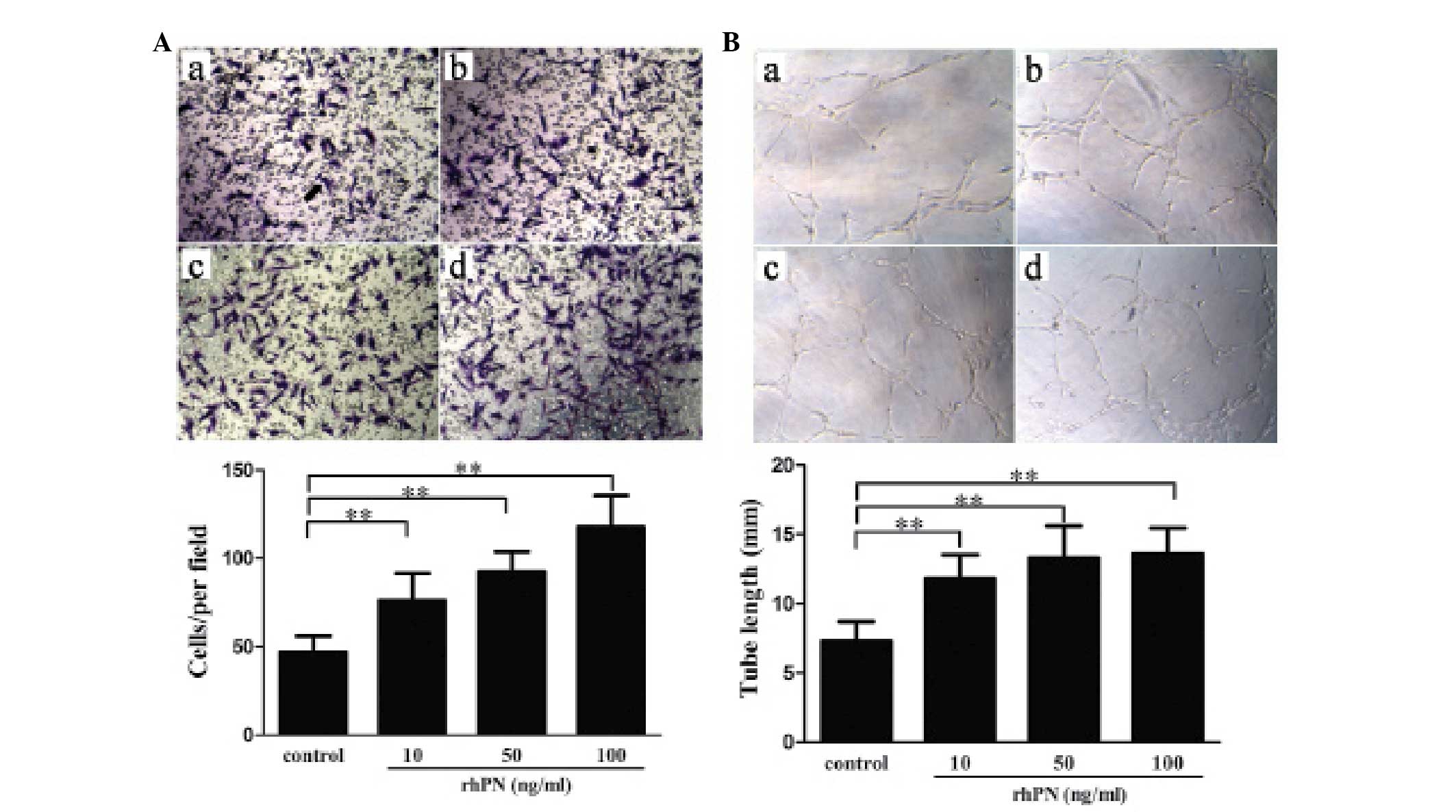
To exclude the effect of other factors in the conditioned medium,
the effect of rhPN treatment at different concentrations on HUVEC
migration and tube formation was examined. Contrary to the effects
of conditioned medium from the KFs with shRNA periostin-knockdown,
treatment with rhPN dose-dependently promoted migration (Fig. 4A) and tube formation (Fig. 4B).
