Introduction
Cholesteatomas are gradually expanding, destructive
lesions of the temporal bone (1).
The presence of destructive epithelial lesions of the temporal bone
results in the erosion of adjacent bone structures, and leads to
various complications, including otalgia, malodorous otorrhea and
hearing loss (2). There are two
types of cholesteatomas: Congenital and acquired (2). The common feature of these two types
of cholesteatomas is the migration of keratinized
hyperproliferative squamous epithelium, from a fibrous stroma into
the middle ear and mastoid cavity (2). A previous study demonstrated that
keratinocyte proliferation and migration is mediated by growth
factors and their receptors (1).
An upregulation of epidermal growth factor (EGF) and its receptor
(EGFR), and of keratinocyte growth factor (KGF) and its receptor
(KGFR) have previously been reported in cholesteatomas (3–6).
Further studies have suggested that an upregulation of these growth
factors and their receptors induces cell proliferation of
keratinocytes in cholesteatomas (7–9). In
addition to growth factors, the possible roles of microRNAs (miRNA)
in the formation of cholesteatomas have recently been proposed
(10,11).
miRNAs are evolutionarily conserved, small
non-coding RNA molecules, and are considered to be important
post-transcriptional modifiers (12). miRNAs have significant roles in the
regulation of cellular proliferation, differentiation, apoptosis
and oncogenesis (13). Previous
studies have shown that miRNAs are strongly associated with the
development of cholesteatomas (10,11).
Friedland et al (11),
demonstrated an upregulation of human miR-21, and a reduction of
its targets, phosphatase and tensin homologue (PTEN) and programmed
cell death protein (PDCD4) in cholesteatomas. In our previous
study, it was shown that the levels of miR-21 and let-7a miRNA were
significantly increased, particularly in pediatric patients
(10). Furthermore, the expression
levels of PTEN and PDCD4 were decreased in cholesteatoma tissues
(10). These findings support the
potential roles of miR-21 and let-7a miRNA in the pathogenesis of
cholesteatomas.
The present study aimed to investigate the functions
of let 7a in cholesteatoma keratinocytes using let 7a miRNA mimics
and a let 7a inhibitor. In particular, the role of let-7a miRNA on
cellular proliferation, apoptosis and migration in cholesteatoma
keratinocytes was evaluated.
Materials and methods
Tissue collection
The present study was approved by the Ethics
Committee of the First Affiliated Hospital of Zhengzhou University
(Zhengzhou, China). Written informed consent was obtained from all
of the patients, or from the parents of the patients, prior to
surgery. Cholesteatoma tissues were collected from 20 cholesteatoma
patients (aged 18–70 years), and normal postauricular skin
specimens from the same patients served as the control tissues. The
samples were transported to the laboratory immediately following
surgery.
Cell culture and miRNA transfection
Cholesteatoma tissues were transferred into 5 ml
keratinocyte free media (KSFM; Gibco-BRL, Carlsbad, CA, USA). The
tissues were then treated with 0.5 ml collagenase (20 mg/ml), and
dissociated into single cells. The suspension was centrifuged for 5
min at 287 × g (Thermo Labofuge 400R; Thermo Fisher Scientific,
Waltham, MA, USA). The pellets were resuspended in 10 ml KSFM
supplemented with penicillin and streptomycin (Sigma-Aldrich, Santa
Clara, CA, USA, 10ml/l vol/vol). The cells were cultured in a
humidified incubator containing 5% CO2 at 37°C. The
mimics, inhibitor and control miRNA were purchased from GenePharma,
Co., Ltd., Shanghai, China. Cells were transfected with let 7a
inhibitor, let 7a mimics or a negative control miRNA according to
the manufacturer’s instructions. In brief, 2.5 μl
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA) was mixed with 50 μl serum-free medium in a
sterile tube. Following incubation for 15 min, 15 pmol mimic,
inhibitor or control miRNA was added to the mixture for a further
15 min. The mixture was subsequently added to the 24-well plates
and total RNA was extracted on the third day following
transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cholesteatoma tissues from each surgical specimen
(~30 mg) were homogenized using a syringe and needle.
High-molecular-weight DNA may be sheared by passing the lysate
through a 20-gauge needle, attached to a sterile plastic syringe,
at ≥5–10 times until a homogeneous lysate was achieved. Increasing
the volume of lysis buffer may be required to facilitate handling
and minimize sample loss. Cells were lysed using TRIzol®
reagent (Invitrogen Life Technologies), according to the
manufacturer’s instructions. Total miRNAs were extracted with the
miRNeasy kit (GenePharma, Co., Ltd., Shanghai, China). qPCR was
performed using a 7500 Fast Real-time PCR system (Applied
Biosystems Life Technologies, Foster City, CA, USA). Briefly, 1 μg
total RNA was reverse-transcribed into cDNA using a kit purchased
from GenePharma Co., Inc., and amplified by qPCR using
gene-specific primers. The sequences of the primers used were as
follows: Forward: 5′-ACGTTGTGTAGCTTATCAGACTG-3′ and reverse:
5′-AATGGTTGTTCTCCACACTCTC-3′ for miR-21; forward:
5′-CGATTCAGTGAGGTAGTAGGTTGT-3′ and reverse:
5′-TATGGTTGTTCTGCTCTCTGTCTC-3′ for let-7a; and forward:
5′-ATTGGAACGATACAGAGAAGATT-3′ and reverse:
5′-GGAACGCTTCACGAATTTG-3′ for U6. The PCR conditions were as
follows: 95°C for 3 minutes, followed by 40 cycles of 95°C for 12
sec and 62°C for 40–60 seconds. For all of the reactions,
no-template controls and random RNA preparations were also
subjected to reverse transcription, in order to verify the absence
of genomic DNA amplification. The relative miRNA expression levels
were calculated using the 2−ΔΔCT method.
