Introduction
Radiation-induced lung injury typically presents
with two distinct subsequent clinical phases, interstitial
pneumonia and fibrosis, which frequently occur following completion
of radiation therapy for thoracic neoplasia. This complex process
is regulated by mutually dependent cellular lineages and a
multitude of biologically active molecules. Transforming growth
factor-β1 (TGF-β1) is an important growth factor among the
molecules that are expressed in tissues following radiation
exposure and a positive correlation has been observed between the
severity of radiation-induced lung injury and TGF-β1 signal
activation (1,2). Lung fibroblasts are one of the main
cells in which TGF-β1 is highly expressed, thus injury may be
prevented by inhibiting the expression of TGF-β1. Since its
identification by Fire et al (3), RNA interference (RNAi) has been used
to guide sequence-specific gene silencing of target mRNAs.
In the present study, the RNAi strategy was used to
downregulate TGF-β1 by constructing a small interfering RNA (siRNA)
plasmid vector, termed TGF-β1-siRNA. The effects of TGF-β1-siRNA on
the proliferation and differentiation of lung fibroblast, HFL-I,
cells the intervention effects of the expression vector on the
radiation-induced lung injury were then investigated. The aim of
the present study was to identify effective treatment options to
assist in the prevention and/or management of radiation-induced
lung injury.
Materials and methods
Construction of the TGF-β1-siRNA
expression vector
According to Reynolds et al (4), three synthetic siRNAs targeting human
TGF-β1 mRNA (GenBank accession no. NM000660), with a length of
19–21 nucleotides, were synthesized by GenePharma Co., Ltd.
(Shanghai, China), of which one effective siRNA sequence
(5′-GCAGAGTACACACAGCATA-3′) was adopted for the subsequent
experiments.
In vitro experiment
Cell culture and shRNA
transfection
The human embryonic lung fibroblast, HFL-I, was
obtained from the cell bank of the Chinese Academy of Sciences
(Shanghai, China), and were maintained in F12K supplemented with
10% fetal bovine serum and antibiotics (100 U/ml penicillin G and
100 U/ml streptomycin sulfate; Gibco, Grand Island, NY, USA) at
37°C in 5% CO2. HFL-I cells were resuspended using 1 ml
trypsin, and plated in six-well plates at a density of
1.2×105 cells/well. After a period of 24 h, the siRNA
duplexes were mixed with Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) in Opti-MEM® I reduced
serum medium for 20 min at room temperature to enable complex
formation. The total volume (250 μl) of transfection mixture was
then added to the six-well plates, which were randomly divided into
the positive interference group, which was transfected with
TGF-β1-siRNA, the negative control group, which was transfected
with empty vectors and the blank control group without
transfection. Following 6 h incubation, the medium was replaced by
4 ml Opti-MEM® I containing 5% fetal bovine serum and
the cells were incubated for another 42 h prior to harvesting for
reverse transcription quantitative PCR (RT-qPCR) analysis.
RT-qPCR
The total RNA was isolated from 1×106
cells of each well using TRIzol® reagent (Invitrogen
Life Technologies). Total RNA (1 μg) was reverse-transcribed into
cDNA using AMV Reverse Transcriptase (Promega, Madison, WI, USA).
Oligonucleotide primers were designed for the specific
amplification of TGF-β1 and the internal control β-actin according
to the sequences published in GenBank (Table I). Amplifications were performed
using the FTC-2000 (Funglyn Biotech, Inc., Toronto, ON, Canada)
sequence detection system, using SYBR-Green I (ShineGene, Shanghai,
China). The thermal profile was as follows: 94°C for 4 min followed
by 35 cycles of 94°C for 20 sec, 60°C for 30 sec and 72°C for 30
sec. The TGF-β1 mRNA level in each sample, relative to that of
β-actin mRNA, was calculated using the 2−ΔΔCt formula.
The levels of β-actin were not changed in any of the experimental
conditions (Table I).
 | Table INucleotide sequences of primers used
for quantitative polymerase chain reaction and product sizes. |
Table I
Nucleotide sequences of primers used
for quantitative polymerase chain reaction and product sizes.
| Gene | Primer sequence
(5′-3′) | Amplicon size
(bp) |
|---|
| β-actin |
AGCACAGAGCCTCGCCTTT | 258 |
|
AGGGTGAGGATGCCTCTCTT | |
| TGF-β1 |
GTACCTGAACCCGTGTTGCT | 486 |
|
GTCCTTGCGGAAGTCAATGT | |
Enzyme linked immunosorbent assay
(ELISA)
The supernatant of the cultured cells was collected
48 h after transfection and the concentrations of TGF-β1 were
measured using an ELISA kit (R&D Systems, Inc., Minneapolis,
MN, USA) according to the manufacturer’s instructions. The standard
immunoreagent was diluted with sample dilution to 31.2, 62.5, 125,
250, 500, 1,000 and 2,000 ng/ml as a series multiproportion
dilution. The absorbance values were determined at 450 nm using a
Bio-Rad Model 450 microplate reader (Bio-Rad, Hercules, CA, USA)
and a standard curve was established to calculate the
concentrations of TGF-β1 accordingly.
Annexin V apoptosis detection
assay
The HFL-I cells were plated onto 6-well plates at a
density of 4×105/well and randomly divided into the
positive interference group (transfected with TGF-β1-siRNA),
negative control group (transfected with empty vectors) and control
group (without transfection), with two replicates in each.
Following incubation of the cells for 72 h, apoptosis was analyzed
using a BD FACSAria flow cytometer (BD Biosciences, San Jose, CA,
USA), using a fluorescein isothiocyanate (FITC) Annexin V/Dead Cell
Apoptosis kit with FITC Annexin V and propidium iodide (Invitrogen
Life Technologies).
In vivo experiment
Animals and experimental design
In total, 384 adult, female, specific-pathogen-free
C57BL/6 mice (~8 weeks old), were purchased from Vital River
Laboratory (Beijing, China). The mice were maintained in a cage,
each containing between four and six mice, supplied with standard
laboratory food and water. The mice were randomly divided into the
following four groups: control without any treatment (24 mice),
radiation alone (120 mice); radiation followed by transfection with
empty vectors (120 mice) and radiation followed by transfection
with the TGF-β1-siRNA vector (120 mice). The empty vectors and
TGF-β1-siRNA vectors were transfected into the lung of the mice in
the transfection groups, respectively, on days 1, 7, 14, 28 and 60
of radiation (24 mice/time point). The present study was performed
in strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health and the animal experimental procedures were approved by the
Institutional Animal Care and Use Committee of Suzhou University
(Suzhou, China).
