Introduction
Diabetes mellitus is a serious chronic disease, with
an increasing worldwide incidence each year. According to
statistics from the World Health Organization, the number of
patients with diabetes reached 346,000,000 in 2011 (1). One of the microvascular complications
of diabetes, diabetic retinopathy (DR) and, in particular,
proliferative diabetic retinopathy (PDR), threatens the vision of
patients with diabetes and is the leading cause of blindness among
working-age adults (2).
Angiogenesis is the key pathological feature of PDR
(3). Advanced glycation
end-products (AGEs) are proteins or lipids that are
non-enzymatically glycated and oxidized following contact with
aldose sugars (4). Animal
experiments have revealed that foods rich in AGEs are a risk factor
for angiogenesis-associated diseases (5). AGEs promote angiogenesis by
increasing the expression of vascular endothelial growth factor
(VEGF) in vitro and there is a positive correlation between
the levels of AGE and PDR in vivo (6). These findings suggest that AGE are
involved in the pathogenesis of PDR.
β2-Glycoprotein I (β2-GPI), also termed
apolipoprotein H, is a phospholipid-binding plasma protein, which
consists of five homologous repeated units. β2-GPI is the major
autoantigen in patients with antiphospholipid syndrome (7). β2-GPI has anti-angiogenetic
properties (8) and one of its
mutants, in which domain V of domains I–IV is absent (DI-IV), has
the potential to produce more marked effects (9).
Our previous study was the first, to the best of our
knowledge, to demonstrate that while β2-GPI and DI-IV inhibited
angiogenesis in human umbilical vein endothelial cells, DI-IV had a
more marked effect (10). Another
study found that DI-IV inhibits angiogenesis by downregulating VEGF
signaling pathways (11).
Therefore, DI-IV may provide a potential treatment for diabetic
retinopathy.
The present study investigated whether DI-IV was
able to inhibit AGE-induced angiogenesis in RF/6A rhesus macaque
choroid-retinal vascular endothelial cells and examined the
potential mechanisms involved.
Materials and methods
Materials and reagents
The RF/6A cell line was purchased from the China
Center for Type Culture Collection (Wuhan, China). DI-IV was
purchased from Tianjin LuoSai Technology Development Co., Ltd.
(Tianjin, China) and was produced as previously described (12). AGE-bovine serum albumin (BSA) was
purchased from Biovision (Mountain View, CA, USA). Dulbecco’s
modified Eagle’s medium (DMEM) was purchased from Gibco-BRL (Grand
Island, NY, USA). Fetal bovine serum (FBS) and pancreatin were
purchased from Beijing Solarbio Technology, Ltd. (Beijing, China).
CellTiter 96® AQueous One Solution regent and Matrigel
were purchased from BD Biosciences (Bedford, MA, USA). Monoclonal
antibodies to VEGF receptor (VEGFR)-1, VEGFR-2, protein kinase B
(Akt), extracellular signal-regulated kinase (ERK), phosphorylated
(p)-Akt and p-ERK, anti-mouse immunoglobulin (Ig)G and anti-rabbit
IgG labeled with horseradish peroxidase (HRP) were all purchased
from Cell Signaling Technology, Inc. (Beverly, MA, USA). All other
reagents used were purchased from Sigma (St. Louis, MO, USA).
RF/6A cell culture
The RF/6A cells were cultured in high-glucose DMEM
containing 10% FBS and incubated at 37°C in 5% CO2. The
cells were seeded at a density of 5×104 cells/ml and
divided into the following five treatment groups: 100 μg/ml BSA as
a control, 100 μg/ml AGE, 100 μg/ml AGE+10 nm DI-IV, 100 μg/ml
AGE+100 nm DI-IV and 100 μg/ml AGE+400 nm DI-IV.
RF/6A proliferation assay
The RF/6A cells were seeded onto 96-well tissue
culture plates (100 μl/well; 5×104 cells/ml) and
incubated in the regular medium and incubation conditions described
above for 24 h and subsequently with serum-free medium (starved)
for 12 h.
Following removal of the growth medium, the cells
were incubated for an additional 72 h in medium supplemented with
AGE and/or DI-IV, as described above. Subsequently, 20 μl CellTiter
96® AQueous One Solution regent, mixed with 100 μl
growth medium, was added to each well and the cells were incubated
at 37°C for between 1 and 4 h. The absorbance of each well at 490
nm was measured using a microplate reader (BioTek Synergy 2;
BioTek, Winooski, VT, USA).
In vitro wound healing assay
The RF/6A cells were seeded onto 48-well plates,
which were coated with 50 μl 0.1% gelatin. When the cells had
formed a monolayer, a wound, termed the ‘denuded area’, was
introduced by clearing an area of the monolayer with a micropipette
tip. The culture medium in the well was carefully removed and the
cells were washed twice with phosphate-buffered saline (PBS) prior
to adding medium containing AGE and/or DI-IV, as described
previously. After 18–24-h incubation, the cells were washed twice
with PBS and fixed with 10% (w/v) formalin for 10 min, followed by
staining with eosin for 3 min. To determine cell migration into the
denuded area (cell number/mm2), images were captured
using microscopy with a camera (Leica DM4000 B; Leica Microsystems,
Wetzlar, Germany) and analyzed using ImagePro-Plus software
(Version 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
In vitro matrigel angiogenesis assay
Briefly, 96-well plates were coated with cold
Matrigel (70 μl /well), which was left to polymerize at 37°C for
~30 min. Subsequently, 100 μl RF/6A suspension (5×104
cells/ml) was seeded onto the Matrigel and cultured for 24 h in
medium supplemented with AGE and/or DI-IV, as described previously.