Cell cycle analysis
For cell cycle analysis, the control and transfected
cells were washed with phosphate-buffered saline (PBS; Gibco-BRL),
fixed with 90% ethanol overnight at 4°C and incubated with RNase
(Sigma-Aldrich) at 37°C for 30 min. The nuclei of the cells were
stained with propidium iodide (PI) for an additional 30 min. A
total of 104 nuclei were examined in a FACSCalibur™ flow
cytometer (BD Biosciences, San Jose, CA, USA). The experiments were
performed in triplicate. The results were presented as a percentage
of the cells in a particular phase.
5-Ethynyl-2′-deoxyuridine (EdU)
incorporation assay
A total of 10 μM EdU (Cat. no. C10310; Ribobio, Co.,
Ltd., Guangzhou, China) was added to the cultured cells for 24 h.
The cells were then washed with PBS and fixed with 4%
paraformaldehyde (Solarbio, Beijing, China). The cells were
incubated with 2 mg/ml glycine and washed with PBS twice. Following
permeabilization with PBS containing 0.5% Triton X-100
(Sigma-Aldrich) and extensive washing with PBS, the cells were
incubated with Apollo® staining solution (Guangzhou Ribo
Bio, Guangzhou, China) for 30 min. The cells were washed a further
three times with PBS containing 0.5% Triton X-100, and then
incubated with Hoechst 33342 (Sigma-Aldrich) for 10 min. The images
of the staining were captured using an Olympus BX43 fluorescent
microscope (Olympus Corp., Tokyo, Japan).
Annexin V-fluorescein isothiocyanate
(FITC) apoptosis assay
To quantify let-7a miRNA-induced apoptosis, Annexin
V/propidium iodide and phycoerythrin (PIPE) staining was performed.
The apoptotic rate of the cells was evaluated by flow cytometric
analysis. Briefly, the cells were treated with the let-7a
inhibitor, let-7a mimics or the negative control miRNA. The treated
cells were collected and subjected to Annexin V/PI staining using
an Annexin V-FITC Apoptosis Detection kit (BioVision, Inc.,
Milpitas, CA, USA), according to the manufacturer’s instructions.
The fluorescence was measured by fluorescence-activated cell
sorting (FACSCalibur; BD Biosciences).
Terminal deoxynucleotidyl
transferase-mediated dUTP-digoxigenin nick end-labeling (TUNEL)
staining
The apoptosis of the cholesteatoma keratinocytes
transfected with the let-7a inhibitor, let-7a mimics or negative
control miRNA was detected using a TUNEL kit (Roche, Shanghai,
China). Briefly, the cells were incubated with terminal
deoxynucleotidyl transferase enzyme solution for 60 min, washed
twice with PBS and incubated for 30 min with
4′,6-diamidino-2-phenylindole (Yeasen, Shanghai, China). The images
of the staining were captured using a fluorescent microscope.
Cell invasion assay
The invasive abilities of the cells were examined
using 6-well Transwell plates (Corning, Inc., New York, NY, USA).
The cells transfected with let-7a inhibitor, let-7a mimics or
negative control miRNA were removed from the culture flasks and
resuspended in serum-free medium (5×105 cells/ml). A
total of 200 μl of each cell suspension was added to the upper
chambers of Transwell plates. The chambers were incubated for 48 h
at 37°C. The filters were then stained with
hexamethylpararosaniline staining solution (Yeasen). The upper
surfaces of the filters were scraped twice with cotton swabs to
remove non-migrated cells. The experiment was repeated three times,
and the migrated cells were counted in five different fields per
filter.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software, version 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical significance was determined by two-tailed Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference. All experiments were repeated ≥3 times and
the data are expressed as the mean ± standard deviation.
Results
Let-7a miRNA suppresses growth by
inhibiting cell proliferation of cholesteatoma keratinocytes
To investigate the function of let-7a during the
development of cholesteatoma keratinocytes, the cell number of
miRNA-transfected cholesteatoma keratinocytes was compared
(Fig. 1). The cell number was
reduced when the cells were transfected with mimics of let-7a
(Fig. 1A). Whereas, the cell
number was increased when the cells were transfected with the
inhibitor of let-7a (Fig. 1A).
These data suggest that let-7a may restrict the growth of
cholesteatoma keratinocytes. An EdU incorporation assay was used to
determine whether let-7a impacts the proliferation of cholesteatoma
keratinocytes. The number of proliferative EdU-positive
(EdU+) cells increased following transfection with the
let-7a inhibitor, as compared with the miRNA control (Fig. 1Ba and Bg). Whereas, the number of
EdU+ cells was decreased following transfection with
let-7a mimics (Fig. 1Bd and Bg).
The number of EdU+ cells was significantly increased
following transfection with the let-7a inhibitor, and significantly
decreased following transfection with let-7a mimics (Fig. 1C).