Periostin promotes HUVEC migration and
tube formation by activating the ERK1/2 and FAK pathways
To clarify the mechanism of periostin-promoted
angiogenesis, the present study examined the involvement of several
intracellular signaling molecules, including FAK, Akt and ERK in
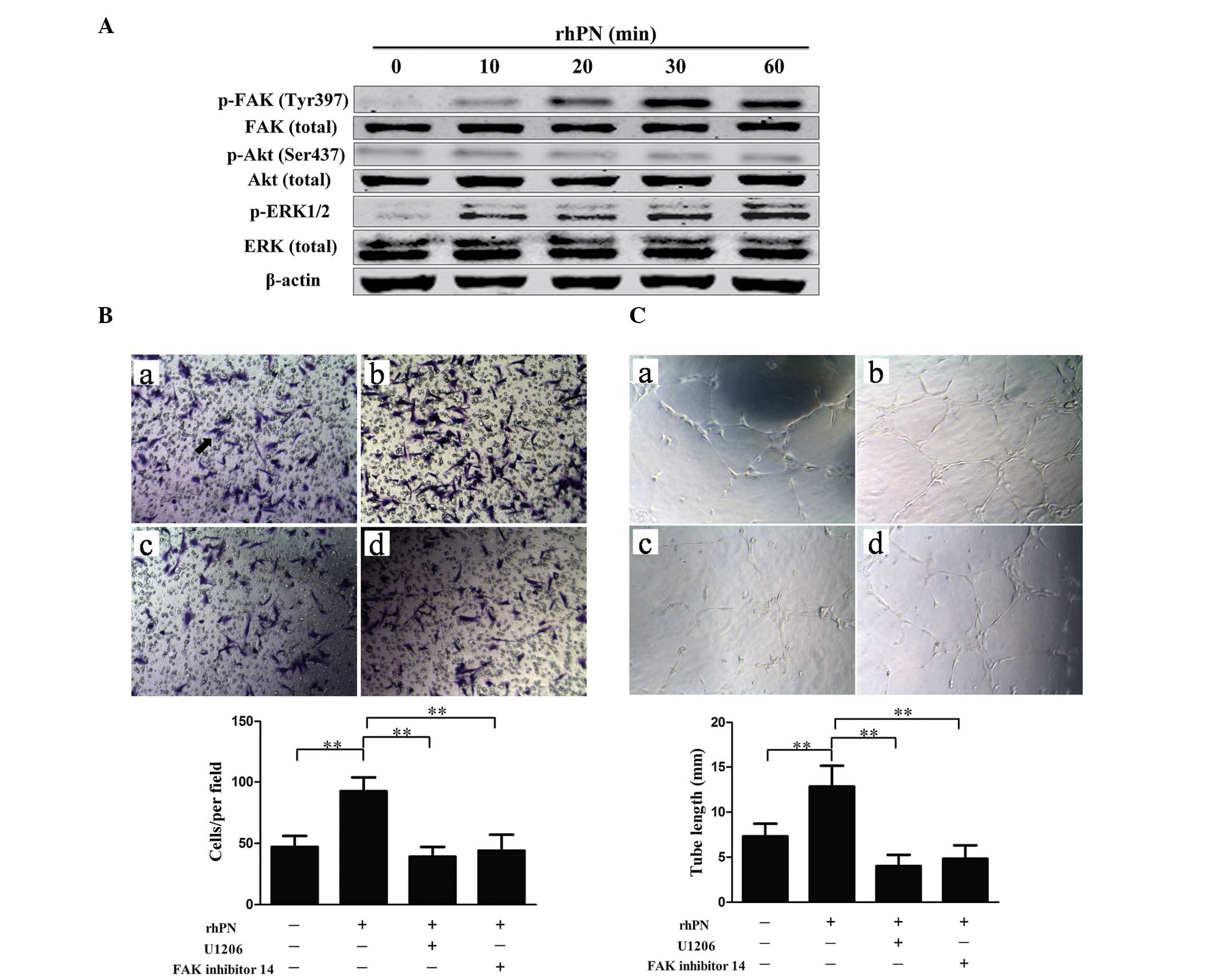
the HUVECs following incubation with rhPN. Incubation with rhPN
increased the phosphorylation of FAK and ERK (Fig. 5A). To demonstrate the involvement
of FAK and ERK in periostin-promoted angiogenesis, capillary tube
formation was examined following treatment with either FAK
inhibitor 14 or ERK inhibitor (U0126) together with rhPN. Treatment
with FAK inhibitor 14 or U0126 suppressed FAK and ERK activity,
respectively. FAK inhibitor 14 and U0126 inhibited the
periostin-promoted migration (Fig.
