Introduction
All of the clinical and experimental studies on
adiponectin (APN) markedly suggest that it is a critical vascular
protective molecule and its reduction may contribute to vascular
injury in metabolic disorder-associated diseases (1–5).
Adiponectin is an adipose-specific plasma protein that has
important roles in atherosclerosis, obesity and type 2 diabetes
(6–8). Experimental studies have demonstrated
that adiponectin has anti-inflammatory and anti-atherogenic
properties. Previous studies have demonstrated that adiponectin
overexpression reversed abnormal neointimal thickening in
adiponectin-deficient mice and alleviated atherosclerotic lesions
in apolipoprotein E-deficient (ApoE−/−) mice. Okamoto
et al (9) reported that
injection of an adenovirus containing adiponectin into
ApoE−/− mice significantly reduced lesions within the
aortic sinus region, and the results suggested that adiponectin may
mediate its anti-atherogenic effects by adhering to macrophages and
actin-negative cells (endothelial cells) within the fatty streak
lesions. The study also revealed that adiponectin decreased the
size of lipid droplets, vascular cell adhesion protein 1 expression
and class A scavenger receptor without affecting lipid profiles in
the plasma (9).
Oxidative stress, comprising the production of
·O2− and its derivative peroxynitrite
(ONOO−), contributes to the onset of atherosclerosis
(10). The levels of circulating
adiponectin are inversely correlated with the plasma levels of
oxidized low-density lipoprotein (LDL) in patients with type 2
diabetes and coronary artery disease, which suggests that low
adiponectin levels are associated with an increased oxidative state
in the arterial wall (11).
Reduced adiponectin levels have also been correlated with increased
levels of systemic oxidative stress (12). Furthermore, elevated endothelial
reactive oxygen species (ROS) production stimulated by multiple
agonists, including oxidized adiponectin and high glucose levels,
was dose-dependently suppressed in vitro by recombinant
globular adiponectin (13–14). Adiponectin improved endothelial
function in hyperlipidemic rats (15) and protected hearts against
ischemia/reperfusion injury by reducing oxidative/nitrative stress
(16). Therefore, adiponectin may
be a potent anti-oxidative/nitrative stress agent. However, to the
best of our knowledge, whether adiponectin overexpression reduces
atherosclerotic plaque by acting on oxidative/nitrative stress has
not been investigated to date.
Therefore, the present study aimed to determine the
relevance of adiponectin and its attenuation of oxidative stress.
It was identified that adiponectin may reduce atherosclerotic
plaque and improve the stability of plaques in ApoE−/−
mice with atherosclerosis by inhibiting inducible nitric oxide
synthase (iNOS) and oxidative/nitrative stress.
Materials and methods
Animals
The adenovirus producing the full-length adiponectin
was constructed with use of the Adenovirus (Ad) Expression Vector
kit (Takara, Kyoto, Japan) as described previously (17). From the effects of different titers
of Ad-APN on serum adiponectin concentration in mice by ELISA, the
optimal concentration of Ad-APN of 2×108 plaque-forming
units [pfu] of infection was used.
Male ApoE−/− mice (10–12 weeks old;
Beijing University, Beijing, China) were housed at room temperature
(24°C) under a 12-h dark/light cycle and received a high-fat diet
(0.25% cholesterol and 15% cocoa butter) for 12 weeks. Carotid
atherosclerotic lesions were induced by surgical placement of
perivascular constrictive silica collars (Shandog Medical
Instrument Institute, Shandong, China) on the left common carotid
arteries as described by our group previously (17). Eight weeks following surgery, the
mice were divided into three groups for treatment (n=30/group):
Phosphate-buffered saline (PBS), Ad-β-galactosidase (Ad-β-gal) and
Ad-APN groups. The silica collars on the left common carotid
arteries were removed carefully. PBS, Ad-β-gal and Ad-APN were
trickled into the silica collar parts of common carotid arteries of
mice. Two weeks following treatment, the mice were sacrificed and
carotids were excised. The experiment conformed with the Guide for
the Care and Use of Laboratory Animals published by the US National
Institutes of Health (NIH Publication no. 85–23, revised 1985) and
Shandong University guidelines (Shandong, China). This study was
approved by the ethics committee of Jinan Central Hospital
affiliated to Shandong University (Jinan, China).
Micro-ultrasonography
The Vevo770 ultrasonography system (Visualsonics,
Toronto, Canada; 55-MHz scan head, 4.5-mm focus, axial resolution
30 μM) was used to measure the baseline parameters of the left
carotid artery at the beginning of the experiment prior to and
following transfection as previously described (18).
Histology
Frozen carotid-artery cross-sections (10-μM thick)
embedded in optimal cutting temperature compound (OCT; Sakura
Finetechnical Co., Ltd., Tokyo, Japan) following overnight fixation
in 10% formalin were mounted on slides. Three sections (200-μM
apart) for each mouse were stained with Oil-red O (Ameresco, Solon,
OH, USA) and Masson trichrome (Maxim-Bio, Fujian, China). The
corresponding sections were measured by use of an automated image
analysis system (Image-Pro Plus 5.0, Media Cybernetics, Rockville,
MD, USA) attached to a color CCD video camera (BX51; Olympus Corp.,
Tokyo, Japan).
Determination of total nitric oxide (NO)
in carotid tissue
The carotid tissue was rinsed and homogenized. The
content of NO in the supernatant was determined using the Griess
Reagent method (Nitric Oxide Assay kit, Beyotime, Shanghai; Siever
280i NO Analyzer) as described previously (17).