Tube formation was quantified by determining the mean vessel length
in three randomly selected fields using ImagePro-Plus image
analysis software.
Analysis of the mRNA expression of VEGF
and VEGFR with reverse transcription quantitative polymerase chain
reaction (RT-qPCR)
The RF/6A cells were seeded and incubated for 24 h,
as previously defined. Following culture with serum-free medium
overnight, AGE and/or DI-IV were added to the medium. After 48 h
incubation, the total cellular RNA was extracted using TRIzol
reagent according to the manufacturer’s instructions. The final RNA
concentrations were determined by their optical density (OD) at 260
nm and integrity was verified by ethidium bromide staining of
ribosomal 18S and 28S bands on a 2% agarose gel. The A260/A280
ratios were between 1.8 and 2.0. The total RNA was then reverse
transcribed into cDNA using a SuperScriptTM III
Platinum® Two-Step RT-qPCR kit with
SYBR®Green. The primers for all the genes asessed were
designed using Primer 5.0 software (Premier Biosoft International,
Palo Alto, CA, USA). The primer sequences used were as follows:
VEGF-A, forward 5′-GCCTTGCACACACTTTGTACTGGT-3′ and reverse
5′-TGTCCATAGCTTTCCTCGTGCCT-3′; VEGFR-1, forward
5′-AAGGCCGTGTCATCATTTCCAGAC-3′ and reverse
5′-ACCACTTGATTGTAGGTCGAGGGA-3′; VEGFR-2, forward
5′-GCATGTGCTGACGATCATGGAAGT-3′ and reverse
5′-ACCACGTGACTCTGCTTCTCCTTT-3′; receptor for AGE (RAGE), forward
5′-TGCTTCTAGAATACAGGCCGGACA-3′ and reverse
5′-TCGGTAGTTGGACTTGGTCTCCTT-3′ and β-actin, forward
5′-ATGAAGTGTGACGTGGACATCCGT-3′ and reverse
5′-TCTCCTTCTGCATCCTGTCAGCAA-3′. The PCR cycling conditions on the
Rotor-Gene TM 3000 (Corbett Life Sciences, San Francisco, CA, USA)
were set as follows: UCG activation at 50°C for 2 min and 95°C for
2 min, 45 cycles at 94°C for 5 sec, 55°C for 10 sec and 72°C for 10
sec. Melting curve analysis was performed between 55 and 95°C by
monitoring SYBR Green I fluorescence at 0.5°C increment increases
at 10 sec intervals. All sample measurements were performed in
triplicate. β-actin primers were included to correct for
sample-to-sample variation and for each experimental sample, the
mRNA levels were normalized to those of β-actin.
Western blot analysis for signaling
pathway analysis
The RF/6A cells were seeded as previously described,
incubated for 24 h and then cultured with serum-free medium
overnight. As mentioned above, cells were divided into five groups.
The incubation was terminated by adding 1 ml cold PBS containing
100 μM sodium vanadate (Sigma). The samples were then placed on
ice, washed with ice-cold PBS and lysed in radioimmunoprecipitation
assay buffer containing 50 mm Tris (pH 7.4), 150 mm NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 2 mM sodium pyrophosphate,
25 mM β-glycerophosphate, 1 mM EDTA, 1 mm
Na3VO4 and 0.5 μg/ml leupeptin. The cells
were incubated on ice for 5 min and were then detached and briefly
sonicated. The lysates were clarified by centrifugation (12,000 xg,
20 min, 4°C) and the protein content in the supernatant was
measured using a Micro BCATM Protein kit (Invitrogen
Life Technologies, Carlsbad, CA, USA), according to the
manufacturer’s instructions. Aliquots (30 μg protein/lane) of the
total protein were resolved using NuPAGETM 4–12%
Bis-Tris gel and were blotted onto a nitrocellulose transfer
membrane. The membrane was inhibited with 2% BSA in 20 mM Tris-HCl
(pH 7.6), 137 mm NaCl and 0.01% Tween-20 (TBST) for 1 h at room
temperature, followed by incubation with the specific primary
antibodies, VEGFR-1, VEGFR-2, p-ERK1/2, total-ERK1/2, Akt and p-Akt
(Thr 308). Following washing with TBST, the membrane was reprobed
with either HRP-anti-mouse or anti-rabbit IgG (1:1,000) in 2% BSA
in TBST for 60 min at room temperature and then exposed to a
high-performance chemiluminescence film (Amersham Pharmacia
Biotech, Buckinghamshire, UK). The film was developed and
densitometry was performed using the Bio-Rad Gel Doc 2000 analysis
system (Bio-Rad, Hercules, CA, USA). The relative density of the
bands was calculated from the areas under the peaks on plots of OD
and compared with that of β-actin in each sample.