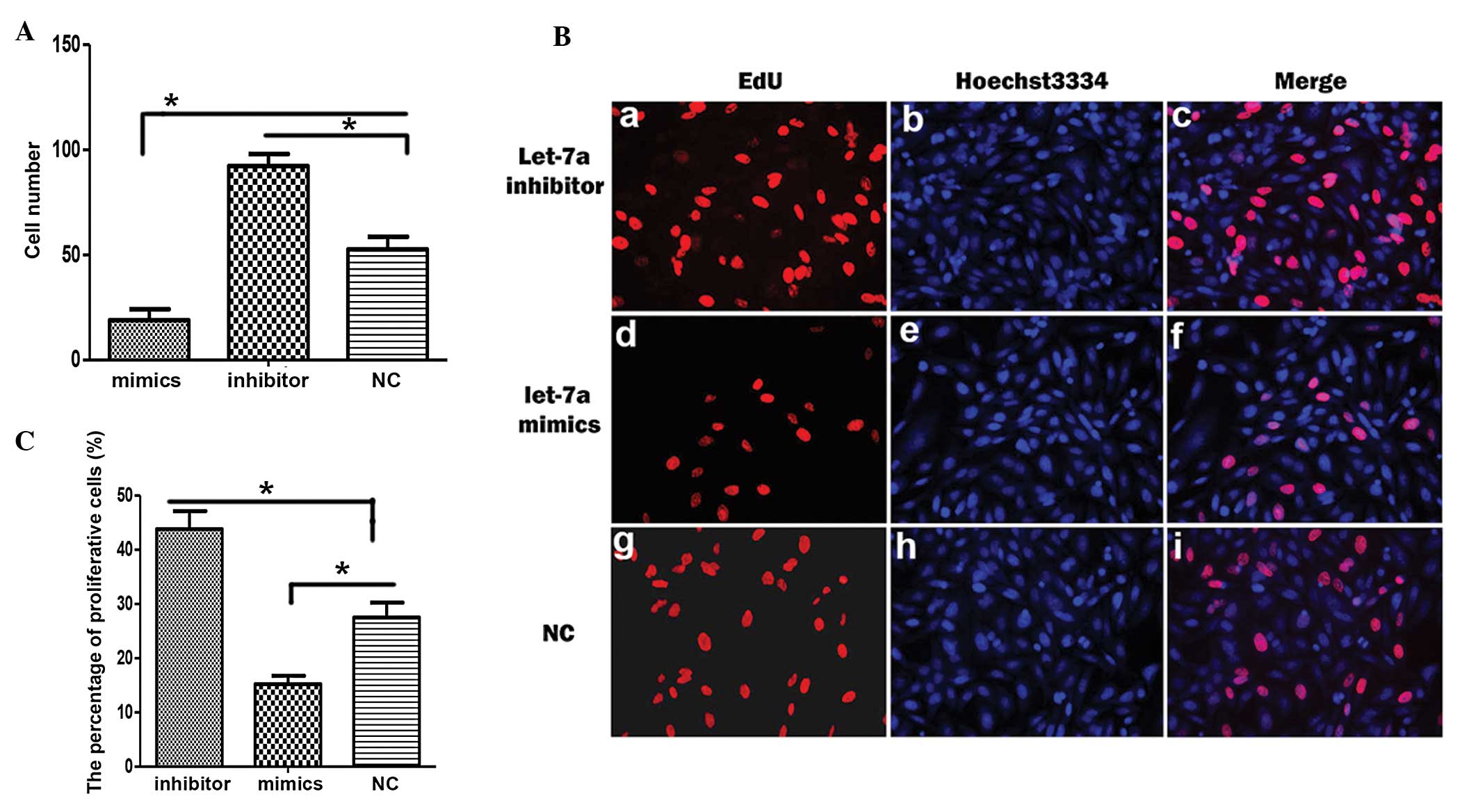 | Figure 1Let-7a inhibits proliferation of
cholesteatoma keratinocytes. (A) The number of cholesteatoma
keratinocytes was counted following transfection with let-7a
mimics, let-7a inhibitor or control miRNA (NC). (B) The
representative images of 5-ethynyl-2′-deoxyuridine (EdU)
immunostaining (red; a, d, g), Hoechst 3334 staining (blue; b, e,
h) and their merged images (c, f, i) in cholesteatoma keratinocytes
transfected with a let-7a inhibitor, let-7a mimics or NC. (C)
Statistical analysis of the percentage of proliferative cells
following transfection with a let-7a inhibitor, let-7a mimics or
NC. Data are represented as the mean ± standard deviation.