Quantification of superoxide
production
In situ superoxide content was detected by
dihydroethidium staining (DHE; Molecular Probes, Carlsbad, CA) as
described previously(19). The
carotid tissue superoxide content was determined by
lucigenin-enhanced luminescence (20).
Immunoblotting
The protein from the carotid tissue homogenates was
separated by SDS-PAGE, transferred to nitrocellulose membranes and
incubated with monoclonal antibodies against adiponectin, iNOS
(both Upstate Biotechnology, Inc., Lake Placid, NY, USA) and
β-actin (Cell Signaling Technology, Inc., Beverly, MA, USA), then
horseradish peroxidase-conjugated anti-mouse immunoglobulin G
antibody (1:2,000, Cell Signaling Technology, Inc., Danvers, MA,
USA) for 1 h. The blots were developed by using a supersignal
chemiluminescence kit (Pierce Biotechnology, Inc., Rockford, IL,
USA) and visualized using a Kodak Image Station 400 (Amersham
Pharmacia, Deisenhofen, Germany). The sample loadings were
normalized with anti-β-actin polyclonal antibody (Sigma-Aldrich,
St. Louis, MO, USA) and quantification involved use of the Image
Station 2000R system (Eastman Kodak, Rochester, NY, USA).
Immunohistochemistry
Paraformaldehyde-fixed tissues were cut into
semi-thin sections 4–5-μM thick and stained with the following
antibodies: mouse anti-macrophage/monocyte monoclonal (clone
MOMA-2; 1:500; Millipore, Billerica, MA, USA), mouse anti-human
anti-α-actin (1:500; Millipore) and mouse anti-nitrotyrosine (clone
1A6; 1:200; Millipore). Immunohistochemistry staining was developed
using a Vectastain ABC kit (Vector Laboratories, Burlingame, CA,
USA).
Determination of plasma APN
concentration
Plasma adiponectin levels were determined by use of
a mouse adiponectin ELISA kit (Phoenix Pharmaceuticals, Inc.,
Belmont, CA, USA) according to the manufacturer’s instructions.
Quantification of tissue nitrotyrosine
content
The nitrotyrosine content in the carotid tissue,
indicating in vivo ONOO− formation, an index of
nitrative stress, was determined by use of a nitrotyrosine ELISA
kit (Cell Sciences, Canton, MA, USA) according to the
manufacturer’s instructions.
Statistical analysis