5B) and tube formation (Fig.
5C) of the HUVECs, which suggested that periostin-induced
angiogenesis is mediated by ERK1/2 and FAK activity.
Periostin affects VEGF and Ang-1
secretion in KFs
To investigate whether periostin affected the
expression of other pro-angiogenic factors, the present study
examined the expression of two angiogenic factors, VEGF and Ang-1,
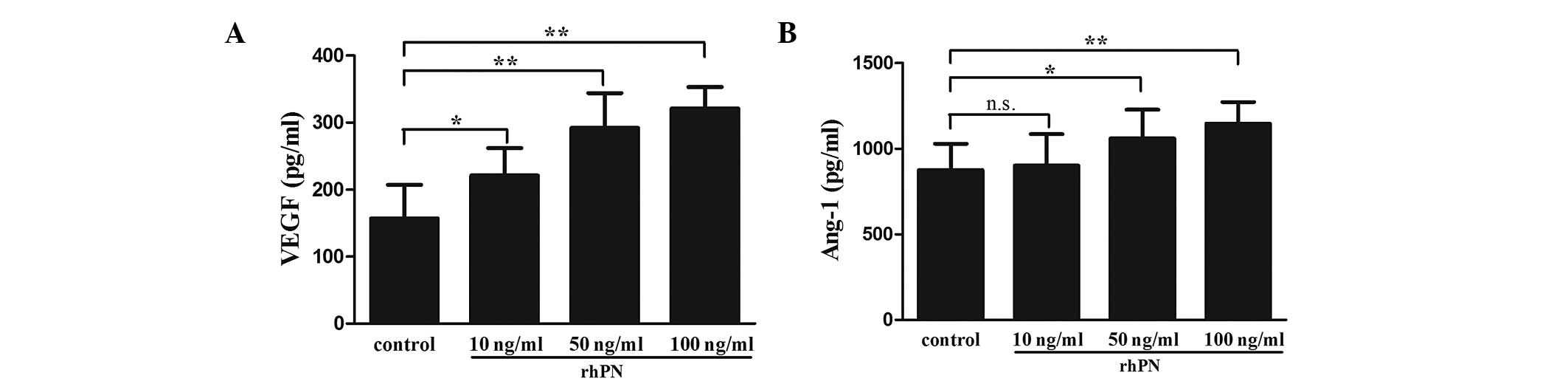
in the KFs following treatment with rhPN. Treatment with rhPN
dose-dependently promoted the secretion of VEGF in the KFs
(Fig. 6A). The expression of Ang-1
was increased following treatment with 50 or 100 ng/ml rhPN,
however no difference was observed with 10 ng/ml rhPN (Fig. 6B).
Discussion
The presents study demonstrated that the level of
periostin and blood vessel density were higher in human keloid
tissue compared with normal tissue and a positive correlation was
observed between periostin level and blood vessel density. In
addition, the increased periostin level in the KFs promoted
angiogenesis by increasing the migration and tube formation of
HUVECs via the ERK1/2 and FAK signal pathways. Periostin also
affected the expression of VEGF and Ang-1 in the KFs and periostin
may be a promising therapeutic target for treatment of keloids and
other angioproliferative diseases.
Although keloids are defined as a dermal benign
disorder, their behavior resembles that of tumor cells, with
keloids exhibiting biological characteristics of tumor cells,
including bioenergetics, hyperproliferation and invasion (21,22).
The induction of angiogenesis is considered important in the
cascade of tumor invasion (23).