The mice were restrained on the treatment table with
specific jigs and a 23 mm thick paraffin block was placed above the
thoraces of the animal to obtain an even distribution of radiation
dose, using lead shields for radiation protection of the head and
abdomen. A dose of 12 Gy to the entire thorax was delivered in a
single fraction at the anterior field using a linear accelerator
(Primus; Siemens AG, Munich, Germany). The radiation parameters
were a beam energy of 6 MV, an X-ray source-surface distance of 100
cm and a field size of 10 cm2 to provide adequate
coverage of the entire lung.
The irradiated mice were then anesthetized using
3.8% chloral hydrate (10 ml/kg) and fixed upright prior to
injection with the specific TGF-β1-siRNA vector or empty vector
(109 pfu/0.11 ml).
Sampling
A total of three mice from each group were sampled
at days 2, 15 and 28 and at week 8, 12, 16, 20 and 24 of
transfection. Initially, 2 ml blood was obtained immediately from
the heart and placed into an anticoagulant tube containing
ethylenediaminetetraacetic acid (BD Biosciences). Following
standing for 30 min and centrifugation at 1,500 × g for 15 min, the
serum was frozen at −70°C. The hilum of the left lung was then
ligated and 4 ml physiological saline was injected following
endotracheal intubation. This was then removed by suction following
standing for 3 min standing and was repeated three times.
Bronchoalveolar lavage fluid was collected and centrifuged at 3,000
× g for 4 min and the supernate was then stored at −70°C. The left
lung was fixed in 10% formaldehyde solution (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) for 24 h, dehydrated, embedded
in paraffin (Sinopharm Chemical Reagent Co., Ltd.) and sectioned on
a microtome (Leica, Wetzlar, Germany). The sections were then
stained with hematoxylin and eosin (H&E) and Van-Gieson (VG)
(Zhongshan Golden Bridge Bio-technology, Beijing, China) for the
observation of pulmonary fibrosis. In addition, the right lung was
stored in liquid nitrogen.
Immunohistochemistry
Slides were deparaffinized, rehydrated though a
graded series of ethanol and treated with 3%
H2O2 in H2O to quench endogenous