All data are expressed as the mean ± standard error.
Comparisons between the various groups were analyzed by one-way
analysis of variance followed by Dunnett’s post-hoc test using SPSS
16.0 software (International Business Machines, Armonk, NY, USA).
P<0.05 (two-sided) was considered to indicate a statistically
significant difference.
Results
Ad-APN transfection increased APN levels
in carotid arteries of ApoE−/− mice
On the 7th day following virus injection, the
expression of virus-targeted genes was detectable in the mice,
without loss of weight. As demonstrated in Fig. 1A–C, ~50% GFP expression in the
Ad-APN plaques and 52% in the Ad-β-gal plaques but not in PBS
plaques indicated effective transfection.
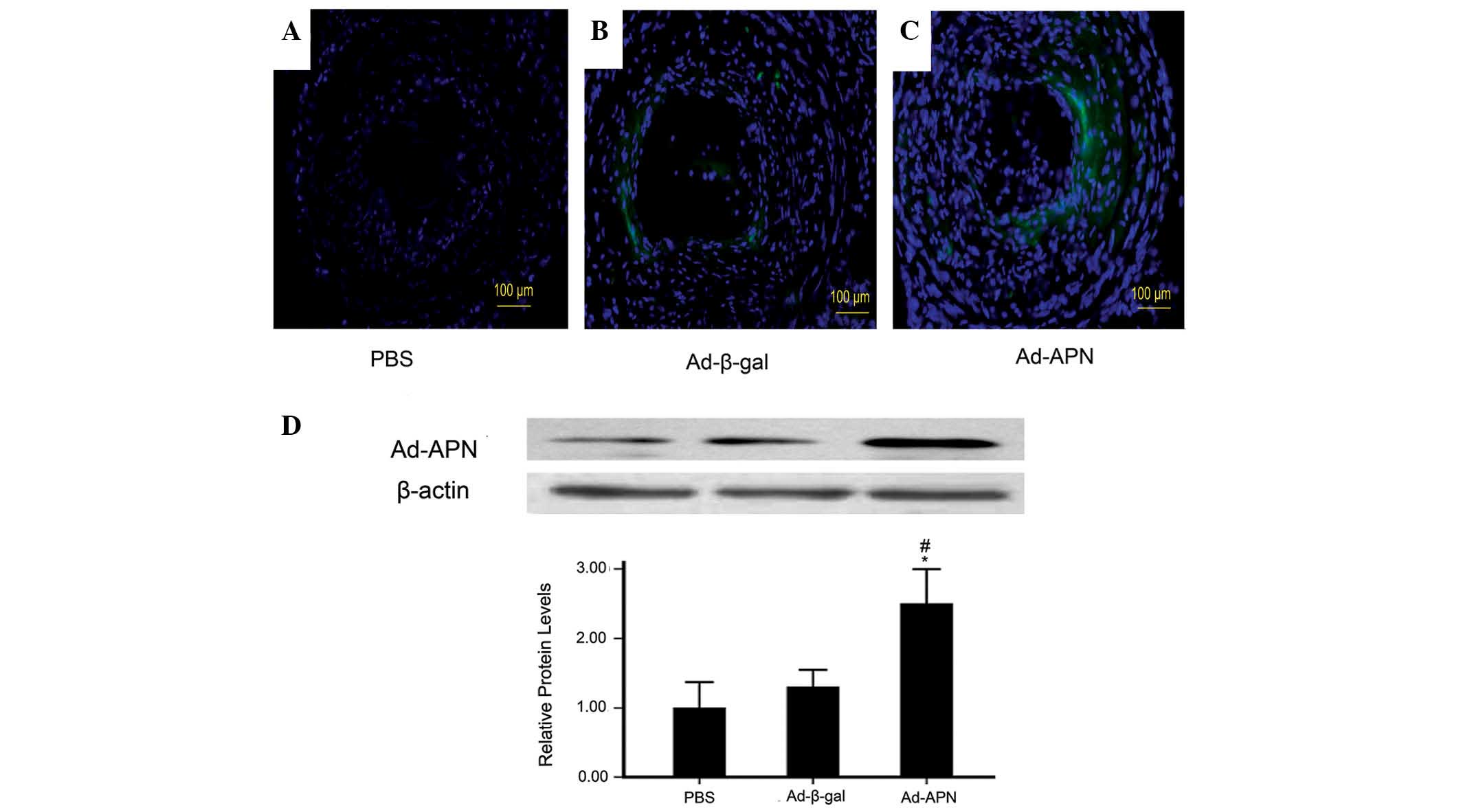 | Figure 1APN levels in atherogenic plaque in
ApoE−/− mice. Green fluorescence protein expression in
atherogenic plaque seven days following injection with (A) PBS, (B)
Ad-β-gal and (C) Ad-APN (magnification, ×10). (D) Relative APN
levels in atherogenic plaque in PBS, Ad-β-gal and Ad-APN groups.
Values are expressed as the mean ± standard deviation.
*P<0.05 vs. control; #P<0.05 vs.
Ad-β-gal. PBS, treated with PBS; Ad-β-gal, transfected with empty
vector; Ad-APN, transfection with Ad-APN. ApoE−/−,
apolipoprotein E-deficient; Ad-β-gal, Ad-β-galactosidase; PBS,
phosphate-buffered saline; APN, adiponectin; Ad, adenovirus. |
Protein levels of adiponectin in the carotid tissue
were further studied. The results demonstrated that protein levels
of adiponectin in Ad-APN plaques were higher than those in the
Ad-β-gal and PBS plaques (2.5±0.2 vs. 1.3±0.1; P<0.05; 2.5±0.2
vs. 1.0±0.15; P<0.05; Fig. 1D).
There was no significant difference between the control groups
(1.0±0.15 vs. 1.3±0.1; P>0.05; Fig.
1D). Therefore, Ad-APN transfection efficiently increased the
levels of adiponectin in mouse carotid arteries.
APN reduces atherosclerotic lesions in
carotid arteries in ApoE−/− mice
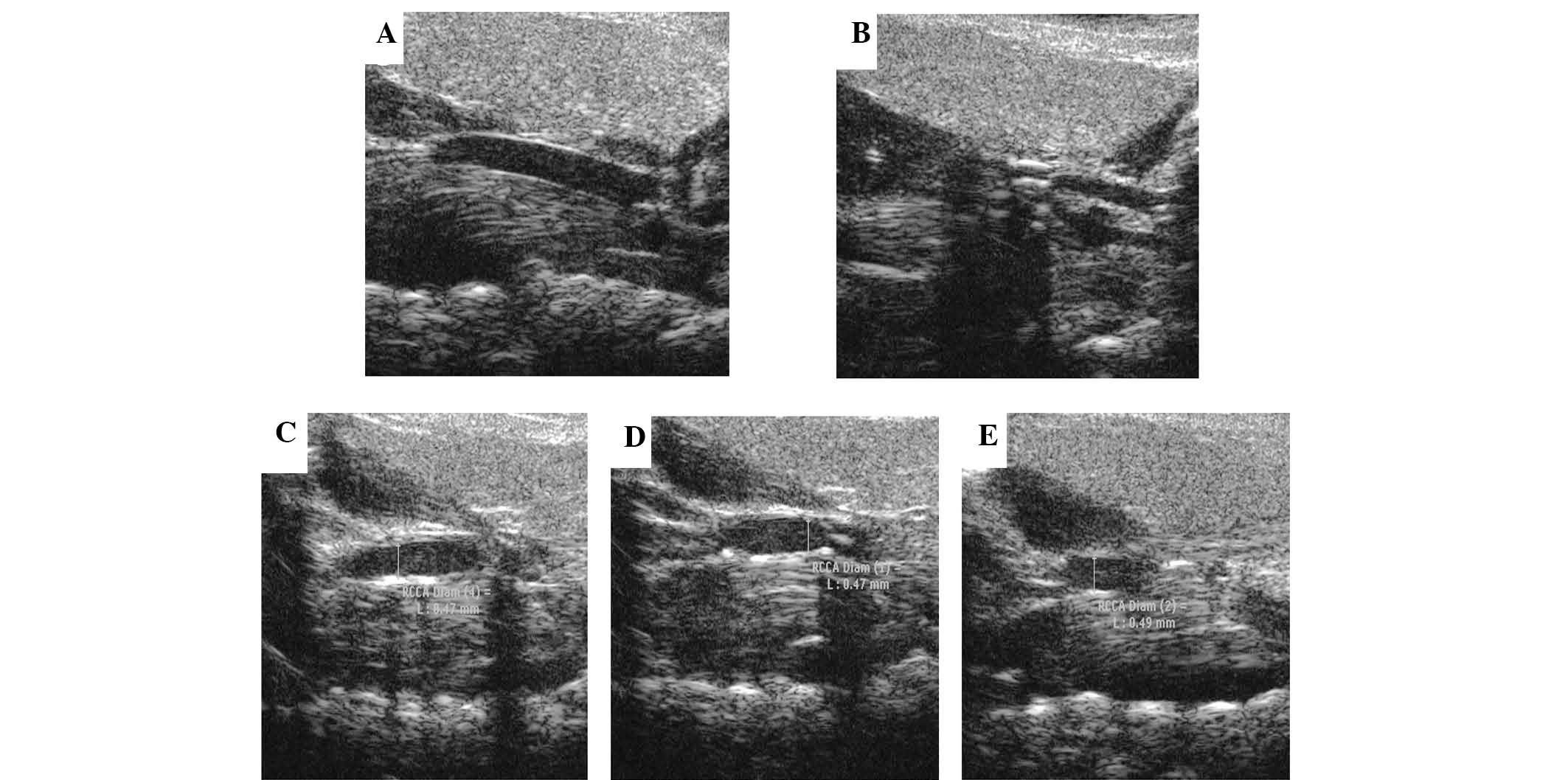
The present study used micro-ultrasonography to
evaluate the plaques. Prior to surgery, the carotid intima of
control mice was smooth (Fig. 2A).