Statistical analysis
Statistical analysis was conducted using SPSS
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
the mean ± standard deviation. An independent sample t-test,
one-way analysis of variance and least significant difference-t
test were used to evaluate significant differences. P<0.05 was
considered to indicate a statistically significant difference.
Homogeneity of variance was assessed prior to statistical
analysis.
Results
DI-IV inhibits the AGE-induced
proliferation of RF/6A cells
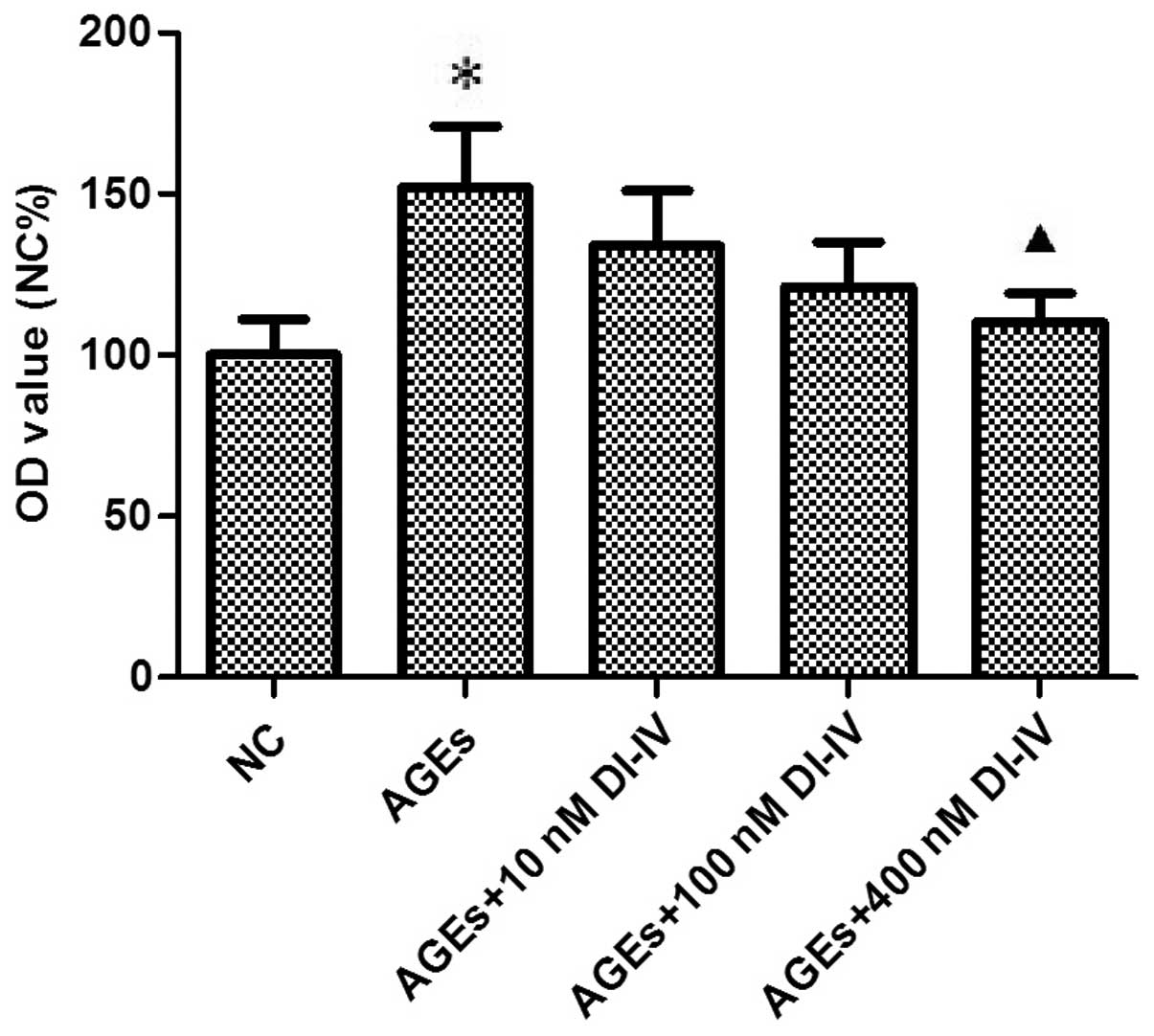
Fig. 1 shows that 3
days of incubation with AGE stimulated the proliferation of RF/6A
cells ~1.5-fold (P<0.05) and that DI-IV inhibited this effect
(P<0.05) at 400 nm.