peroxidase activity. The specimens were incubated overnight at 4°C
with rabbit polyclonal antibody against mouse TGF-β1 (1:100; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) using the
ULtraSensitive™ S-P Mouse kit-9701 and was detected with
diaminobenzidine staining (Fuzhou Maixin Biotechnology Co., Ltd.,
Fuzhou, China) used as chromogen. Subsequently, 10 randomly
selected fields on each slide were observed and images were
captured using an Olympus BX51 microscope image acquisition system
(Olympus, Tokyo, Japan). The pulmonary interstitial surface density
and integral optical density were quantified using Image-Pro Plus
6.0 software (Media Cybernetics, Silver Spring, MD, USA) and
deposition of collagen in the lung tissue was observed by VG
staining.
ELISA
The levels of TGF-β1 in the serum and the
bronchoalveolar lavage fluid were determined using ELISA kits
(R&D Systems, Minneapolis, MN, USA) according to the
manufacturer’s instructions.
Statistical analysis
Data are expressed as the mean ± standard deviation.
The differences between any two groups were determined by analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
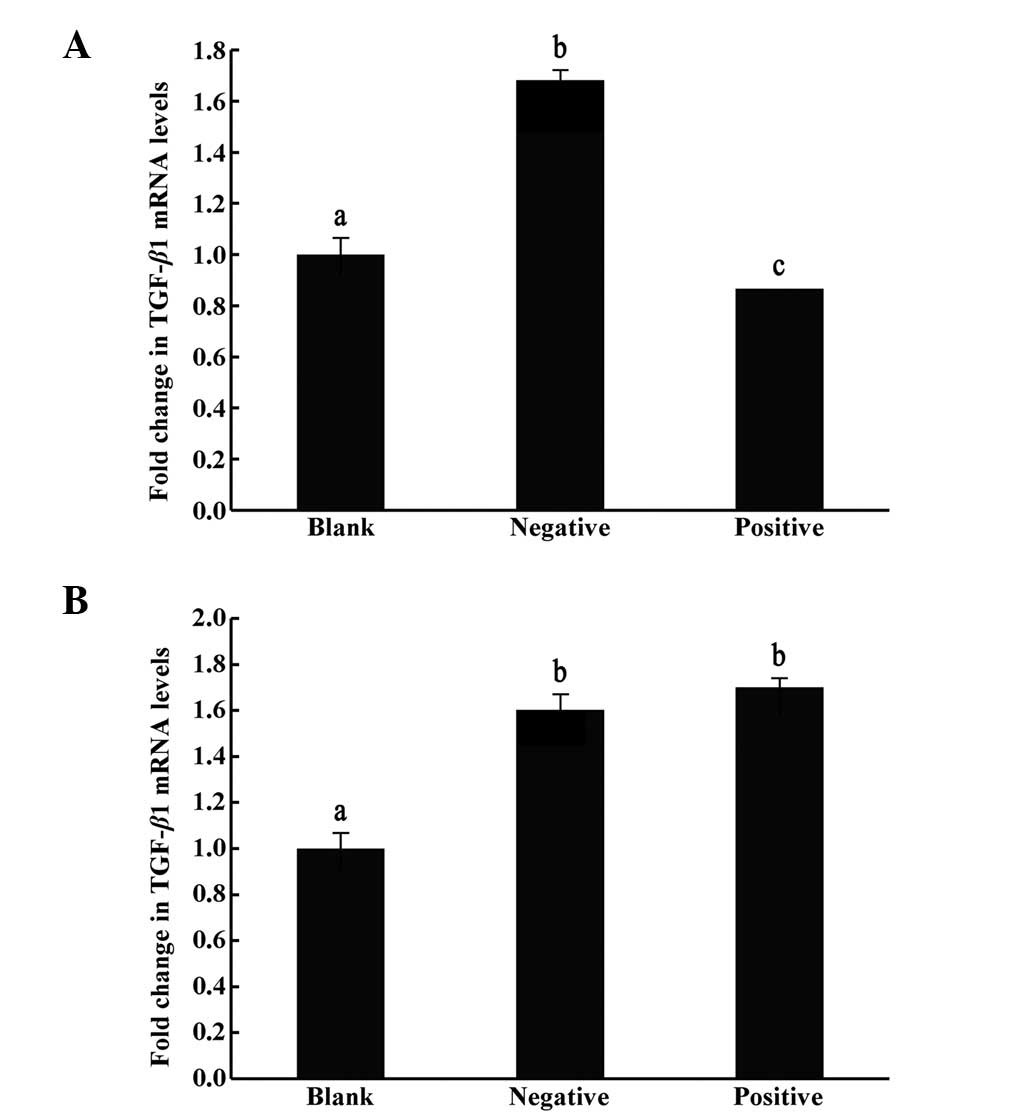
Effects of TGF-β1-siRNA on human
embryonic lung fibroblast HFL-I cells in vitro
TGF-β1 mRNA levels in HFL-I cells
quantified by RT-qPCR
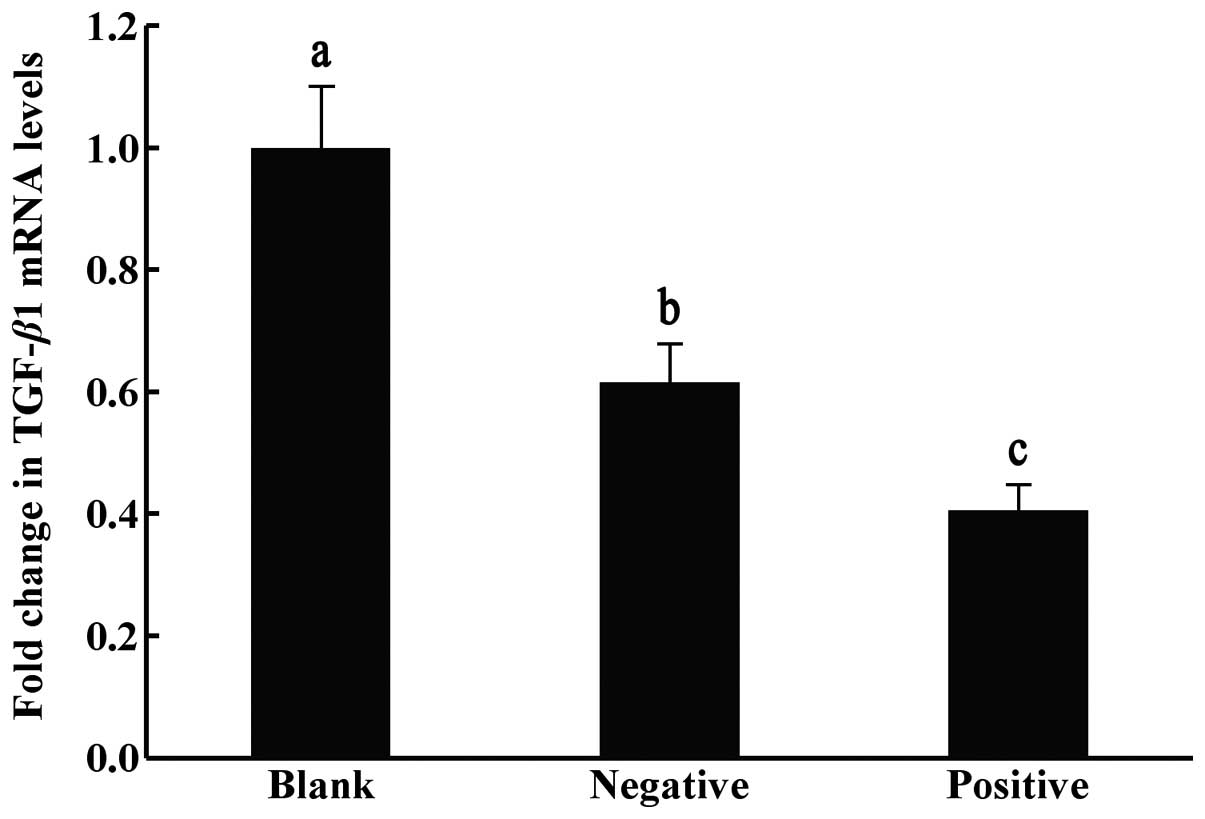
The relative ratios of mRNA expression of TGF-β1
between the blank control with no transfection, the negative
control transfected with empty vectors and the positive
interference group transfected with TGF-β1-siRNA, were
significantly different (P<0.05). The expression levels of the
target gene TGF-β1 were lowest in the TGF-β1-siRNA injection group,
suggesting that the constructed interference plasmid exerted
interference effects (Fig. 1).
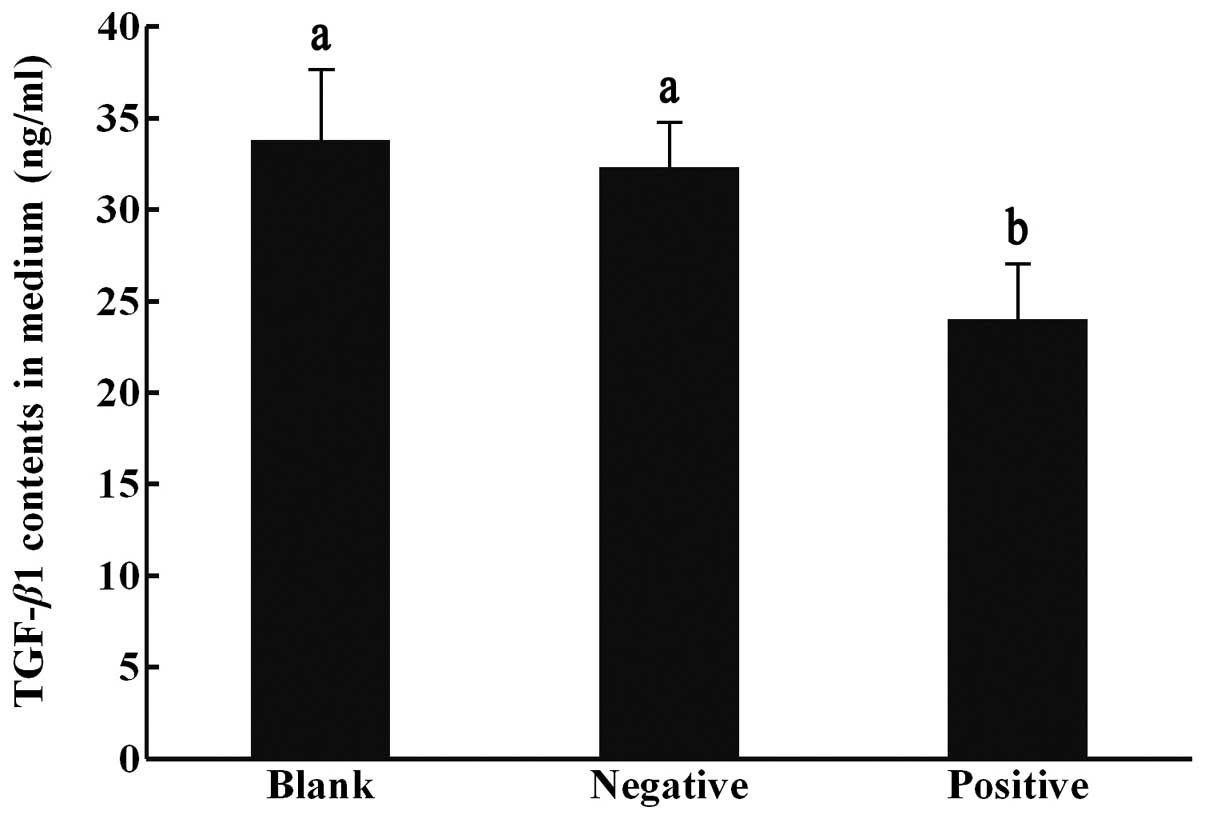
TGF-β1 protein contents in the cell
culture medium measured by ELISA
The contents of TGF-β1 measured in the blank
control, negative control and positive interference groups were
33.8±3.8, 32.3±2.4 and 24.0±3.0 ng/ml, respectively. The levels of
TGF-β1 were lowest in the positive interference group, which
differed significantly from those of the other groups (P<0.05;
Fig. 2).