Six weeks following surgery, abundant atherosclerotic lesions were
present in the lumen (Fig. 2B).
The carotids were also compared following collar removal and 14
days of PBS treatment to demonstrate that this remained the same
(Fig. 2B and C). The results
revealed that the removal of the collar did not improve
atherosclerosis itself, which was improved by Ad-APN only.
Following two weeks of transfection, the lesion sizes in the APN
group were significantly lower than those in the Ad-β-gal and PBS
groups, while there was no significant difference between the
control groups (Fig. 2C–E).
Based on the micro-ultrasonography results, the
plaque composition was further analyzed (Fig. 3A). Transfection with Ad-APN did not
decrease the lipid contents, as there was no significant difference
between any of the groups (82 vs. 86%, P>0.05; 82% vs. 90%,
P>0.05; 86 vs. 90%, P>0.05; Fig.
3B). Ad-APN transfection produced a higher macrophage content
than Ad-β-gal transfection (73 vs. 68%; P<0.05), with no
difference between the control groups (68 vs. 0.27%; P>0.05;
Fig. 3C). Transfection with Ad-APN
produced a greater vascular smooth muscle cell (VSMC) content than
transfection with Ad-β-gal (63 vs. 36%; P<0.05), while there was
no significant difference between the control groups (36 vs. 38%,
P>0.05; Fig. 3D).
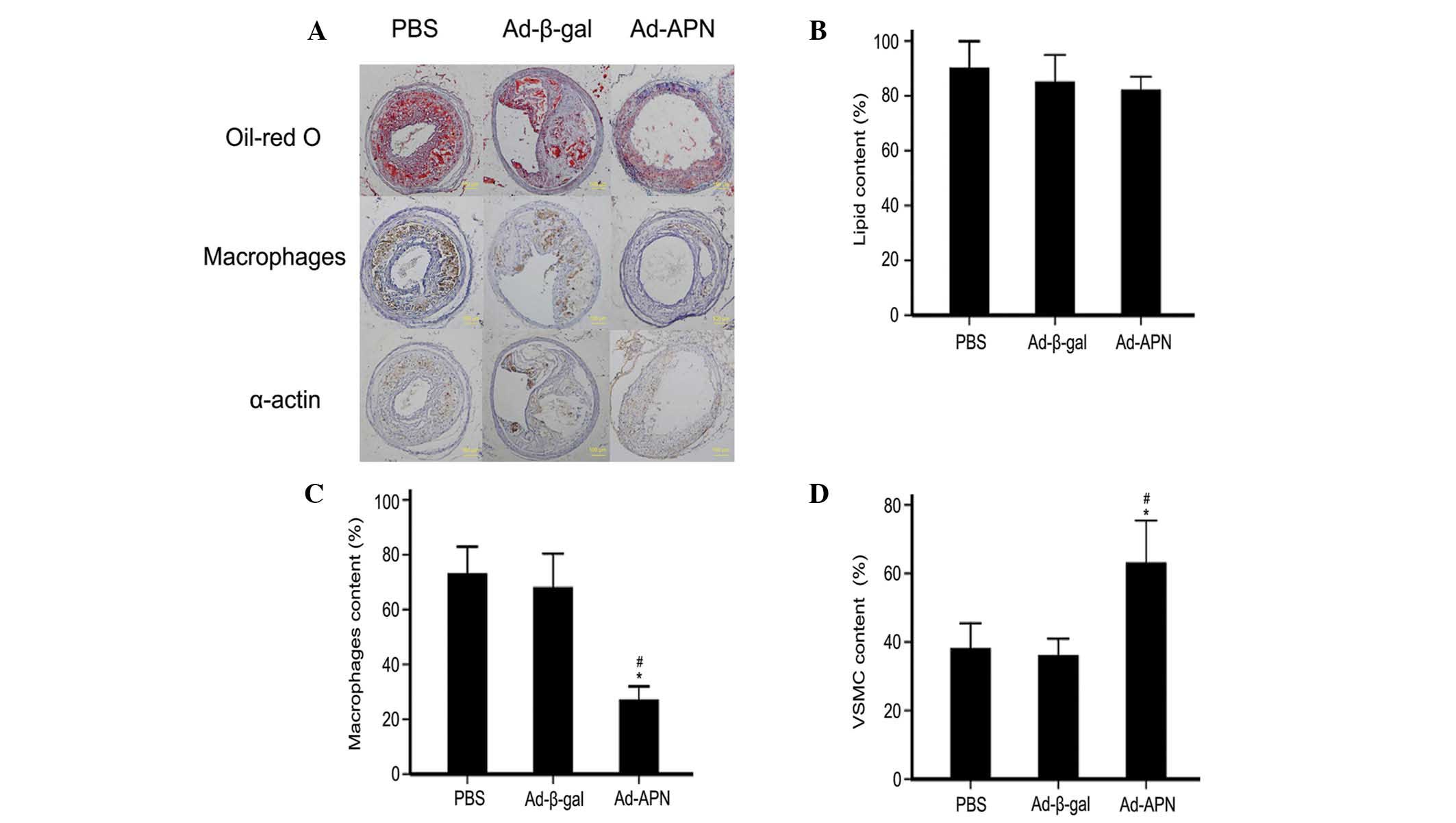 | Figure 3Effect of APN on plaque composition
in carotid atherosclerotic lesions in ApoE−/− mice. (A)
Representative microscopic images of the types of atherosclerotic
plaques (magnification, ×20). The plaque composition was comparable
among treatment groups (PBS, Ad-β-gal transfection and Ad-APN
transfection) two weeks following transfection. (B–D)
Quantification of the immunohistochemical stains. (B) Lipid content
assessed by Oil-red O staining; (C) immunohistochemical detection
of macrophages; and (D) α-actin, for smooth muscle in
atherosclerotic lesions. PBS, transfection with PBS; Ad-β-gal,
transfection with empty vector; Ad-APN, transfection with Ad-APN.
ApoE−/−, apolipoprotein E-deficient; PBS,
phosphate-buffered saline; APN, adiponectin; Ad, adenovirus; VSMC,
vascular smooth muscle cell. |
APN decreases superoxide, NO and
ONOO− production in ApoE−/− mice
Oxidative and nitrative stresses are primary factors