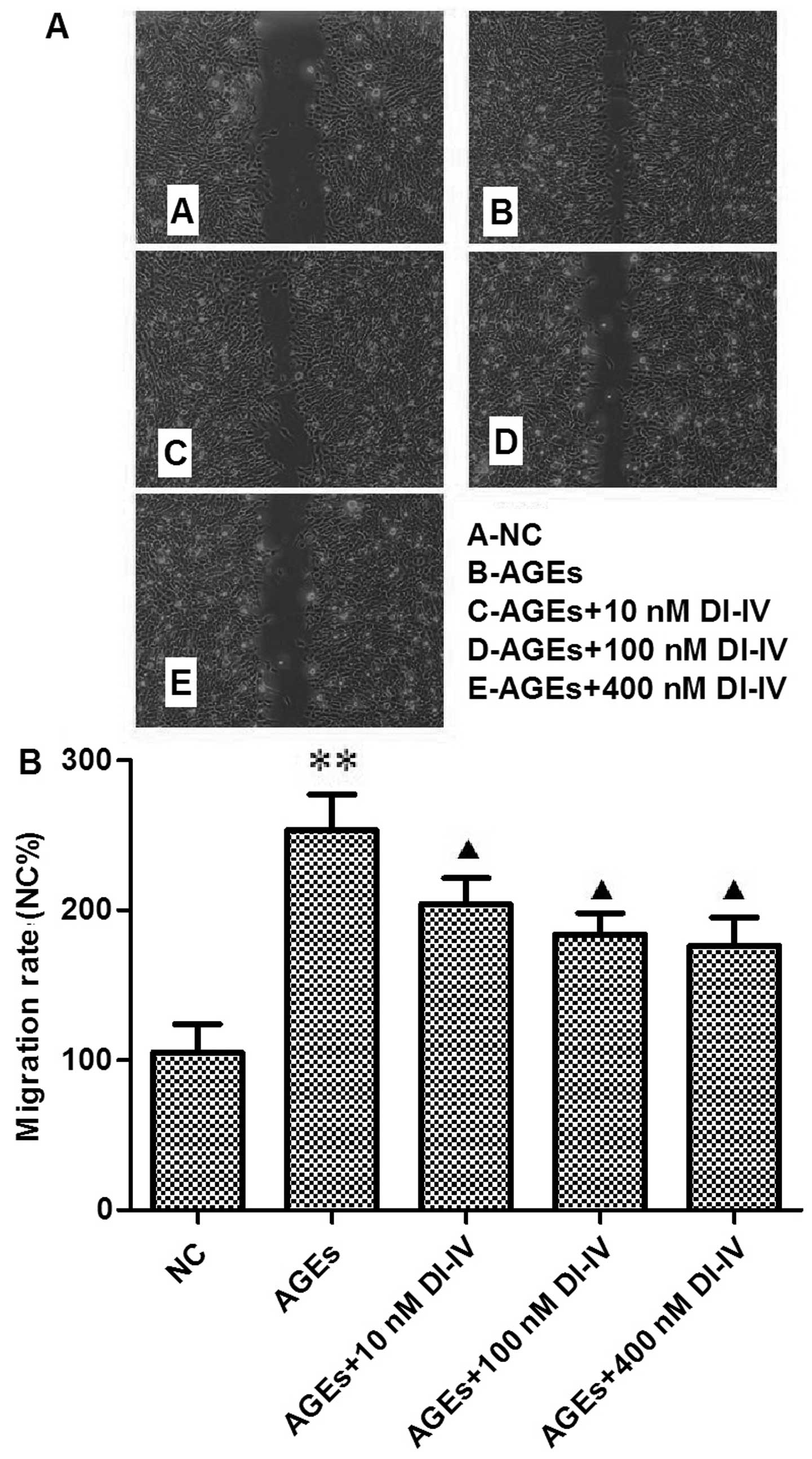
DI-IV inhibits the AGE-induced migration
of RF/6A cells
After 18–24 h incubation with AGE, more cells had
migrated into the denuded area. Quantification analysis revealed a
2.5-fold increase in RF/6A cell migration (P<0.01). DI-IV
inhibited this change in a dose-dependent manner (P<0.05;
Fig. 2A and B).
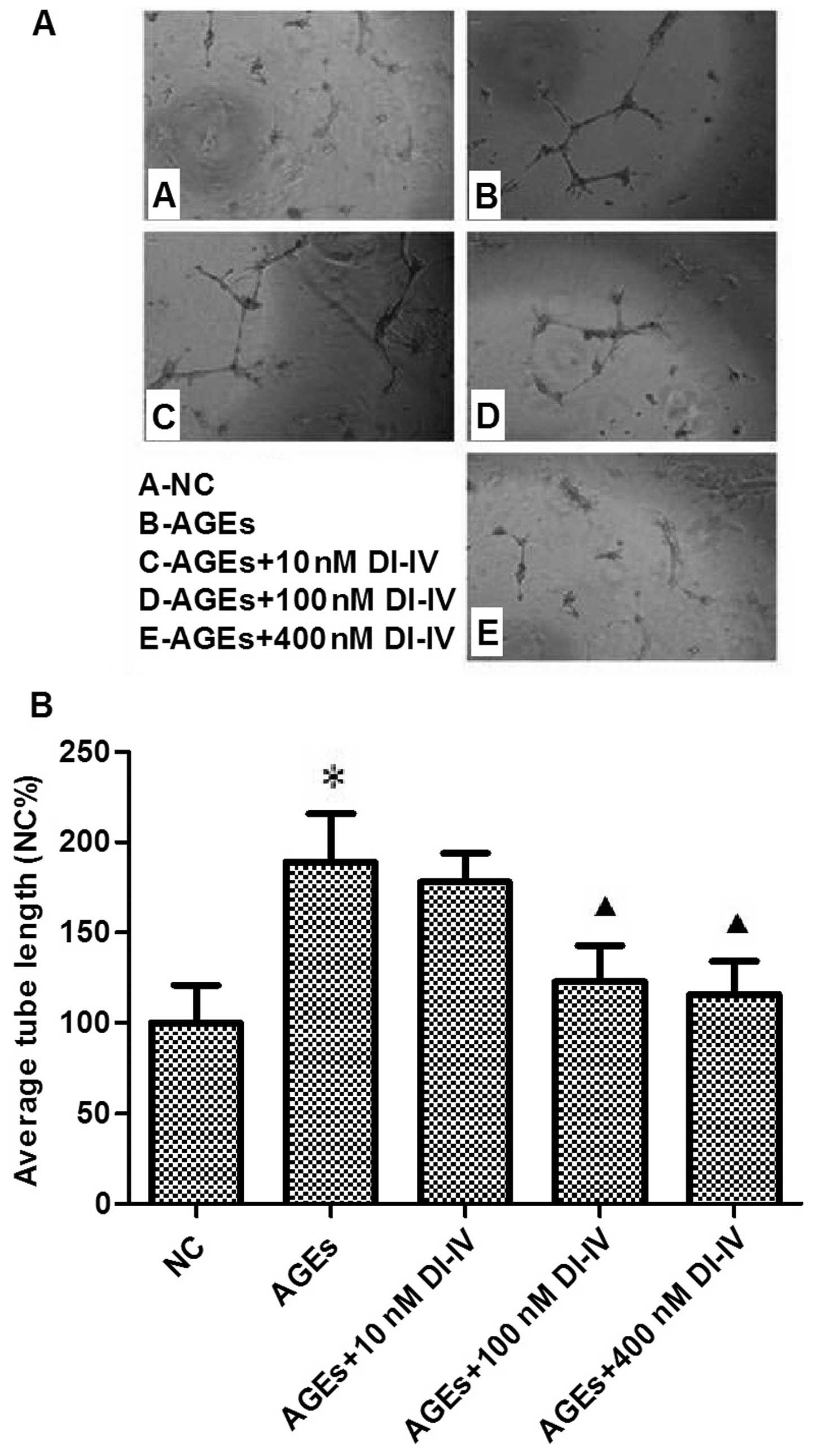
DI-IV inhibits AGE-induced tube formation
in RF/6A cells
AGE induced a 1.7-fold increase in tube formation in
the Matrigel angiogenesis assay. DI-IV inhibited this effect of
AGE, however the suppression was only statistically significant
(P<0.05) at 100 and 400 nm DI-IV. At these concentrations, the
AGE-induced increase in tube formation was eradicated. (P<0.05;
Fig. 3A and B).
DI-IV inhibits the AGE-induced mRNA
expression of VEGFR-2
Our previous study identified that DI-IV inhibits
human umbilical vein cell angiogenesis through the VEGF pathway
(8). To determine whether DI-IV
inhibited AGE-induced RF/6A angiogenesis through a similar
mechanism, the transcript levels of VEGF and its receptors were
measured. Incubation of RF/6A cells with AGE for 48 h significantly
increased the mRNA expression of VEGF, VEGR-2 and RAGE (P<0.01
or P<0.05). DI-IV inhibited the AGE-induced increase in VEGFR-2
mRNA at DI-IV concentrations of 100 and 400 nm, respectively
(P<0.05), whereas the increases in the mRNA expression of VEGF
and RAGE were not inhibited by DI-IV. By contrast, the mRNA
expression of VEGFR-1 was not affected by AGE or by DI-IV (Fig. 4).
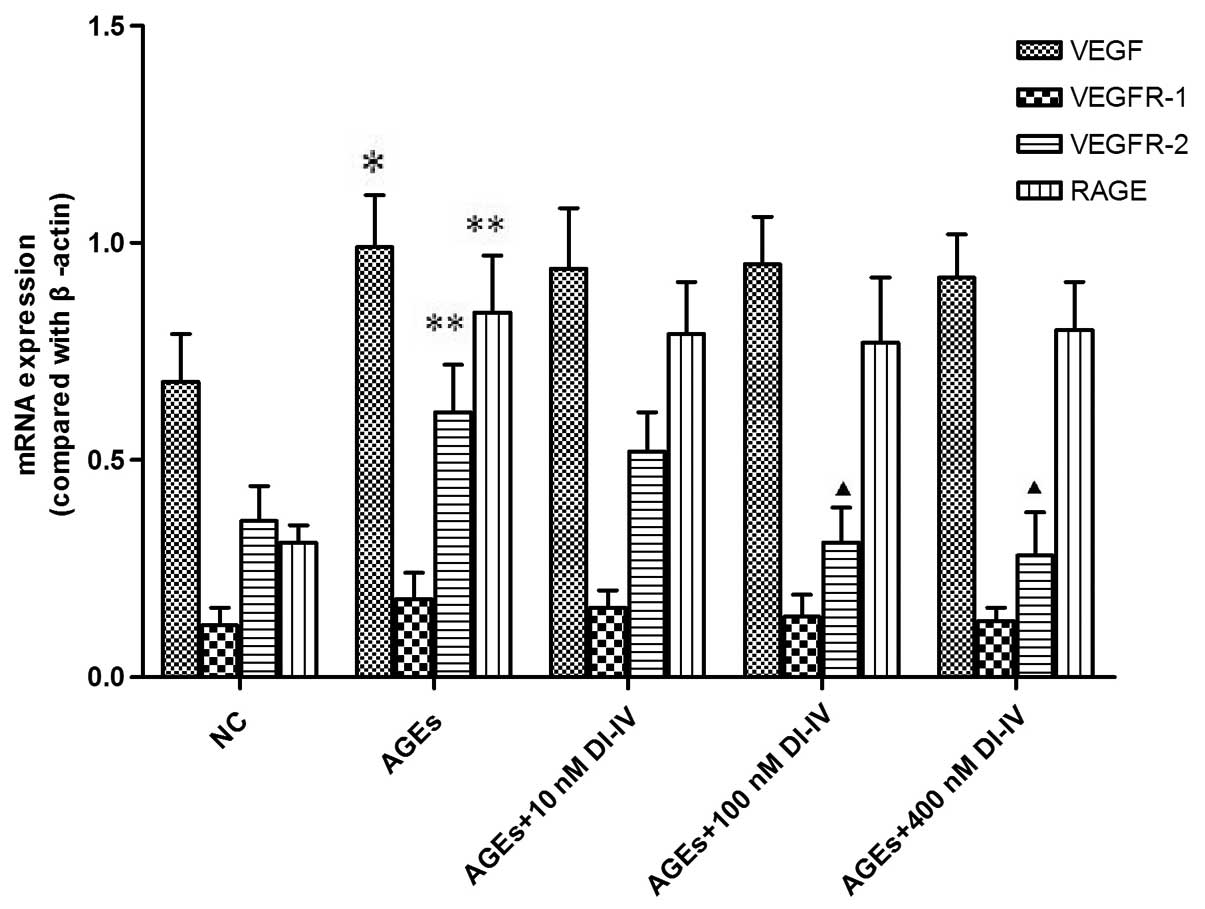 | Figure 4DI-IV inhibits AGE-induced increases
in the mRNA expression level of VEGFR-2. The rhesus macaque