*P<0.05, Student’s t-test. |
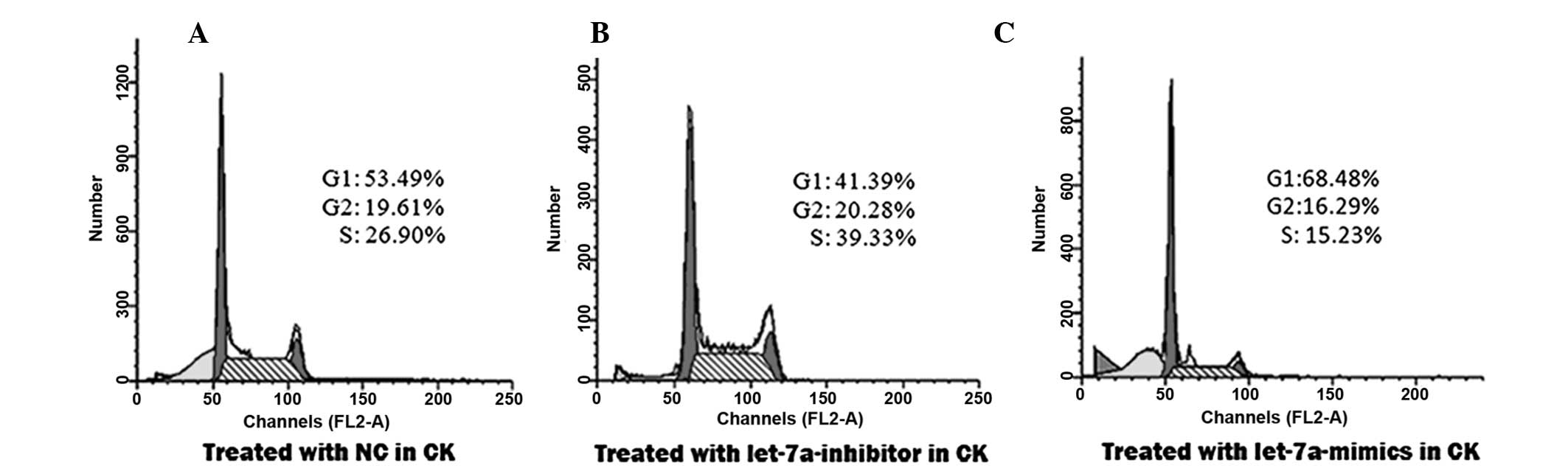
To confirm that let-7a inhibited cellular
proliferation by regulating the cell cycle progression of the
cholesteatoma keratinocytes, cell cycle analysis was performed. The
distribution of the cells in the different phases of the cell cycle
was examined by FACS, 48 h post-transfection with the various
miRNAs. The percentage of cells in the S phase increased from
26.90% in the control group (Fig.
2A), to 39.33% in the group transfected with the let-7a
inhibitor (Fig. 2B). This
percentage was reduced to 15.23% in the group transfected with
let-7a mimics (Fig. 2C).
Concordantly, the percentage of cells in the G2/M phase increased
from 19.61% in the control group (Fig.
2A), to 20.28% in the group transfected with the let-7a
inhibitor (Fig. 2B). The
percentage of cells in the G2/M phase was reduced to 16.29% in the
group transfected with let-7a mimics (Fig. 2C), as compared with the control
group. Conversely, the percentage of cells in the G0/G1 phase was
reduced from 53.49% in the control group (Fig. 2A), to 41.39% following transfection
with the let-7a inhibitor (Fig.
2B). The percentage of cells in the G0/G1 phase was increased
to 68.48% following transfection with the let-7a mimics (Fig. 2C), as compared with the control.
These results suggest that let-7a promotes the arrest of
cholesteatoma keratinocytes at the G0/G1 phase.
These data demonstrate that let-7a suppresses
proliferation of keratinocytes, by promoting cell cycle arrest in
the G0/G1 phase.
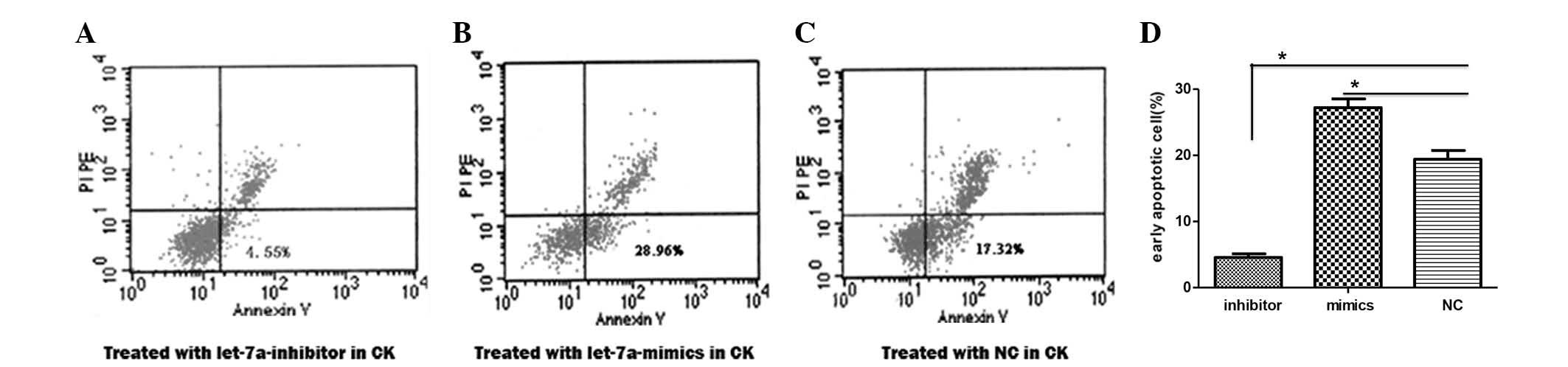
Let-7a miRNA induces cell apoptosis of
cholesteatoma keratinocytes
To explore whether let-7a impacts cell growth by
regulating apoptosis, early apoptosis was examined in the
cholesteatoma keratinocytes using an Annexin V-FITC Apoptosis
Detection kit. The percentage of cells displaying the features of
early apoptosis was elevated from 17.32% in the control cells
(Fig. 3A), to 28.96% in the cells
transfected with let-7a mimics (Fig.