responsible for atherosclerosis. To further assess the mechanism of
altered atherosclerotic lesions in ApoE−/− mice,
superoxide and NO production were detected. As summarized in
Fig. 4, Ad-APN transfection
reduced the enhanced superoxide production in the carotid tissue of
ApoE−/− mice, compared with that in the
Ad-β-gal-transfected mice (2.47.1±0.9 vs. 4.43±0.6 RLU/mg/s);
P<0.05; Fig. 4A), while there
was no difference between the control groups (4.43±0.6 vs. 4.10±0.2
RLU/mg/s; P>0.05; Fig. 4A). In
addition, NO production was decreased in the Ad-APN-treated mice as
compared with the Ad-β-gal-treated mice (22±3 vs. 96±5 nmol/g;
P<0.05; Fig. 4B), with no
difference between the control groups (96±5 vs. 97±4 nmol/g;
P>0.05; Fig. 4B). Therefore,
adiponectin reduced atherosclerosis in ApoE−/− mice,
possibly through reducing superoxide production and preventing NO
destruction. In addition, the content of ONOO−, the
biradical reaction product of superoxide and NO, was detected by
measurement of nitrotyrosine, a marker of in vivo
ONOO− production. It was identified that the
nitrotyrosine content was reduced in the Ad-APN-treated mice
compared with that in the Ad-β-gal-treated mice (189±12 vs. 331±13
ng/g; P<0.05; Fig. 4C), with no
difference between the control groups (331±13 vs. 325±25 ng/g;
P>0.05; Fig. 4C).
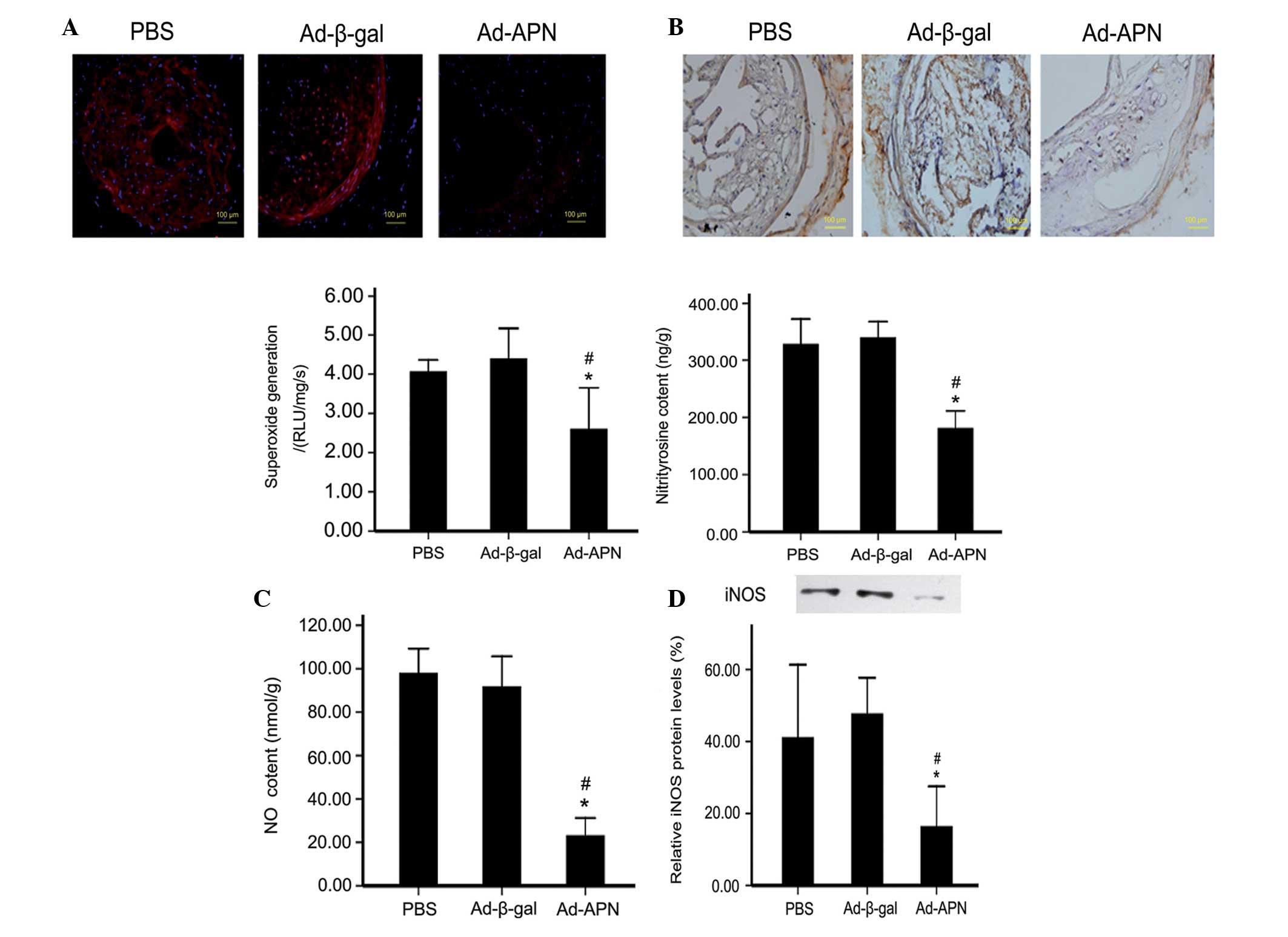 | Figure 4Effect of APN on oxidative/nitrative
stress in carotid atherosclerotic lesions in ApoE−/−
mice. (A) Detection of superoxide content (magnification, ×40). (B)
Immunohistochemical detection of nitrotyrosine and nitrotyrosine
content in carotid tissues (magnification, ×40). (C) NO content.
(D) Western blot analysis of iNOS protein levels. The values are
the mean ± standard deviation. *P<0.05 vs. control;
#P<0.05 vs. Ad-β-gal. PBS, treated with PBS;
Ad-β-gal, transfected with empty vector; Ad-APN, transfected with
Ad-APN. iNOS, inducible nitric oxide synthase; ApoE−/−,
apolipoprotein E-deficient; PBS, phosphate-buffered saline; APN,
adiponectin; Ad, adenovirus. |
Treatment with adiponectin decreases NO
production by downregulating iNOS expression in ApoE−/−
mice
The aforementioned results suggested that the
reduced superoxide production and prevention of NO destruction may
contribute to the vasculoprotective effect of APN in
ApoE−/− mice. To identify the molecular candidates