choroid-retinal vascular endothelial cells were incubated with AGE
(100 g/ml) alone or with DI-IV (10, 100 or 400 nm) for 48 h. Total
cellular RNA was extracted from the cultured cells using TRIzol
reagent. The mRNA expression levels of VEGF, VEGFR-2, VEGFR-1 and
RAGE were analyzed with reverse transcription quantitative
polymerase chain reaction. The mRNA levels were compared with
β-actin. AGE (100 g/ml) significantly increased the mRNA expression
levels of VEGF, VEGFR-2 and RAGE compared with the NC. DI-IV (100
and 400 nm) significantly reduced the AGE-induced increase in the
mRNA expression level of VEGFR-2, but not VEGF or RAGE. Values are
expressed as the mean ± standard deviation. *P<0.05
and **P<0.01, compared with the NC group;
▲P<0.05, compared with the AGE group. AGE, advanced
glycation end products; NC, normal control of RF/6A cells cultured
with 100 μg/ml bovine serum albumin; DI-IV, domain V mutant of
β2-glycoprotein I; VEGF, vascular endothelial growth factor; VEGFR,
VEGF receptor; RAGE, receptor for advanced glycation end
products; |
DI-IV inhibits the expression of VEGFR-2
and the phosphorylation of Akt and ERK1/2 induced by AGE
Incubation of the RF/6A cells with AGE for 48 h
significantly increased the expression of VEGFR-2 and the
phosphorylation of Akt and ERK1/2 (P<0.05); DI-IV inhibited this
effect of AGE at concentrations of 100 nm and 400 nm (P<0.05).
By contrast, the expression levels of Akt and ERK were not affected
by AGE or DI-IV (Fig. 5A–D).
 | Figure 5DI-IV inhibits AGE-induced
phosphorylation of ERK1/2 and Akt (Thr 308). (A) Representative
images and quantitative analyses of VEGFR-1 and VEGFR-2; (B)
Representative images and quantitative analyses of p-ERK1/2,