To maintain the invasive characteristics and ability to recur,
tumor-like keloids require the generation of numerous new blood
vessels to maintain a steady supply of oxygen and nutrients. The
histological characteristics of keloids also demonstrate tumor
properties. In general, keloids can be divided into three areas,
which exhibit distinct histological features: a central stable
portion, an erythematous border and a normal skin periphery
(1,22). The central stable portion features
a local state of hypoxia due to the overproliferation of
endothelial cells and the deposition of abundant collagen leading
to capillary occlusion. The border contains proliferative
fibroblasts and an increased density of blood vessels, which invade
the peripheral normal skin. In the present study, blood vessel
density, examined by staining with CD31 and CD105, was greater in
the keloid tissues compared with the normal tissues, consistent
with previous studies by Amadeu et al (24) and Appleton et al (25). CD31 and CD105 are endothelial
antigens, which have been used as direct markers of the degree of
neoangiogenesis. In the present study, the increased periostin
level in keloids was correlated with the expression of CD31 and
CD105, which suggested that periostin may promote angiogenesis in
keloids.
Fibroblasts are responsible for the construction and
remodeling of extracellular components and angiogenesis is an
essential process in the progression of keloids (19,25).
Previous studies have demonstrated that several growth factors and
cytokines are regulated in KFs and certain types, including VEGF
and TGF-β, promote angiogenesis in keloids (5). The present study revealed that the
synthesis and secretion of periostin was greater in the KFs
compared with the NFs. Conditioned medium from KFs promoted the
migration and tube formation of HUVECs and rhPN promoted
angiogenesis in a dose-dependent manner. Notably, knock down of
periostin decreased the HUVEC migration and angiogenesis stimulated
by the conditioned medium. Periostin promotes the survival of
endothelial cells and angiogenesis in certain types of cancer and
angiogenesis and lymphangiogenesis have been observed to correlate
with periostin in non-small cell lung cancer (26). Additionally, acquired periostin in
breast cancer promotes tumor angiogenesis by upregulating the
expression of endothelial growth factor receptor 2 (18) and use of a periostin antibody
significantly inhibits tumor growth and angiogenesis in vivo
(27). Therefore, periostin
promotes angiogenesis to directly affect the development of keloids
and is, therefore, a novel pro-angiogenic factor involved in
angiogenesis in keloids.
The present study examined the possible mechanism
underlying the action of periostin in endothelial cells. Previous
studies revealed that growth factors stimulate angiogenesis via
activating kinase signaling pathways, including PI3K/AKT, ERK1/2,
FAK and p38/MAPK, to regulate endothelial cell migration, survival
and vascular permeability (28,29).
In the present study, periostin promoted HUVEC migration and tube
formation by activating the ERK1/2 and FAK pathways. The ERK
pathway is activated by various stimuli, including mitogen kinases
and cell survival factors and also regulates the cell cycle of
endothelial cells. FAK is a cytoplasmic tyrosine kinase, which is
important in integrin-mediated signal transduction. Upregulation in
its expression has been observed in several cancer cells and it is
important in tumor angiogenic activity and progression (30). The interaction between periostin
and integrin triggers intracellular signaling, which promotes the
tube formation and migration of lymphatic endothelial cells during
lymphangiogenesis (26). The
results of the present study demonstrated that treatment with
either ERK1/2 or FAK inhibitors reduced the migration and tube
formation of HUVECs, which suggested that the periostin-activated
ERK1/2 and FAK pathways are involved in angiogenesis.
Regulation of angiogenesis in keloids is complex and
is controlled by a variety of pro-angiogenic factors. VEGF is the
most potent angiogenic factor due to its high specificity to
endothelial cells (31). It is
closely associated with keloid pathogenesis. Previous studies have
demonstrated that VEGF production is abundant in the underlying
dermis of keloids and that the expression of VEGF is higher in
keloid-derived fibroblasts compared with normal skin fibroblasts
in vitro (5). In the
present study, periostin promoted the secretion of VEGF in the KFs,
which was similar to its expression in periodontal ligament cells
and lymphatic endothelial cells. Ang-1, a main ligand for Tie2, is
an angiogenic growth factor that specifically functions to induce
endothelial migration, tube formation and survival (32). In addition, Ang-1 acts to
cooperatively stimulate VEGF, which accelerates the closure of
endothelial cell scratch-wounds. The present study also found that
periostin increased the expression of Ang-1 in the KFs and
periostin may indirectly promote angiogenesis by increasing the
expression of VEGF and Ang-1.
Appropriate regulation of periostin is required
throughout the repair process as dysregulation of periostin leads
to excess proliferation, including the formation of hypertrophic
scars and keloids or even tumors. Our previous study found that
periostin was highly expressed in keloids and gradually increased
between normal skin and hypertrophic scars to keloids (11). Accumulating evidence indicates that
the expression of periostin is positively correlated with tumor
grade and stage in several types of tumor, including hepatocellular
carcinoma, colorectal cancer and prostate cancer (33,34).