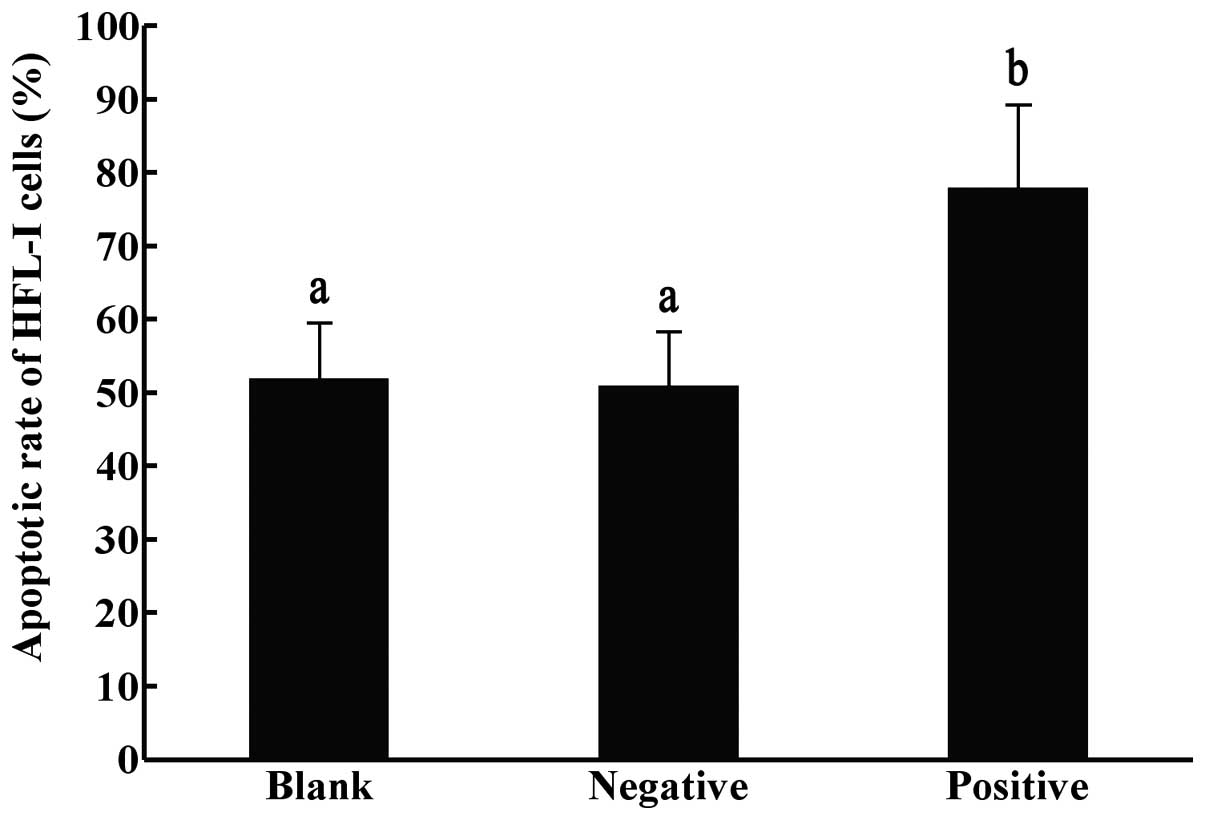
Effect of TGF-β1-siRNA on the
apoptosis of HFL-I cells measured by Annexin V
Following transfection for 72 h, the apoptotic rate
of the HFL-I cells in the positive interference group (78%) was
significantly higher compared with those of the negative and blank
control groups (51 and 52% respectively; P<0.05), while no
significant differences were observed between the negative control
and the blank control groups (Fig.
3).
Effects of TGF-β1-siRNA on
radiation-induced lung injury in vivo
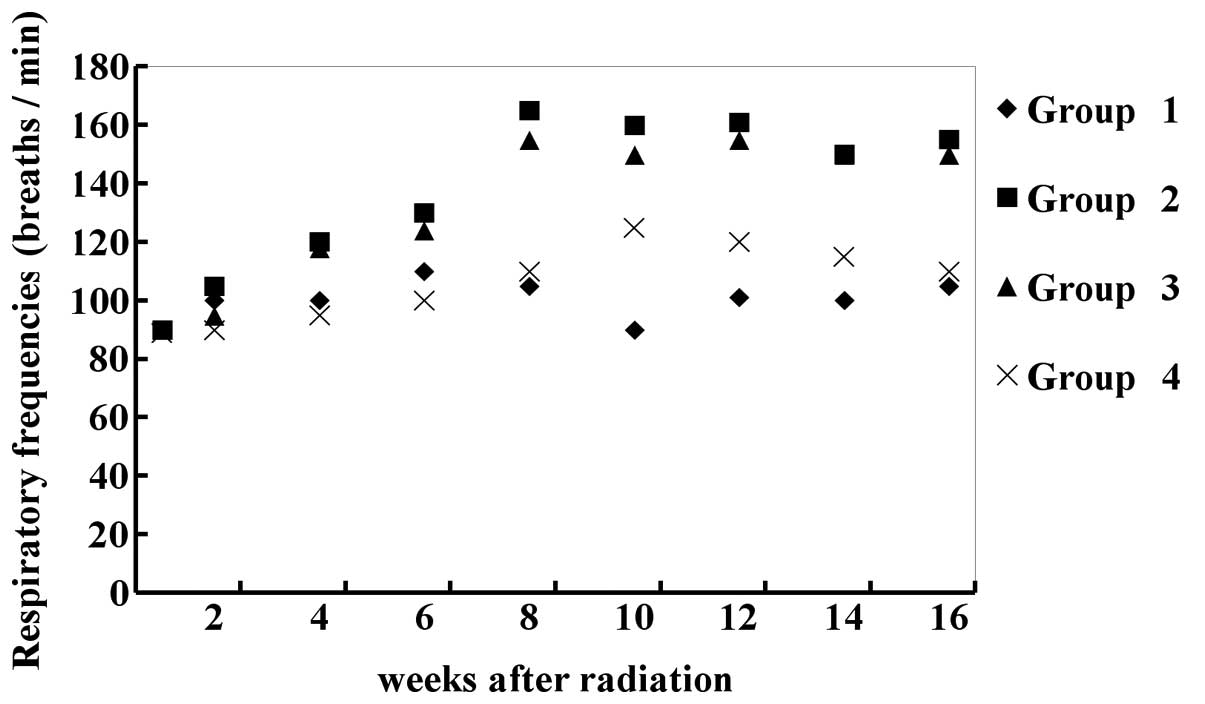
General conditions of the experimental
mice
All the mice were weighed every 2 weeks following
radiation, which were between 19 and 25 g. The mice were in good
condition with normal tail-lift reflection and no hair loss, skin
edema or rupture was observed in the irradiated area. The
respiratory frequencies of the irradiated mice without transfection
increased significantly to 165±13 breaths/min (~80%) in the eighth
week, compared with the control group without any treatment and the
result was similar in the irradiated mice transfected with empty
vectors. In the mice transfected with TGF-β1-siRNA, the respiratory
frequencies increased significantly from the 1st day of radiation
to 125±5 breaths/min (~20%) in the 10th week (P<0.01), compared
with the control and no dyspnea observed (Fig. 4).