total-ERK1/2, Akt and p-Akt. Rhesus macaque choroid-retinal
vascular endothelial cells were incubated with AGE (100 g/ml) alone
or with DI-IV (10, 100 or 400 nm) for 48 h. Total protein was
extracted from the cultured cells and protein expression was
assessed using western blot analysis. AGE increased the expression
of VEGFR-2 and the phosphorylation of Akt (Thr 308) and ERK1/2
compared with the NC. DI-IV inhibited the effect of AGE. By
contrast, the expression of Akt and ERK was not affected by AGE or
DI-IV. Values are expressed as the mean ± standard deviation.
*P<0.05, compared with the NC group;
▲P<0.05, compared with the AGEs group; ▲, P<0.05.
AGE, advanced glycation end products; NC, normal control of RF/6A
cells cultured with 100 μg/ml bovine serum albumin; DI-IV, domain V
mutant of β2-glycoprotein I; ERK, extracellular signal-regulated
kinase; p-, phosphorlyated. |
Discussion
PDR is one of the most severe microvascular
complications for diabetic patients and is the major cause of
acquired blindness. One important pathogenic mechanism in PDR is
neovascularization, which is promoted by various factors, including
hypoxia, local inflammation and angiogenic factors, including VEGF
and FGF-2 (13,14). VEGF, particularly VEGF-A, is a key
factor promoting angiogenesis by binding with its receptors,
VEGFR-1 and VEGFR-2. VEGFR-2 has a more dominant role in
angiogenesis, while VEGFR-1 is involved mainly in the migration of
macrophages and vascular endothelial cells (15). VEGF activates VEGFR and the
downstream phosphoinositide 3-kinase (PI3K)/Akt and
mitogen-activated protein kinase (MAPK)/ERK1/2 signaling pathways
(16,17) and promotes angiogenesis.