3B). Conversely, this number was reduced to 4.55% in the cells
transfected with the let-7a inhibitor (Fig. 3C). The percentage of early
apoptotic cells was significantly decreased in the cells
transfected with the let-7a inhibitor, as compared with the cells
transfected with the control miRNA (Fig. 3D). Whereas, the percentage was
significantly increased in the cells transfected with let-7a mimics
(Fig. 3D).
To confirm the effects of let-7a on cell apoptosis,
the apoptotic rate of the cholesteatoma keratinocytes transfected
with the different miRNAs were examined using TUNEL. The number of
TUNEL-positive cells was decreased in the cholesteatoma
keratinocytes transfected with the let-7a inhibitor and increased
in the keratinocytes transfected with let-7a mimics (Fig. 4A). The percentage of apoptotic
cells following transfection with the let-7a inhibitor or let-7a
mimics was significantly decreased and increased, respectively
(Fig. 4B). These results suggest
that let-7a induces cell apoptosis of cholesteatoma
keratinocytes.
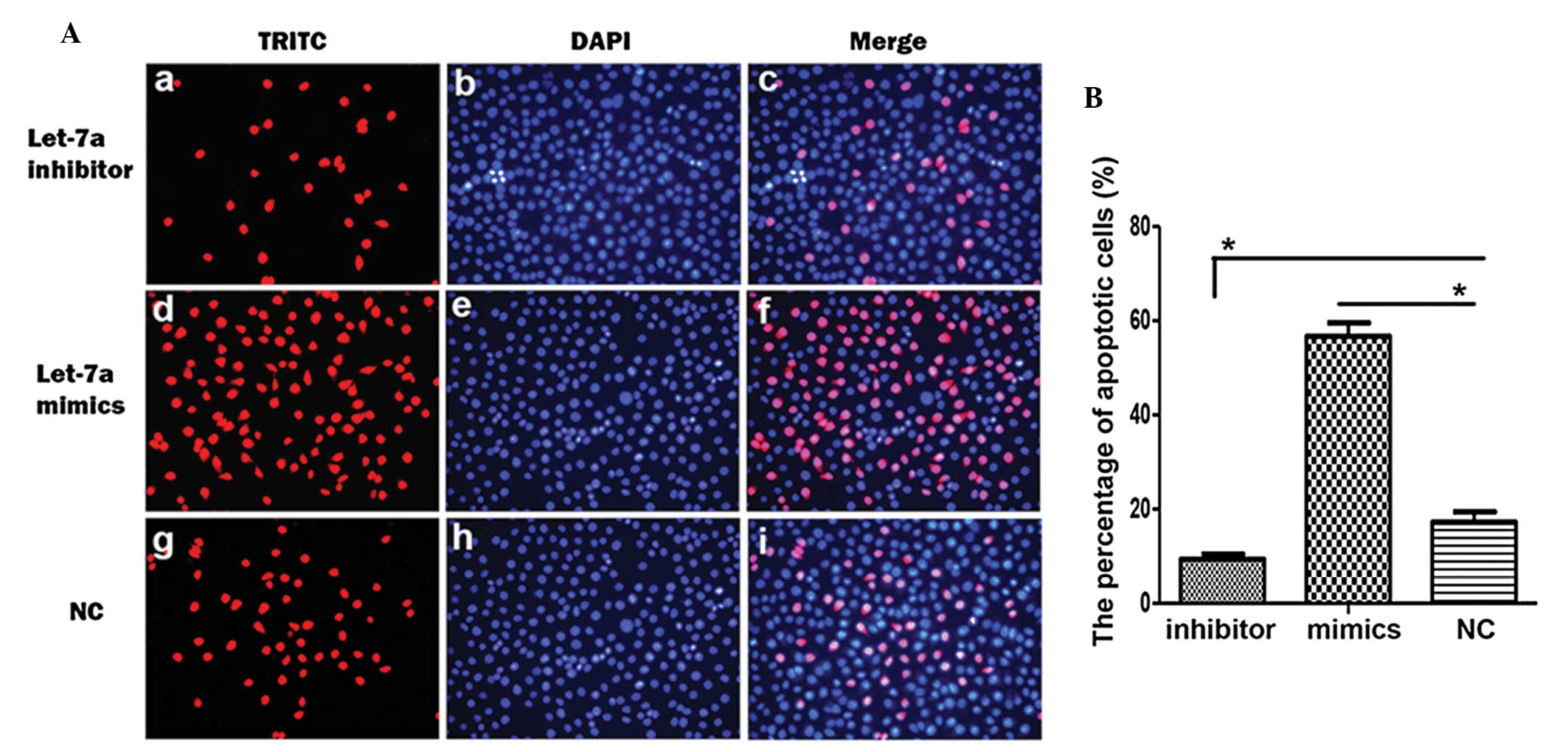 | Figure 4Let-7a promotes cell apoptosis of
cholesteatoma keratinocytes. (A) The representative images of
terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin
nick end-labeling (TUNEL) label (red; a, d, g),
4′,6-diamidino-2-phenylindole (DAPI) staining (blue b, e, h) and
their merged images (c, f, i) in cholesteatoma keratinocytes
transfected with let-7a inhibitor, let-7a mimics or control miRNA
(NC). (B) Statistical analysis of the percentage of apoptotic cells