Histopathological changes of the
lung
Normal lung morphology was observed in the control
mice following H&E staining. In the irradiated mice without
transfection, thickened alveolar walls, hemangiectasis, hyperemia
of the pulmonary capillaries and a low level of inflammatory cell
infiltration were observed on the second day of radiation. Focal
inflammatory infiltration was observed from day 15 onward and
pulmonary edema with local pulmonary consolidation became more
marked in the week 8 and 12 of radiation (Fig. 5A–D). The levels of inflammation and
edema were less marked in the mice transfected with TGF-β1-siRNA on
the first day of radiation (Fig.
5E–H). Compared with that of the control without any treatment,
the surface density of pulmonary interstitial collagen fibres in
mice without transfection and mice transfected with empty vectors
increased gradually in the 4th and 8th weeks of radiation
(P<0.05), which was significantly reduced in the TGF-β1-siRNA
transfection group (P<0.05; Table
II). The VG staining revealed that the dunkelrosa collagens in
mice transfected with TGF-β1-siRNA, distributed mainly in the
airway and vascular adventitia, were reduced compared with those in
the irradiated mice without transfection on the 2nd and 15th days
of radiation. From the 4th week of radiation onwards, collagen
fibers in the vascular adventitia and the alveolar septum were
increased in mice without transfection, which was allayed to some
extent in the TGF-β1-siRNA transfected mice (Fig. 5I–K). Furthermore, compared with
mice transfected with TGF-β1-siRNA at the later phases of radiation
(28th day; Fig. 5J), fewer
collagen fibers were observed in the vascular adventitia and the
alveolar septum in mice transfected with TGF-β1-siRNA earlier (7th
day; Fig. 5I).
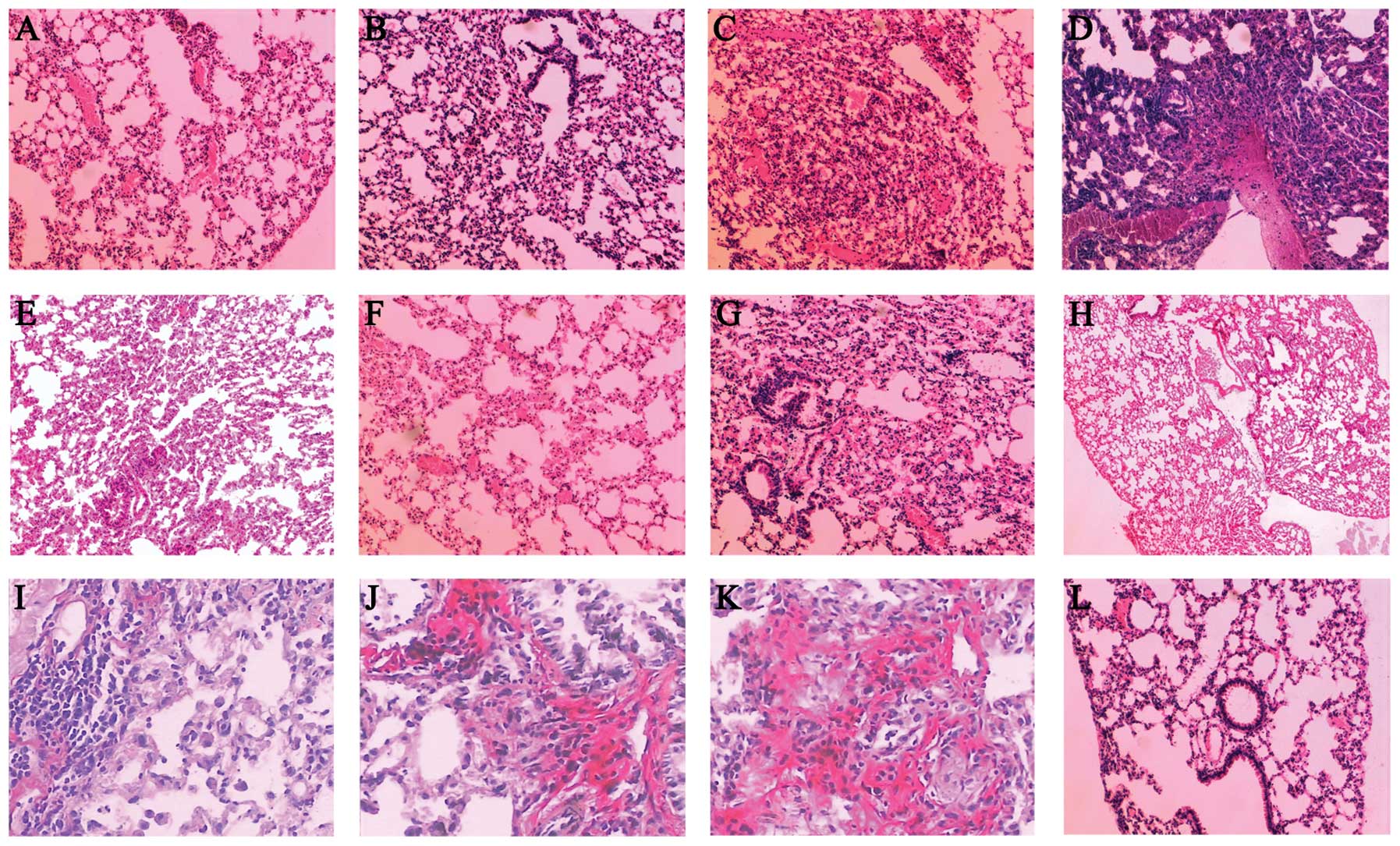 | Figure 5Histopathological changes of the lung
tissues of mice in different treatment groups. (A) On the second
day of radiation, hyperaemia and edema were observed in the
irradiated mice without transfection. (B) On the 15th day of
radiation, inflammation was exacerbated in the irradiated mice
without transfection. (C) At the eighth week of radiation,
thickened alveolar walls and diminished alveolar space were
observed in the irradiated mice without transfection. (D) At the
12th week of radiation, pulmonary fibrosis was observed in the
irradiated mice without transfection. (E) On the second day of
transfection, edema of alveolus pulmonis and a low level of
inflammatory cell infiltration were observed in the mice
transfected with TGF-β1-siRNA on the first day of radiation. (F) On
the 15th day of transfection, hyperaemia and edema were observed in
the mice transfected with TGF-β1-siRNA on the first day of
radiation; (G) At the fourth week of transfection, thickened
alveolar walls and diminished alveolar space were observed in the
mice transfected with TGF-β1-siRNA on the first day of radiation;
(H) At the 12th week of transfection, the injured lung tissue
recovered in the mice transfected with TGF-β1-siRNA on the first
day of radiation. (I) At the fourth week of transfection, fewer
collagenous fibers in the vascular adventitia and alveolar septum
were observed in the mice transfected with TGF-β1-siRNA on the