responsible for this effect, the iNOS protein expression was
determined. It was identified that iNOS activity was reduced in the
Ad-APN-treated mice (P<0.05; Fig.
4D) compared with the Ad-β-gal-treated mice, with no difference
between the control groups (P>0.05; Fig. 4D).
Discussion
Numerous studies have demonstrated that adiponectin
is an important antiatherogenic adipocytokine that inhibits insulin
resistance, inflammation and oxidative stress (21–25).
In the present study, several important observations were noted.
Firstly, adiponectin overexpression in atherosclerotic
ApoE−/− mice significantly reduced the size and area of
carotid atherosclerotic lesions and positively changed the
formation of the lesions. Secondly, it was identified that
inhibiting superoxide production, preserving NO from destruction
and blocking the formation of toxic ONOO− are major
mechanisms by which adiponectin exerts its antiatherosclerotic and
vascular protective effects. Finally, adiponectin exerted an
inhibitory effect on iNOS expression. Therefore, adiponectin
reduces atherosclerosis, at least in part by antioxidative and
antinitrative mechanisms, and may therefore be useful in the
treatment of this condition.
Adiponectin is secreted by adipose tissue and has a
significant role in the development of cardiovascular diseases. The
incidence of cardiovascular mortality is increased in patients with
low plasma adiponectin compared with that with higher plasma
adiponectin levels (26).
Furthermore, low adiponectin levels are associated with endothelial
dysfunction and inflammation (27–29).
Adiponectin infiltrates rapidly into the subendothelial space of
the vascular wall when the endothelial barrier of the arterial wall
is injured by balloon angioplasty (30) Another study documented that the
overexpression of adiponectin actually reduced atherosclerosis
through attenuating endothelial inflammatory response and
macrophage-to-foam cell transformation in vivo (9). A previous study by our group also
reported that transfection of Ad-APN in carotid arteries decreased
the size and formation of atherosclerotic lesions (18). However, whether adiponectin reduces
atherosclerosis by inhibiting iNOS and the resulting
oxidative/nitrative stress remains to be confirmed by future
studies.