RF/6A is a long-term culture of a spontaneously
transformed endothelial cell line derived from the choroid-retina
of the rhesus macaque fetus (18).
The morphology, growth, ultrastructure, immunocytochemistry
(immunofluorescence) and immunodiffusion of these cells
distinguishes them as endothelial in origin (18). The cells demonstrate prolonged
expression of VEGF and VEGFRs (19). Due to their ease of culture and
rapid transfer, this cell line is widely used in the investigation
of PDR and other retina-associated diseases (19–22).
AGE is important in diabetic retinopathy,
particularly in PDR (14,15). It is reported that AGE promotes
neovascularization by increasing the expression of VEGF (mainly
VEGF-A) in endothelial cells and consequently inducing tube
formation of the retinal microvessel endothelial cells (23). AGE can potentially induce retinal
ganglion cells to express VEGF-A and inhibit apoptosis and are,
thus, important in the pathogenesis of diabetic retinopathy
(24). The results of the present
study revealed that AGE significantly increased the mRNA expression
of VEGF and RAGE, promoted the proliferation and migration of
vascular endothelial cells and stimulated neovascularization in the
RF/6A cells. These results led to the hypothesis that AGE promoted
the expression of VEGF through interactions with RAGE and was,
therefore, important in retinal neovascularization in patients with
diabetes mellitus.
The present study demonstrated that AGE
significantly increased the expression of VEGF and VEGFR-2 in
vascular endothelial cells, however it had no effect on the
expression of VEGFR-1. VEGFR-2 is the key receptor regulating
VEGF-mediated angiogenesis (25).
In the present study, the expression of VEGFR-2 mRNA and protein
were examined. The results revealed that the proliferation,
migration and tube formation of the RF/6A cells were significantly
increased in the AGE group, concomitant with increases in the
expression of VEGFR-2 mRNA and VEGFR-2. In the DI-IV treatment
group, the proliferation, migration and tube formation of the RF/6A
cells were decreased, as was the expression of VEGFR-2 mRNA and
VEGFR-2. Thus, although AGE promoted the expression of VEGF, it
also upregulated VEGFR-2 and activated its downstream PI3K/Akt and
MAPK/ERK1/2 signaling pathways to inhibit angiogenesis.
Our previous study revealed that DI-IV inhibits
human umbilical vein cell angiogenesis by downregulating the
expression of VEGFR-2 in endothelial cells and by inhibiting the
phosphorylation of VEGF downstream effector molecules in the
MAPK/ERK1/2 and PI3K/Akt pathways. Nakagawa et al further
identified the potential anti-angiogenic effect of plasmin-nicked
β2-GPI (26). The findings of the
present study demonstrated that DI-IV inhibited the AGE-induced
angiogenesis and reduced the AGE-induced cell proliferation and
migration. DI-IV also inhibited the dose-dependent AGE-induced
increase of VEGFR-2, but not that of VEGF. Since DI-IV also had no
effect on the expression of RAGE, the inhibitory effect of DI-IV on
AGE-induced angiogenesis must be through the VEGF-A/VEGFR-2
pathway, but not the AGE-RAGE axis. Therefore the present study
demonstrated that DI-IV inhibited AGE-induced RF/6A
neovascularization by downregulating VEGFR-2 and its downstream
effector molecules in the MAPK/ERK1/2 and PI3K/Akt pathways.
In conclusion, AGE promoted neovascularization by
binding with RAGE and increasing the expression of VEGF, followed
by activation of VEGFR-2 and its downstream PI3K/Akt and
MAPK/ERK1/2 pathways. DI-IV had no effect on RAGE or VEGF, however
it exerted anti-angiogenic action by downnegulating the expression
of VEGFR-2 and its downstream PI3K/Akt and MAPK/ERK1/2 pathways.
Therefore, the use of compounds with a similar action to DI-IV
provide a potential approach for the treatment of PDR.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 30971393 and 81070645),
the Tianjin Natural Science Fund (no. 10JCYBJC12000) and the
Science and Technology Fund of Tianjin Health Bureau (nos. 09KZ01
and 2012KG135).
References
|
1
|
Danaei G, Finucane MM, Lu Y, Singh GM,
Cowan MJ, Paciorek CJ, et al; Global Burden of Metabolic Risk
Factors of Chronic Diseases Collaborating Group (Blood Glucose).