seventh day of radiation. (J) At the fourth week of transfection,
collagenous fibers in the vascular adventitia and alveolar septum
formed in the mice transfected with TGF-β1-siRNA on the 28th day of
radiation. (K) On the fourth week of radiation, collagenous fibers
in the vascular adventitia and alveolar septum were increased in
the mice without transfection. (L) Normal lung tissue.
TGF-β1-siRNA, transforming growth factor-β1-small interfering
RNA. |
 | Table IIVariations in the surface density of
pulmonary interstitial collagen fibers and the TGF-β1 staining
intensity positive for radiation-induced lung injury. |
Table II
Variations in the surface density of
pulmonary interstitial collagen fibers and the TGF-β1 staining
intensity positive for radiation-induced lung injury.
| Surface density of
pulmonary interstitial collagen fibers (%)a | TGF-β1 staining
intensity of positive reaction (%)b |
|---|
|
|
|
|---|
| Group | 2nd day | 15th day | 4th week | 8th week | 12th week | 2nd day | 15th day | 4th week | 8th week | 12th week |
|---|
| 1 | 13.1±6.7 | 13.1±6.7 | 13.1±6.7 | 13.1±6.7 | 13.1±6.7 | 0.19±0.15 | 0.19±0.1 | 0.19±0.1 | 0.19±0.1 | 0.19±0.1 |
| 2 | 36.2±13.1 | 43.4±8.1 | 51.3±3.4 | 54.1±8.4 | 73.9±4.7 | 0.52±0.3 | 0.53±0.3 | 0.79±0.5 | 0.80±0.5 | 0.83±0.3 |
| 3 | 33.2±3.1 | 42.2±1.7 | 46.3±5.1 | 56.6±3.4 | 66.2±8.5 | 0.53±0.2 | 0.52±0.2 | 0.72±0.3 | 0.82±0.3 | 0.82±0.5 |
| 4 | 25.5±7.5 | 21.5±8.6 | 23.6±5.6 | 21.8±5.2 | 21.0±9.7 | 0.29±0.15 | 0.29±0.3 | 0.19±0.2 | 0.26±0.2 | 0.22±0.3 |
Immunohistochemistry
Few areas of TGF-β1 positive reaction were detected
in the alveolar septum, fine bronchial smooth muscle, vascular
smooth muscle, endothelium and vascular surroundings of the control
lung tissue, suggesting that the expression of TGF-β1 is normally
weak or absent. In the irradiated mice without transfection,
radiation for 4 and 8 weeks significantly enhanced the expression
of TGF-β1 in the above-mentioned regions and, in particular, high
levels of expression were found in the alveolar epithelial cells
and pulmonary interstitial macrophages. Compared with this group,
the positive area in the TGF-β1-siRNA transfected mice was markedly
decreased (P<0.05; Table
II).
Levels of TGF-β1 in the serum and
bronchoalveolar lavage fluid measured by ELISA
Changes in the levels of TGF-β1 in the serum and
bronchoalveolar lavage fluid were almost in accordance with the
histopathological changes observed in the lung tissues.
Specifically, the levels of TGF-β1 in the serum of irradiated mice
without transfection increased with time, which increased
significantly 4 weeks and peaked 8 weeks after radiation, compared
with the control (P<0.05). The levels of TGF-β1 in the serum of
irradiated mice transfected with TGF-β1-siRNA also increased
gradually, however it differed significantly compared with that in
the mice irradiated without transfection (P<0.05). Changes in
the levels of TGF-β1 in the bronchoalveolar lavage fluid were
similar with that in the serum, however, a reduction was observed
following the peak in the fourth week of radiation (Table III).
 | Table IIIChanges in the levels of TGF-β1 in the
serum and bronchoalveolar lavage fluid (ng/ml). |
Table III
Changes in the levels of TGF-β1 in the
serum and bronchoalveolar lavage fluid (ng/ml).