Evidence suggests that common risk factors for
atherosclerosis increase the risk of free ROS production from
endothelial cells and from smooth muscle and adventitial cells
(31). Hypercholesterolemia,
diabetes mellitus, arterial hypertension, smoking, age and nitrate
intolerance increase the production of free ROS, which includes
superoxide anion (O2−), hydroxyl radical
(OH−) and ONOO−. Superoxide reacts with NO at
a near diffusion-limited rate, which is three times faster than the
reaction between superoxide and superoxide dismutase (32). This reaction causes the
inactivation of NO, a cytoprotective and vasodilatory molecule, and
results in the formation of ONOO−, a highly reactive and
cytotoxic molecule (33). However,
adiponectin may significantly reverse this course. The present
study provided direct evidence that adiponectin reduced the
enhanced superoxide production in the carotid tissue of
ApoE−/− mice. It was demonstrated that NO production was
decreased in Ad-APN-treated mice. The content of ONOO−,
the biradical reaction product of superoxide and NO, was detected
by measurement of nitrotyrosine, a marker of in vivo
ONOO− production, and the nitrotyrosine content was
reduced with adiponectin pretreatment. Therefore, adiponectin
reduced atherosclerosis in ApoE−/− mice, likely through
reducing superoxide production and preserving NO destruction.
Oxidative/nitrative stress due to increased iNOS and
superoxide production and subsequent cytotoxic ONOO−
production are early hallmarks of vascular injury in patients with
atherosclerosis. The physiological or pharmacological
concentrations of NO exert significant cardioprotective effects,
whereas the reaction product of NO and ·O2−,
ONOO−, is highly cytotoxic. Several lines of evidence
indicated that adiponectin improves endothelial function by its
antioxidative/antinitrative properties and by enhancing eNOS
activity, blocking iNOS and NADPH oxidase expression and
ONOO− production (1,34–36).
A previous study by our group on cultured adventitial fibroblasts
also demonstrated that adiponectin inhibited iNOS activity and
reduced excessive NO production (17). In the present study, it was
identified that adiponectin downregulated iNOS expression in
carotid tissues. Levels of iNOS expression were increased in the
carotid artery of ApoE−/− mice and reduced significantly
with overexpression of adiponectin. Further studies that examine
these mechanisms may reveal novel signaling pathways that regulate
iNOS activity.
In conclusion, these results demonstrated that
adiponectin, as a unique cytokine, reduced atherosclerosis and
attenuates oxidative/nitrative stress by blocking iNOS, superoxide
and ONOO− production. The present study took a different
approach and provided evidence that adiponectin expression
significantly reduced atherosclerosis associated with
oxidative/nitrative stress.
Acknowledgements
This study was supported by The National Basic
Research Program of China (973 Program, 2011CB503906), Development
Program of China (863 Program, 2007AA02Z448), the National Natural
Science Foundation of China (nos. 30728025 and 30970709), National
Natural Science Funds for Young Scholar (no. 81200211), grants from
the Shandong Young Scientists Award Fund (no. BS2012SW003) and
grants from Scientific and technology Development program of Jinan
(nos. 201003125 and 200905035).
References
|
1
|
Li R, Wang WQ, Zhang H, et al: Adiponectin
improves endothelial function in hyperlipidemic rats by reducing
oxidative/nitrative stress and differential regulation of eNOS/iNOS
activity. Am J Physiol Endocrinol Metab. 293:E1703–E1708. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Okui H, Hamasaki S, Ishida S, et al:
Adiponectin is a better predictor of endothelial function of the
coronary artery than HOMA-R, body mass index, immunoreactive
insulin, or triglycerides. Int J Cardiol. 126:53–61. 2008.
View Article : Google Scholar
|
|
3
|
Lee S, Park Y, Dellsperger KC and Zhang C:
Exercise training improves endothelial function via
adiponectin-dependent and independent pathways in type 2 diabetic
mice. Am J Physiol Heart Circ Physiol. 301:H306–H314. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee S, Zhang H, Chen J, Dellsperger KC,
Hill MA and Zhang C: Adiponectin abates diabetes-induced
endothelial dysfunction by suppressing oxidative stress, adhesion
molecules, and inflammation in type 2 diabetic mice. Am J Physiol
Heart Circ Physiol. 303:H106–H115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ouchi N, Ohishi M, Kihara S, et al:
Association of hypoadiponectinemia with impaired vasoreactivity.