following transfection of the choleasteatoma keratinocytes with
let-7a inhibitor, let-7a mimics or NC. Data are represented as the
mean ± standard deviation. *P<0.05, Student’s
t-test. |
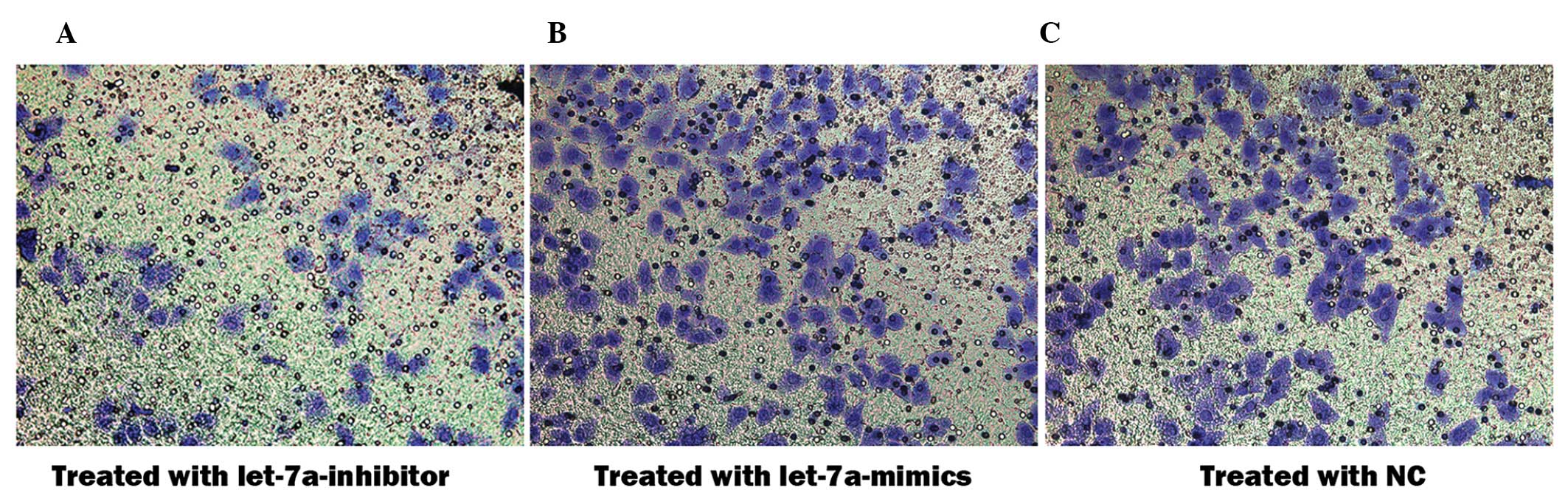
Let-7a miRNA inhibits cell invasion of
cholesteatoma keratinocytes
An aim of the present study was to determine whether
let-7a affects cell invasion of cholesteatoma keratinocytes.
Therefore, Matrigel™ invasion assays were performed using 8.0 μm
pore-size Transwell plates, which allow cell migration across the
filter. The number of cholesteatoma keratinocytes that migrated
across the Matrigel™ and the insert was significantly altered
following transfection with the various miRNAs (Fig. 5A–C). The percentage of migrated
cells transfected with the let-7a inhibitor was 1.75 times higher,
as compared with the cells transfected with the negative control
miRNA (92.3±5.7 vs 52.7±6.0; P<0.05) and 4.87 times higher as
compared with those transfected with let-7a mimics (92.3±5.7 vs
18.9±5.3; P<0.05). These results indicate that let-7a prevents
invasion and migration of cholesteatoma keratinocytes.
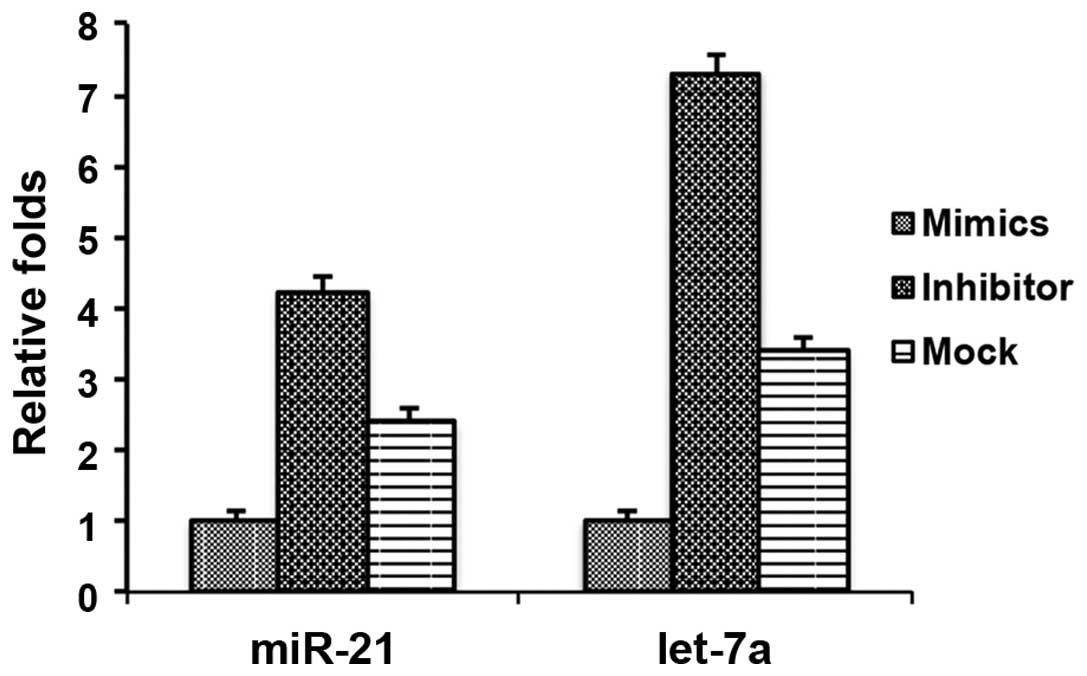
Let-7a miRNA affects the expression of
miR-21 in cholesteatoma keratinocytes
Considering the important roles of let-7a in the
pathogenesis of cholesteatomas, it may be beneficial to identify
the downstream targets regulated by let-7a. Previous studies
demonstrated that high expression levels of miR-21 in cholesteatoma
tissues regulated cell proliferation and apoptosis (10,11).