| Serum TGF-β1a | Bronchoalveolar
lavage fluid TGF-β1b |
|---|
|
|
|
|---|
| Group | 2nd day | 15th day | 4th week | 8th week | 12th week | 2nd day | 15th day | 4th week | 8th week | 12th week |
|---|
| 1 | 3.68±0.96 | 3.68±0.96 | 3.68±0.96 | 3.68±0.96 | 3.68±0.96 | 3.41±0.89 | 3.41±0.89 | 3.41±0.89 | 3.41±0.89 | 3.41±0.89 |
| 2 | 14.74±2.16 | 24.94±2.16 | 29.32±3.20 | 42.14±3.78 | 38.63±4.94 | 4.98±1.12 | 10.40±1.21 | 9.85±2.11 | 9.99±1.02 | 8.44±2.23 |
| 3 | 14.2±3.1 | 24.2±2.73 | 26.31±5.12 | 41.60±3.47 | 34.24±8.58 | 4.78±2.32 | 10.98±1.92 | 9.98±3.12 | 8.98±2.32 | 7.18±1.64 |
| 4 | 9.85±1.58 | 13.86±1.14 | 24.68±2.18 | 35.61±2.55 | 33.59±3.43 | 3.64±1.35 | 5.87±2.31 | 4.44±2.54 | 4.82±1.26 | 3.94±2.41 |
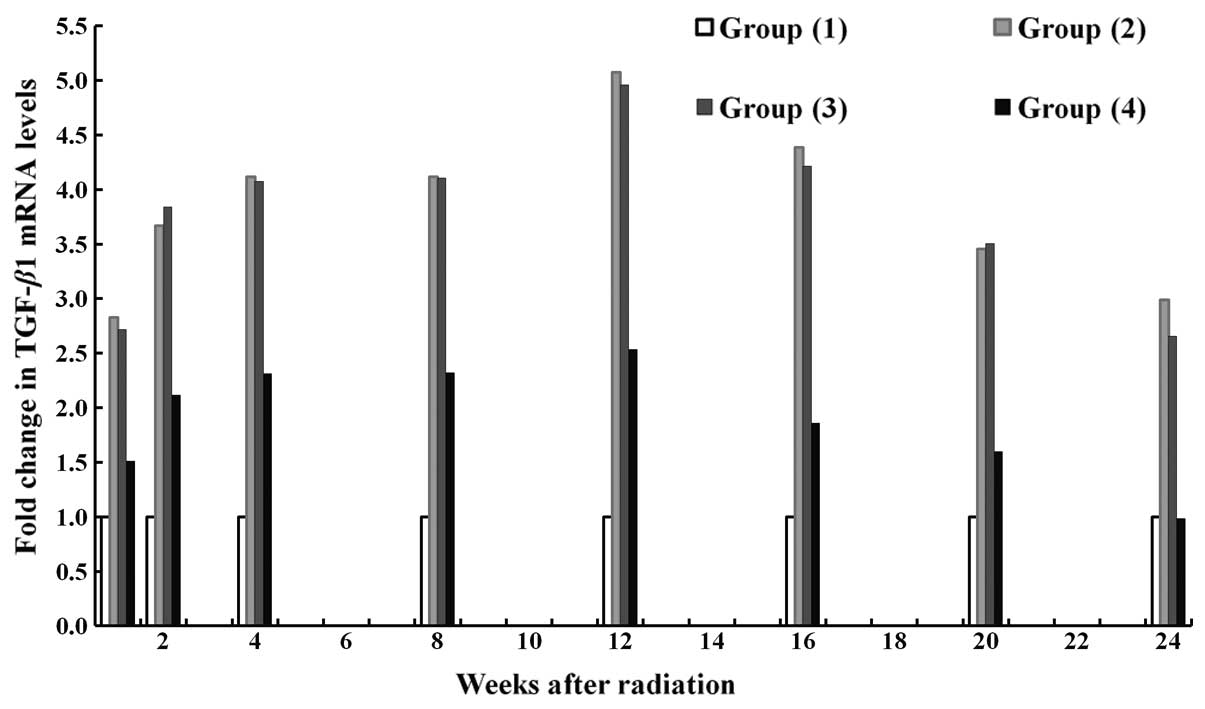
Changes in mRNA expression levels of
TGF-β1 quantified by RT-qPCR
Following radiation, the mRNA expression of TGF-β1
was significantly upregulated in mice without any transfection and
in those transfected with empty vectors, compared with the control
(P<0.05). In general, the mRNA expression levels of TGF-β1 in
mice transfected with TGF-β1-siRNA were markedly lower compared
with those in the mice without transfection or in those transfected
with empty vectors. This difference was significant in the fourth,
eighth, 12th and 16th week of radiation (P<0.05). The mRNA
expression of TGF-β1 was upregulated from the 8th week of
radiation, peaked at the 12th week and was then downregulated.
Notably, in the 12th week of radiation, the mRNA expression levels
of TGF-β1 were highest in the radiated non-transfection group,
followed by the TGF-β1-siRNA transfection group and then the
controls (Fig. 6). Furthermore,
the mRNA expression levels of TGF-β1 were lower in mice transfected
with TGF-β1-siRNA (Fig. 7A) on the
first day following radiation compared with those transfected with
TGF-β1-siRNA at later phases of radiation (28th day; Fig. 7B).
Discussion
Radiation-induced lung injury is one of the most
common complications following thoracic radiochemotherapy, of which
radiation pneumonitis and pulmonary fibrosis represent acute and
late phases, respectively. It can markedly decrease the quality of
life and even the life span of patients with a thoracic tumor. This
injury may be induced by various factors, involving the injured
cells targeted by radiation and profibrotic cytokines produced by
damaged and activated cells (5).
In the later phases, cytokine-mediated proliferation, activation
and differentiation of fibroblasts into myofibroblasts occur with
associated collagen deposition, which may cause respiratory
failure. Currently, several pharmaceuticals are used in the
management of radiation pneumonitis in the early phase, including
glucocorticoids, non-steroidal anti-inflammatory drugs, adjuvant
interferon R and other drugs, which relieve lung injury. However,
the formation of a mass of pulmonary fibrosis remains possible in
the late phase.
Previous investigations have demonstrated that
between a few hours and a few days following radiation, quantities
of growth factors are synthesized and secreted, which continues for
several months. These early changes have profound impacts on
pathological and physiological changes in the late phase. Cytokines
including TGF-β1, platelet-derived growth factor (PDGF), tumor
necrosis factor-α (TNF-α), interleukin-1 (IL-1) and insulin-like
growth factor-1 are involved in pulmonary fibrosis, by which
multi-cellular interactions are mediated to initiate and maintain
the process of fibrosis. TGF-β1, one of the factors regulating the
growth of various human epithelial cells, has a wide range of
biological effects on cell growth, differentiation, extracellular
matrix deposition and immune response (6). TGF-β1 is considered to be closely
associated with the formulation and maintenance of radiation
pulmonary fibrosis, since increased levels of TGF-β1 are detected
in pulmonary fibrosis induced by bleomycin, cyclophosphamide and
radiation (7–10). TGF-β1 stimulates the synthesis of
IL-1, TNF-α and PDGF by inflammatory cells or fibroblast,
suggesting the importance of this factor in the cytokine network,
while its own production also exists in an autocrine manner. TGF-β1
is also responsible for the regulation of extracellular matrix
(ECM) by two different mechanisms (2). It enhances ECM synthesis by inducing
the synthesis of collagen and various other extracellular matrix
components, including fibronectin and it can reduce ECM
degradation, partially by inhibiting the expression of proteolytic
enzyme. Chiang et al (11)
reported that in mice receiving radiation, pulmonary fibrosis or
subacute radiation pneumonia develops and TGF-β1 is upregulated,
particularly in the late phases of pulmonary fibrosis.