The results of the present study demonstrated that
periostin-induced angiogenesis underlies the pathology of keloids
and the level of periostin may affect the process of hyperplasia
and regression.
In conclusion, the present study revealed that the
expression of periostin was increased in human keloid tissue and
correlated with blood vessel density. The upregulated level of
periostin promoted angiogenesis by activating the ERK1/2 and FAK
signaling pathways and increasing the secretion of VEGF and Ang-1.
Therefore, periostin may promote angiogenesis in keloids and be a
key factor in their development. These findings may contribute to
an improved understanding of keloid pathogenesis and may provide a
novel therapeutic target in the treatment of keloids and other
angioproliferative diseases.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 30973126) and the Ministry of
Education Doctoral Foundation of the People’s Republic of China
(no. 20130001110095). The authors would like to thank the teachers
of the Medical Research Center of Peking University Third Hospital
for their assistance and Laura Smales for providing language
assistance.
References
|
1
|
Bran GM, Goessler UR, Hormann K, Riedel F
and Sadick H: Keloids: Current concepts of pathogenesis (Review).
Int J Mol Med. 24:283–293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alster TS and Tanzi EL: Hypertrophic scars
and keloids: etiology and management. Am J Clin Dermatol.
4:235–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Love PB and Kundu RV: Keloids: an update
on medical and surgical treatments. J Drugs Dermatol. 12:403–409.
2013.PubMed/NCBI
|
|
4
|
Ishiko T, Naitoh M, Kubota H, et al:
Chondroitinase injection improves keloid pathology by reorganizing
the extracellular matrix with regenerated elastic fibers. J
Dermatol. 40:380–383. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujiwara M, Muragaki Y and Ooshima A:
Upregulation of transforming growth factor-beta1 and vascular
endothelial growth factor in cultured keloid fibroblasts: relevance
to angiogenic activity. Arch Dermatol Res. 297:161–169. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bux S and Madaree A: Keloids show regional
distribution of proliferative and degenerate connective tissue
elements. Cells Tissues Organs. 191:213–234. 2010. View Article : Google Scholar
|
|
7
|
Gira AK, Brown LF, Washington CV, Cohen C
and Arbiser JL: Keloids demonstrate high-level epidermal expression
of vascular endothelial growth factor. J Am Acad Dermatol.
50:850–853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Trompezinski S, Pernet I and Mayoux C:
Transforming growth factor-beta1 and ultraviolet A1 radiation
increase production of vascular endothelial growth factor but not
endothelin-1 in human dermal fibroblasts. Br J Dermatol.
143:539–545. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mogili NS, Krishnaswamy VR, Jayaraman M,
Rajaram R, Venkatraman A and Korrapati PS: Altered angiogenic
balance in keloids: a key to therapeutic intervention. Transl Res.
159:182–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Syed F and Bayat A: Notch signaling
pathway in keloid disease: enhanced fibroblast activity in a
Jagged-1 peptide-dependent manner in lesional vs. extralesional
fibroblasts. Wound Repair Regen. 20:688–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song ZH and Qin ZL: Expression of
periostin and the effect of hydrocortisone on it in human
fibroblasts of scar. Beijing Da Xue Xue Bao. 40:301–305. 2008.(In
Chinese). PubMed/NCBI
|
|
12
|
Norris RA, Damon B, Mironov V, et al:
Periostin regulates collagen fibrillogenesis and the biomechanical
properties of connective tissues. J Cell Biochem. 101:695–711.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ruan K, Bao S and Ouyang G: The
multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci.
66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu SQ, Lv YE, Lin BH, et al: Silencing of
periostin inhibits nicotine-mediated tumor cell growth and
epithelial-mesenchymal transition in lung cancer cells. Mol Med
Rep. 7:875–880. 2013.PubMed/NCBI
|
|
15
|
Elliott CG, Wang J, Guo X, et al:
Periostin modulates myofibroblast differentiation during
full-thickness cutaneous wound repair. J Cell Sci. 125:121–132.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu M, Fejzo MS, Anderson L, et al:
Periostin promotes ovarian cancer angiogenesis and metastasis.