Therefore it may be hypothesized that let-7a regulates the
expression of miR-21. To determine a potential role for let-7a in
the regulation of miR-21, the effects of the let-7a inhibitor and
mimics were assessed on miR-21 expression in cholesteatoma
keratinocytes. There was a downregulation in the expression levels
of miR-21 in the cholesteatoma keratinocytes transfected with the
let-7a mimics and an upregulation of miR-21 in the cholesteatoma
keratinocytes transfected with the let-7a inhibitor (Fig. 6). As a control, the expression of
let-7a was determined and shown to be downregulated and upregulated
following transfection with let-7a mimics and inhibitor,
respectively (Fig. 6). These data
suggest that let-7a may regulate miR-21, resulting in the effects
on cell proliferation and cell apoptosis.
Discussion
Previous studies of cholesteatoma growth and
proliferation have mainly focused on growth factors and cytokines
(2). Growth factors and their
receptors, such as EGF/EGFR and KGF/KGFR, are highly upregulated
and associated with cell proliferation in cholesteatomas (3–9). In
addition, cytokines including interleukin (IL)-1, IL-6 and tumor
necrosis factor-α have been shown to be overexpressed and have
important roles in the proliferation of cholesteatoma keratinocytes
(14–18). However, the roles of miRNAs, which
are important regulators of protein translation, have yet to be
explored. In previous studies, miRNAs let-7a and miR-21 have been
implicated in regulating the proliferation and apoptosis of
cholesteatomas (10,11); however, there is currently no
direct evidence supporting this. Let-7 is a member of the
tumor-suppressing miRNA family and its expression is limited in the
majority of human malignancies (19). High expression levels of let-7 have
been shown to have antiproliferative effects on cancer cells
(20). In head and neck squamous
cell carcinoma, it has been reported that low expression levels of
let-7d are a prognostic factor for poor survival (21). In numerous tumors of solid organs,
let-7a is downregulated (22,23),
and acts as a tumor suppressor by targeting oncogenes, including
RAS and HMGA2 (24). The present
study was the first, to the best of our knowledge, to provide solid
evidence to support the roles of let-7a against the growth of
cholesteatoma keratinocytes. Let-7a inhibited the proliferation of
cholesteatoma keratinocytes by promoting cell cycle arrest in the
G0/G1 phase. Furthermore, let-7a induced early and late apoptosis
in cholesteatoma keratinocytes. Through its dual roles in
inhibiting proliferation and inducing apoptosis, let-7a was shown
to be capable of suppressing the growth of cholesteatoma
keratinocytes. These findings are concordant with the previous
identification of let-7a as a tumor suppressor in other cancer
cells (20,24). Notably, the present study
identified a novel role of let-7a in preventing migration and
invasion of cholesteatoma keratinocytes. Therefore, it may be worth
examining whether let-7a has similar functions in the inhibition of
migration and invasion of cancer cells in other tumor tissues.
Considering the crucial roles of let-7a in the
pathogenesis of cholesteatoma, it would be beneficial to identify
the downstream targets of let-7a. miR-21, another miRNA, has
previously been shown to be aberrantly overexpressed in
cholesteatomas, and to be involved in proliferation, apoptosis and
cell growth (10,11). The present study showed that
overexpression of let-7a mimics inhibited the expression of miR-21.
Conversely, inhibition of let-7a promoted the expression of miR-21.
The downstream targets of miR-21, PTEN and PDCD4, have previously
been found to be decreased in cholesteatomas, as compared with
normal tissues (10,11). Since PTEN and PDCD4 have roles in
the inhibition of proliferation and induction of apoptosis in
cancer cells (25,26), it may be reasonable to speculate
that let-7a initially downregulates the expression of miR-21.
Downregulation of miR-21 may then induce the expression of PTEN and
PDCD4, resulting in the inhibition of proliferation and induction
of apoptosis in cholesteatomas. The present study revealed that
let-7a regulation of proliferation and apoptosis may be through
controlling the expression of miR-21; however, the downstream
effectors that mediate the role of let-7a on migration and invasion
remain unknown. This question may be addressed in a further
study.
In conclusion, the present study revealed the
essential roles of let-7a in inhibiting growth and invasion of
cholesteatoma keratinocytes. Furthermore, a potential mechanism was
identified, let-7a may regulate miR-21 to control proliferation and
apoptosis of cholesteatoma keratinocytes. These findings indicate
that let-7a is a pivotal regulator of the pathogenesis of
cholesteatomas.
Acknowledgements
The authors of the present study would like to thank
all other members of the laboratory for their technical support and
helpful discussion.