Understanding the molecular mechanisms and signaling
pathways of radiation-induced lung injury make it possible to
intervene and prevent this injury by using certain biological
techniques similar to anticytokine therapy, which has been utilized
as a novel treatment to inhibit pulmonary fibrosis. Considering the
important roles of TGF-β1 in the development of fibrosis induced by
radiation, matrix generation may be inhibited and matrix
degradation may be stimulated by suppressing the activity of TGF-β1
in antifibrotic therapy. As RNAi relies on the sequence-specific
interaction between siRNAs and mRNA, siRNAs can be tailored to
silence almost any gene. It is one of the important mechanisms in
post-transcriptional gene silencing in eukaryotes. The central
process of RNAi is the cleavage of dsRNA into smaller fragments of
a defined length (~21–23 nucleotides) by the enzyme Dicer. RNAi has
been widely used for the analysis of gene function and signal
transduction, representing a potentially promising approach for
gene therapy. It has been reported that the inhibitory effect of
siRNA synthesized via a plasmid or viral vector on the target gene
is similar with that of synthetic siRNA (12).
In the present study, sequence-specific siRNA
targeting TGF-β1 mRNA was constructed and transfected into human
embryonic lung fibroblast HFL-I cells. The target oligonucleotide
fragments were confirmed to have been cloned into the pRNAT plasmid
vector, as expected using an enzyme digestion and sequence
reaction. Compared with the control group, the mRNA expression and
protein levels of TGF-β1 were found to be significantly inhibited
by qPCR and ELISA, respectively. The results of the Annexin V
apoptosis detection assay suggested that the marked increase in
HFL-I cell apoptosis was caused by TGF-β1-siRNA transfection. Taken
altogether, the constructed TGF-β1-siRNA plasmid demonstrated
significant interference effects in vitro.
The in vivo investigation further
demonstrated that radiation significantly elevated the mRNA
expression and protein levels of TGF-β1 in the lung of mice,
indicating that TGF-β1 is important in the genesis and development
of radiation-induced lung injury. Previous studies have
demonstrated that lung fibrosis is inhibited by inhibiting the
TGF-β1 signaling pathway or the application of TGF-β1 monoclonal
antibodies (13). In the present
study, the above-mentioned functional TGF-β1-siRNA was used in a
rodent model of radiation-induced lung injury. The results revealed
that radiation-induced pulmonary edema and alveolar inflammation
were significantly relieved and the mRNA expression levels of
TGF-β1 were downregulated in the irradiated mice transfected with
TGF-β1-siRNA compared with the irradiated mice without transfection
or transfected with empty vectors. These results suggested that the
TGF-β1-siRNA vector had protective effects against
radiation-induced lung injury, possibly by inhibiting the
expression of TGF-β1. In conclusion, the specific TGF-β1-siRNA
vector effectively reduced the expression of TGF-β1 and thereby
inhibited the inflammatory response during radiation-induced
pulmonary injury, which may assist in identifying novel techniques
to prevent radiation-induced lung injury and fibrosis.
Acknowledgements
This study was funded by the Natural Science
Foundation of Jiangsu Province (no. BK2009102), the National
Natural Science Foundation of China (nos. 81402518 and 81472920),
the Jiangsu Provincial Special Program of Medical Science (no.
BL2012046), the Changzhou Scientific Program (nos. ZD200818,
CE20125026, CE20135050, CJ20112019 and ZD201315) and the Open
Program of Jiangsu Provincial Key Laboratory of Radiation Medicine
and Protection (nos. KJS1241 and KJS1242).
References
|
1
|
Anscher MS, Marks LB, Shafman TD, et al:
Risk of long-term complications after TFG-beta1-guided
very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys.
56:988–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Novakova-Jiresova A, Van Gameren MM,
Coppes RP, Kampinga HH and Groen HJ: Transforming growth
factor-beta plasma dynamics and post-irradiation lung injury in
lung cancer patients. Radiother Oncol. 71:183–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fire A, Xu S, Montgomery MK, Kostas SA,
Driver SE and Mello CC: Potent and specific genetic interference by
double stranded RNA in Caenorhabditis elegans. Nature. 391:806–811.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reynolds A, Leake D, Boese Q, Scaringe S,
Marshall WS and Khvorova A: Rational siRNA design for RNA
interference. Nat Biotechnol. 22:326–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schallenkamp JM, Miller RC, Brinkmann DH,
Foote T and Garces YI: Incidence of radiation pneumonitis after
thoracic irradiation: Dose volume correlates. Int J Radiat Oncol
Biol Phys. 67:410–416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim SH, Lim DJ, Chung YG, Cho TH, Lim SJ,
Kim WJ and Suh JK: Expression of TNF-alpha and TGF-beta 1 in the
rat brain after a single high-dose irradiation. J Korean Med Sci.
17:242–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Evans ES, Kocak Z, Zhou SM, et al: Does
transforming growth factor beta1 predict for radiation induced
pneumonitis in patients treated for lung cancer. Cytokine.
35:186–192. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen Y, Williams J, Ding I, et al:
Radiation pneumonitis and early circulatory cytokine markers. Semin
Radiat Oncol. 12:S26–S33. 2002. View Article : Google Scholar
|
|
9
|
Nagashio Y, Ueno H, Imamura M, et al:
Inhibition of transforming growth factor beta decreases pancreatic
fibrosis and protects the pancreas against chronic injury in mice.
Lab Invest. 84:1610–1618. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martin M, Lefaix JL and Delanian S:
TGF-beta1 and radiation fibrosis: a master switch and a specific
therapeutic target? Int J Radiant Oncol Biol Phys. 47:277–290.
2000. View Article : Google Scholar
|
|
11
|
Chiang CS, Liu WC, Jung SM, et al:
Compartmental responses after thoracic irradiation of mice: strain
differences. Int J Radiat Oncol Biol Phys. 62:862–871. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Volarevic M, Smolic R, Wu CH and Wu GY:
Potential role of RNAi in the treatment of HCV infection. Expert
Rev Anti Infect Ther. 5:823–831. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shimizukawa M, Ebina M, Narumi K, Kikuchi
T, Munakata H and Nukiwa T: Intratracheal gene transfer of decorin
reduces subpleural fibroproliferation induced by bleomycin. Am J
Physiol Lung Cell Mol Physiol. 284:L526–L532. 2003.PubMed/NCBI
|