Gynecol Oncol. 119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu F, Shi CH, Zheng J and Liu YB:
Periostin mediates the increased pro-angiogenic activity of gastric
cancer cells under hypoxic conditions. J Biochem Mol Toxicol.
27:364–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shao R, Bao S, Bai X, et al: Acquired
expression of periostin by human breast cancers promotes tumor
angiogenesis through up-regulation of vascular endothelial growth
factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Beer TW, Baldwin HC, Goddard JR, Gallagher
PJ and Wright DH: Angiogenesis in pathological and surgical scars.
Hum Pathol. 29:1273–1278. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu C, Song ZH and Qin ZL: Construction of
periostin shRNA vectors and their effects on the expression of
periostin in fibroblasts. Beijing Da Xue Xue Bao. 42:503–508.
2010.(In Chinese). PubMed/NCBI
|
|
21
|
Vincent AS, Phan TT, Mukhopadhyay A, Lim
HY, Halliwell B and Wong KP: Human skin keloid fibroblasts display
bioenergetics of cancer cells. J Invest Dermatol. 128:702–709.
2008. View Article : Google Scholar
|
|
22
|
Niessen FB, Spauwen PH, Schalkwijk J and
Kon M: On the nature of hypertrophic scars and keloids: a review.
Plast Reconstr Surg. 104:1435–1458. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh CK, Kwon YW, Kim YS, Jang HS and Kwon
KS: Expression of basic fibroblast growth factor, vascular
endothelial growth factor, and thrombospondin-1 related to
microvessel density in nonaggressive and aggressive basal cell
carcinomas. J Dermatol. 30:306–313. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amadeu T, Braune A, Mandarim-de-Lacerda C,
Porto LC, Desmouliere A and Costa A: Vascularization pattern in
hypertrophic scars and keloids: a stereological analysis. Pathol
Res Pract. 199:469–473. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Appleton I, Brown NJ and Willoughby DA:
Apoptosis, necrosis, and proliferation: possible implications in
the etiology of keloids. Am J Pathol. 149:1441–1447.
1996.PubMed/NCBI
|
|
26
|
Takanami I, Abiko T and Koizumi S:
Expression of periostin in patients with non-small cell lung
cancer: correlation with angiogenesis and lymphangiogenesis. Int J
Biol Markers. 23:182–186. 2008.PubMed/NCBI
|
|
27
|
Orecchia P, Conte R, Balza E, et al:
Identification of a novel cell binding site of periostin involved
in tumour growth. Eur J Cancer. 47:2221–2229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Powazniak Y, Kempfer AC, de la Paz
Dominguez M, et al: Effect of estradiol, progesterone and
testosterone on apoptosis- and proliferation-induced MAPK signaling
in human umbilical vein endothelial cells. Mol Med Rep. 2:441–447.
2009.PubMed/NCBI
|
|
29
|
Esfahanian N, Shakiba Y, Nikbin B, et al:
Effect of metformin on the proliferation, migration, and MMP-2 and
-9 expression of human umbilical vein endothelial cells. Mol Med
Rep. 5:1068–1074. 2012.PubMed/NCBI
|
|
30
|
Baek YY, Cho DH, Choe J, et al:
Extracellular taurine induces angiogenesis by activating ERK-,
Akt-, and FAK-dependent signal pathways. Eur J Pharmacol.
674:188–199. 2012. View Article : Google Scholar
|
|
31
|
Eichmann A and Simons M: VEGF signaling
inside vascular endothelial cells and beyond. Curr Opin Cell Biol.
24:188–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koh GY: Orchestral actions of
angiopoietin-1 in vascular regeneration. Trends Mol Med. 19:31–39.
2013. View Article : Google Scholar
|
|
33
|
Lv Y, Wang W, Jia WD, et al: High
preoparative levels of serum periostin are associated with poor
prognosis in patients with hepatocellular carcinoma after
hepatectomy. Eur J Surg Oncol. 39:1129–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ben QW, Zhao Z, Ge SF, Zhou J, Yuan F and
Yuan YZ: Circulating levels of periostin may help identify patients
with more aggressive colorectal cancer. Int J Oncol. 34:821–828.
2009.PubMed/NCBI
|