References
|
1
|
Louw L: Acquired cholesteatoma: summary of
the cascade of molecular events. J Laryngol Otol. 127:542–549.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Preciado DA: Biology of cholesteatoma:
special considerations in pediatric patients. Int J Pediatr
Otorhinolaryngol. 76:319–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Alves AL, Pereira CS, de Carvalho MF,
Fregnani JH and Ribeiro FQ: EGFR expression in acquired middle ear
cholesteatoma in children and adults. Eur J Pediatr. 171:307–310.
2012. View Article : Google Scholar
|
|
4
|
Barbara M, Raffa S, Murè C, et al:
Keratinocyte growth factor receptor (KGF-R) in cholesteatoma
tissue. Acta Otolaryngol. 128:360–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jin BJ, Min HJ, Jeong JH, Park CW and Lee
SH: Expression of EGFR and microvessel density in middle ear
cholesteatoma. Clin Exp Otorhinolaryngol. 4:67–71. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamamoto-Fukuda T, Takahashi H and Koji T:
Expression of keratinocyte growth factor (KGF) and its receptor in
a middle-ear cavity problem. Int J Pediatr Otorhinolaryngol.
76:76–81. 2012. View Article : Google Scholar
|
|
7
|
Kuczkowski J, Bakowska A, Pawelczyk T,
Narozny W and Mikaszewski B: Cell cycle inhibitory protein p27 in
human middle ear cholesteatoma. ORL J Otorhinolaryngol Relat Spec.
68:296–301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Ren H, Ren J, et al: The role of
EGFR/PI3K/Akt/cyclinD1 signaling pathway in acquired middle ear
cholesteatoma. Mediators Inflamm. 2013:6512072013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sakamoto T, Kondo K, Yamasoba T, et al:
Overexpression of ErbB-2 protein in human middle ear
cholesteatomas. Laryngoscope. 114:1988–1991. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen X and Qin Z: Post-transcriptional
regulation by microrna-21 and let-7a microRNA in paediatric
cholesteatoma. J Int Med Res. 39:2110–2118. 2011. View Article : Google Scholar
|
|
11
|
Friedland DR, Eernisse R, Erbe C, Gupta N
and Cioffi JA: Cholesteatoma growth and proliferation:
posttranscriptional regulation by microRNA-21. Otol Neurotol.
30:998–1005. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Djuranovic S, Nahvi A and Green R: A
parsimonious model for gene regulation by miRNAs. Science.
331:550–553. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Akimoto R, Pawankar R, Yagi T and Baba S:
Acquired and congenital cholesteatoma: determination of tumor
necrosis factor-alpha, intercellular adhesion molecule-1,
interleukin-1-alpha and lymphocyte functional antigen-1 in the
inflammatory process. ORL J Otorhinolaryngol Relat Spec.
62:257–265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bujía J, Kim C, Boyle D, et al:
Quantitative analysis of interleukin-1-alpha gene expression in
middle ear cholesteatoma. Laryngoscope. 106:217–220. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bujia J, Kim C, Ostos P, et al:
Interleukin 1 (IL-1) and IL-1-receptor antagonist (IL-1-RA) in
middle ear cholesteatoma: an analysis of protein production and
biological activity. Eur Arch Otorhinolaryngol. 253:252–255. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kato A, Ohashi Y, Masamoto T, et al:
Interleukin-6 and tumour necrosis factor alpha synthesized by
cholesteatoma cells affect mucociliary function in the eustachian
tube. Acta Otolaryngol. 38(Suppl 5): 90–97. 1998.
|
|
18
|
Mehta D, Daudia A, Birchall JP and
Banerjee AR: The localization of matrix metalloproteinases-8 and
-13 in cholesteatoma, deep-meatal and post-auricular skin: a
comparative analysis. Acta Otolaryngol. 127:138–142. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornton JE and Gregory RI: How does Lin28
let-7 control development and disease? Trends Cell Biol.
22:474–482. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong TS, Man OY, Tsang CM, et al: MicroRNA
let-7 suppresses nasopharyngeal carcinoma cells proliferation
through downregulating c-Myc expression. J Cancer Res Clin Oncol.
137:415–422. 2011. View Article : Google Scholar :
|
|
21
|
Childs G, Fazzari M, Kung G, et al:
Low-level expression of microRNAs let-7d and miR-205 are prognostic
markers of head and neck squamous cell carcinoma. Am J Pathol.
174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mayr C, Hemann MT and Bartel DP:
Disrupting the pairing between let-7 and Hmga2 enhances oncogenic
transformation. Science. 315:1576–1579. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Johnson SM, Grosshans H, Shingara J, et
al: RAS is regulated by the let-7 microRNA family. Cell.
120:635–647. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Park SM, Shell S, Radjabi AR, et al: Let-7
prevents early cancer progression by suppressing expression of the
embryonic gene HMGA2. Cell Cycle. 6:2585–2590. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Frankel LB, Christoffersen NR, Jacobsen A,
et al: Programmed cell death 4 (PDCD4) is an important functional
target of the microRNA miR-21 in breast cancer cells. J Biol Chem.
283:1026–1033. 2008. View Article : Google Scholar
|
|
26
|
Zhao H, Dupont J, Yakar S, Karas M and
LeRoith D: PTEN inhibits cell proliferation and induces apoptosis
by downregulating cell surface IGF-IR expression in prostate cancer
cells. Oncogene. 23:786–794. 2004. View Article : Google Scholar : PubMed/NCBI
|