Hypertension. 42:231–234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arita Y, Kihara S, Ouchi N, et al:
Paradoxical decrease of an adipose-specific protein, adiponectin,
in obesity. Biochem Biophys Res Commun. 257:79–83. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ouchi N, Kihara S, Arita Y, et al: Novel
modulator for endothelial adhesion molecules: adipocyte-derived
plasma protein adiponectin. Circulation. 100:2473–2476. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hotta K, Funahashi T, Arita Y, et al:
Plasma concentrations of a novel, adipose-specific protein,
adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc
Biol. 20:1595–1599. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okamoto Y, Kihara S, Ouchi N, et al:
Adiponectin reduces atherosclerosis in apolipoprotein E-deficient
mice. Circulation. 106:2767–2770. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Muscoli C, Cuzzocrea S, Riley DP, et al:
On the selectivity of superoxide dismutase mimetics and its
importance in pharmacological studies. Br J Pharmacol. 140:445–460.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lautamäki R, Rönnemaa T, Huupponen R, et
al: Low serum adiponectin is associated with high circulating
oxidized low-density lipoprotein in patients with type 2 diabetes
mellitus and coronary artery disease. Metabolism. 56:881–886. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Furukawa S, Fujita T, Shimabukuro M, et
al: Increased oxidative stress in obesity and its impact on
metabolic syndrome. J Clin Invest. 114:1752–1761. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ouedraogo R, Wu X, Xu SQ, et al:
Adiponectin suppression of high-glucose-induced reactive oxygen
species in vascular endothelial cells: evidence for involvement of
a cAMP signaling pathway. Diabetes. 55:1840–1846. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Motoshima H, Wu X, Mahadev K and Goldstein
BJ: Adiponectin suppresses proliferation and superoxide generation
and enhances eNOS activity in endothelial cells treated with
oxidized LDL. Biochem Biophys Res Commun. 315:264–271. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li R, Wang WQ, Zhang H, et al: Adiponectin
improves endothelial function in hyperlipidemic rats by reducing
oxidative/nitrative stress and differential regulation of eNOS/iNOS
activity. Am J Physiol Endocrinol Metab. 293:E1703–E1708. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao L, Gao E, Jiao X, et al: Adiponectin
cardioprotection after myocardial ischemia/reperfusion involves the
reduction of oxidative/nitrative stress. Circulation.
115:1408–1416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cai XJ, Chen L, Li L, et al: Adiponectin
inhibits lipopolysaccharide-induced adventitial fibroblast
migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS
pathway. Mol Endocrinol. 24:218–228. 2010. View Article : Google Scholar
|
|
18
|
Li L, Cai XJ, Feng M, et al: Effect of
adiponectin overexpression on stability of preexisting plaques by
inducing prolyl-4-hydroxylase expression. Circ J. 74:552–559. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao ZQ, Corvera JS, Halkos ME, et al:
Inhibition of myocardial injury by ischemic postconditioning during
reperfusion: comparison with ischemic preconditioning. Am J Physiol
Heart Circ Physiol. 285:H579–H588. 2003.PubMed/NCBI
|
|
20
|
Tao L, Liu HR, Gao E, et al:
Antioxidative, antinitrative, and vasculoprotective effects of a
peroxisome proliferator-activated receptor-gamma agonist in
hypercholesterolemia. Circulation. 108:2805–2811. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sulistyoningrum DC, Gasevic D, Lear SA, Ho
J, Mente A and Devlin AM: Total and high molecular weight
adiponectin and ethnic-specific differences in adiposity and
insulin resistance: a cross-sectional study. Cardiovasc Diabetol.
12:1702013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rabe K, Lehrke M, Parhofer KG and Broedl
UC: Adipokines and insulin resistance. Mol Med. 14:741–751. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fisman EZ and Tenenbaum A: Adiponectin: a
manifold therapeutic target for metabolic syndrome, diabetes, and
coronary disease? Cardiovasc Diabetol. 13:1032014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Pu H, Ma C, et al: Adiponectin
abates atherosclerosis by reducing oxidative stress. Med Sci Monit.
20:1792–1800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Essick EE, Wilson RM, Pimentel DR, et al:
Adiponectin modulates oxidative stress-induced autophagy in
cardiomyocytes. PLoS One. 8:e686972013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zoccali C, Mallamaci F, Tripepi G, et al:
Adiponectin, metabolic risk factors, and cardiovascular events
among patients with end-stage renal disease. J Am Soc Nephrol.
13:134–141. 2002.
|
|
27
|
Shimabukuro M, Higa N, Asahi T, et al:
Hypoadiponectinemia is closely linked to endothelial dysfunction in
man. J Clin Endocrinol Metab. 88:3236–3240. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tan KC, Xu A, Chow WS, et al:
Hypoadiponectinemia is associated with impaired
endothelium-dependent vasodilation. J Clin Endocrinol Metab.
89:765–769. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fernández-Real JM, Castro A, Vázquez G, et
al: Adiponectin is associated with vascular function independent of
insulin sensitivity. Diabetes Care. 27:739–745. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okamoto Y, Arita Y, Nishida M, et al: An
adipocyte-derived plasma protein, adiponectin, adheres to injured
vascular walls. Horm Metab Res. 32:47–50. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gozin A, Franzini E, Andrieu V, et al:
Reactive oxygen species activate focal adhesion kinase, paxillin
and p130cas tyrosine phosphorylation in endothelial cells. Free
Radic Biol Med. 25:1021–1032. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huie RE and Padmaja S: The reaction of NO
with superoxide. Free Radic Res Commun. 18:195–199. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beckman JS and Koppenol WH: Nitric oxide,
superoxide, and peroxynitrite: the good, the bad, and the ugly. Am
J Physiol Cell Physiol. 271:C1424–C1437. 1996.
|
|
34
|
Margaritis M, Antonopoulos AS, Digby J, et
al: Interactions between vascular wall and perivascular adipose
tissue reveal novel roles for adiponectin in the regulation of
endothelial nitric oxide synthase function in human vessels.
Circulation. 127:2209–2221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zheng Q, Yuan Y, Yi W, et al:
C1q/TNF-related proteins, a family of novel adipokines, induce
vascular relaxation through the adiponectin
receptor-1/AMPK/eNOS/nitric oxide signaling pathway. Arterioscler
Thromb Vasc Biol. 31:2616–2623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li R, Xu M, Wang X, et al: Reduced
vascular responsiveness to adiponectin in hyperlipidemic rats -
mechanisms and significance. J Mol Cell Cardiol. 49:508–515. 2010.
View Article : Google Scholar : PubMed/NCBI
|