National, regional, and global trends in fasting plasma glucose and
diabetes prevalence since 1980: systematic analysis of health
examination surveys and epidemiological studies with 370
country-years and 2.7 million participants. Lancet. 378:31–40.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fong DS, Aiello LP, Ferris FL and Klein R:
Diabetic Retinopathy. Diabetes Care. 27:2540–2553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller JW, Adamis AP and Aiello LP:
Vascular endothelial growth factor in ocular neovascularization and
proliferative diabetic retinopathy. Diabetes Metab Rev. 13:37–50.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldin A, Beckman JA, Schmidt AM and
Creager MA: Advanced glycation end products: sparking the
development of diabetic vascular injury. Circulation. 114:597–605.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sato T, Wu X, Shimogaito N, et al: Effects
of high-AGE beverage on RAGE and VEGF expressions in the liver and
kidneys. Eur J Nutr. 48:6–11. 2009. View Article : Google Scholar
|
|
6
|
Yamagishi S, Matsui T, Nakamura K, et al:
Olmesartan blocks advanced glycation end products (AGE)-induced
angiogenesis in vitro by suppressing receptor for AGE (RAGE)
expression. Microvasc Res. 75:130–134. 2008. View Article : Google Scholar
|
|
7
|
De Groot PG and Meijers JC:
β(2)-Glycoprotein I: evolution, structure and function. J Thromb
Haemost. 9:1275–1284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Passam FH, Qi JC, Tanaka K, Matthaei KI
and Krilis SA: In vivo modulation of angiogenesis by beta 2
glycoprotein I. J Autoimmun. 35:232–240. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu P, Passam FH, Yu DM, Denyer G and
Krilis SA: Beta2-glycoprotein I inhibits vascular endothelial
growth factor and basic fibroblast growth factor induced
angiogenesis through its amino terminal domain. J Thromb Haemost.
6:1215–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu P, Passam FH, Yu DM, Denyer G and
Krilis SA: Beta2-glycoprotein I inhibits vascular endothelial
growth factor and basic fibroblast growth factor induced
angiogenesis through its amino terminal domain. J Thromb Haemost.
6:1215–1223. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beecken WD, Ringel EM, Babica J, et al:
Plasmin-clipped beta (2)-glycoprotein-I inhibits endothelial cell
growth by down-regulating cyclin A, B and D1 and up-regulating p21
and p27. Cancer Lett. 296:160–167. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iverson GM, Victoria EJ and Marquis DM:
Anti-beta2-glycoprotein I (beta2-GPI) autoantibodies recognize an
epitope on the first domain of beta2-GPI. Proc Natl Acad Sci USA.
95:15542–15546. 1998. View Article : Google Scholar
|
|
13
|
Heo JW, Kim JH, Cho CS, et al: Inhibitory
activity of bevacizumab to differentiation of retinoblastoma cells.
PLoS One. 7:e334562012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nichol D and Stuhlmann H: EGFL7: a unique
angiogenic signaling factor in vascular development and disease.
Blood. 119:1345–1352. 2012. View Article : Google Scholar :
|
|
15
|
Shibuya M: Tyrosine kinase receptor
Flt/VEGFR family: its characterization related to angiogenesis and
cancer. Genes Cancer. 1:1119–1123. 2010. View Article : Google Scholar
|
|
16
|
Huang Q and Sheibani N: High glucose
promotes retinal endothelial cell migration through activation of
Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol.
295:C1647–C1657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elayappan B, Ravinarayannan H, Pasha SP,
Lee KJ and Gurunathan S: PEDF inhibits VEGF- and EPO-induced
angiogenesis in retinal endothelial cells through interruption of
PI3K/Akt phosphorylation. Angiogenesis. 12:313–324. 2009.
View Article : Google Scholar
|
|
18
|
Lou DA and Hu FN: Specific antigen and
organelle expression of a long-term rhesus endothelial cell line.
In Vitro Cell Dev Biol. 23:75–85. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Du W, Yu W, Huang L, Zhao M and Li X:
Ephrin-a4 is involved in retinal neovascularization by regulating
the VEGF signaling pathway. Invest Ophthalmol Vis Sci.
53:1990–1998. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amrite AC, Ayalasomayajula SP, Cheruvu NP
and Kompella UB: Single periocular injection of celecoxib-PLGA
microparticles inhibits diabetes-induced elevations in retinal
PGE2, VEGF, and vascular leakage. Invest Ophthalmol Vis Sci.
47:1149–1160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun T, Cao H, Xu L, et al: Insulin-like
growth factor binding protein-related protein 1 mediates
VEGF-induced proliferation, migration and tube formation of retinal
endothelial cells. Curr Eye Res. 36:341–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grigsby JG, Parvathaneni K, Almanza MA, et
al: Effects of tamoxifen versus raloxifene on retinal capillary
endothelial cell proliferation. J Ocul Pharmacol Ther. 27:225–233.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Zhao J and Gregersen H:
Up-regulated expression of advanced glycation end-products and
their receptor in the small intestine and colon of diabetic rats.
Dig Dis Sci. 57:48–57. 2012. View Article : Google Scholar
|
|
24
|
Lee JJ, Hsiao CC, Yang IH, et al:
High-mobility group box 1 protein is implicated in advanced
glycation end products-induced vascular endothelial growth factor A
production in the rat retinal ganglion cell line RGC-5. Mol Vis.
18:838–850. 2012.PubMed/NCBI
|
|
25
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) dignaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar
|
|
26
|
Nakagawa H, Yasuda S, Matsuura E, et al:
Nicked {beta}2-glycoprotein I binds angiostatin 4.5 (plasminogen
kringle 1–5) and attenuates its antiangiogenic property. Blood.
114:2553–2559. 2009. View Article : Google Scholar : PubMed/NCBI
|