Introduction
Mesenchymal stem cells (MSCs) derived from the human
umbilical cord (HUC), known as HUC-MSCs, represent a prospective
cell source and hold great promise for tissue engineering and
therapy (1,2). Compared with bone marrow MSCs they
exhibit considerable advantages including the lack of ethical
controversy, accessibility, extraction procedures that are painless
for donors, a reduced risk of contamination, osteogenic
differentiation capacities, ex vivo expansion, faster
proliferation and a higher immunomodulatory capacity (2). Furthermore, there are few ethical
restrictions or medico-legal limitations on extracting and applying
these cells (2,3). The ample resources of the cords and
the feasible cryopreservation of HUC-MSCs allow these cells to be
preserved for engineering applications in the future (3). Animal experiments have demonstrated
that HUC-MSCs may be useful in the treatment of neuron disease and
multiple selerosis (3–6). However, the safety and efficacy of
stem cell therapy depends on the mode of cell administration.
Furthermore, techniques aimed at increasing the number of HUC-MSCs
isolated from umbilical cords (UCs) are extremely valuable.
Previous studies have indicated that the intrathecal
treatment of UC-MSCs is safe, less invasive and a more convenient
procedure involving no surgery (7–10).
Currently, the efficiency of UC-MSC transplantation is limited to
the grafting method (7–10). However, the grafting process may
decrease the viability of the UC-MSCs. Therefore, enhancing the
viability of UC-MSCs is the best way to improve the efficiency of
UC-MSC transplantation.
The present study speculated that inhibiting the
apoptosis of UC-MSCs may enhance the viability and survival of
UC-MSCs. The role of the mitogen-activated protein kinase pathway
in the pathogenesis of neurological disorders was also
investigated. A prospective analysis was performed to assess the
safety, therapeutic effect and the technical difficulties of
HUC-MSCs intrathecal infusion in patients.
Patients and methods
Characteristics of participants
In total, 100 patients with neurological disorders
were recruited between December 2006 and May 2010 from The Second
Affiliated Hospital, School of Medicine, Zhejiang University
(Hangzhou, Zhejiang, China). A total of 53 males and 47 females
were enrolled in the present study with a male to female ratio of
1.12:1. The age at diagnosis ranged between 2 and 68 years (median
40 years). In terms of diagnosis, 30 patients had spinal cord
injury, 15 patients had cerebral palsy, 15 patients had
post-traumatic brain syndrome, 8 patients had post-brain infarction
syndrome, 8 patients had spinocerebellar ataxias and 12 patients
had motor neuron disease (Table
I). The local institutional review board of The Second
Affiliated Hospital of Medicine, under the auspices of the National
Ministry of Health, approved application of the technique and
written informed consent was obtained from each patient prior to
initiation of the treatment. Patients were excluded from the
present study if they met the following criteria: i) prior history
of severe allergic reactions; ii) history of, or active,
malignancy; iii) active systemic or severe focal infections,
including HIV and syphilis; iv) active cardiac, pulmonary, renal,
hepatic or gastrointestinal disease; v) coagulopathy or any other
contraindication for lumbar puncture; vi) gastrostomy, tracheostomy
or noninvasive ventilatory support as these can effect the
prognosis and end-point measurements; vii) any severe psychiatric
disorder and viii) any immunodeficiency disease or condition. As
per protocol, the pre- and post-treatment study assessments
included complete blood counts, routine urine tests, analysis of
liver function, renal function, electrolytes, sero-enzymology,
blood glucose, blood lipids, cellular and humoral immunity, routine
cerebrospinal fluid (CSF) and biochemical markers (biochemistry
analyzer and Epics-XL flow cytometer; Beckman Coulter, Inc.
(Pasadena, CA, USA).
 | Table IClinical conditions of the
investigated patients. |
Table I
Clinical conditions of the
investigated patients.
| Condition | Number of
patients |
|---|
| Number of
patients | 100 |
| Spinal cord
injury | 35 |
| Cerebral palsy | 20 |
| Post-traumatic brain
syndrome | 20 |
| Post-brain
infarction syndrome | 9 |
| Spinocerebellar
ataxias | 8 |
| Motor neuron
disease | 8 |
| Patients with
technical difficulties | 31 (31.0%) |
| General anesthesia
supplementation | 18 |
| Taylor’s
approach | 7 |
| Multiple
attempts | 6 |
| Side effects | 22 (22.0%) |
| Headache | 13 |
| Low-grade fever | 5 |
| Low back pain | 2 |
| Lower limb pain | 2 |
| Improvement in
functional indices | 47 (47.0%) |
| Spinal cord
injury | 16 |
| Cerebral palsy | 12 |
| Post-traumatic brain
syndrome | 10 |
| Post-brain
infarction syndrome | 6 |
| Spinocerebellar
ataxias | 2 |
| Motor neuron
disease | 1 |
Characteristics of HUC-MSCs
The isolation, culture and expansion of the HUC-MSCs
were performed as previously described by Gu et al (10). In brief, human umbilical cord (HUC)
was obtained from the Gynecology Department at Renmin Hospital,
Hubei University of Medicine (Shiyan, Hubei, China). Tissue
collection was approved by the Ethics Committee of Renmin Hospital
and informed consent was obtained from the newborns’ parents. The
selected tissue was sliced into 1–2 mm3 pieces and then
incubated with 0.075% collagenase type II (Sigma, St. Louis, MO,
USA) for 30 min and with 0.125% trypsin (Gibco-BRL, Grand Island,
NY, USA) for 30 min. To obtain the cell suspensions, the treated
tissue was passed through a 100 mm filter. The low glucose
Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL) and 5% fetal
bovine serum (FBS; HyClone, Logan, UT, USA), supplemented with 10
ng/ml epidermal growth factor (Sigma), 100 mg/ml streptomycin
(Sigma), 10 ng/ml vascular endothelial growth factor (Sigma), 100
U/ml penicillin and 2 mmol/l glutamine (Gibco-BRL) were employed in
the present study. For the cultural condition, the cells were
cultured in an appropriate atmosphere at 37°C with 5%
CO2. Once the cell monolayer was formed, flow cytometric
analysis was used for the detection of CD29, CD105, CD44 and CD166
positive cells and for CD34, CD14, CD45, CD38 and HLA-DR negative
cells.
Cell administration
Patients’ computed tomography scans and/or magnetic
resonance images of the spine and brain were reviewed prior to the
procedure, and the intervertebral space between lumbar vertebrae
three and four was selected for HUC-MSCs placement. HUC-MSCs were
administered via intrathecal injection by lumbar puncture. Each
patient received cell transplantation four to six times depending
on the patient’s condition, within an interval of 5–7 days. CSF (2
ml) was removed and replaced by 2 ml of cell suspension during the
intrathecal injection. The needle was maintained in the same
position for 5 min prior to withdrawal. Pediatric and uncooperative
patients were administered general anesthesia prior to performing
lumbar puncture. The present study evaluated the number of
attempts, localization of subarachnoid space and postprocedural
complications. All patients were monitored in the wards for 24 h
and hydrated with 3 liters of fluid; ambulation was allowed 8 h
postprocedure. Short and long term functional evaluation was
performed using the Hauser Ambulation Index (HAI) by the HUC-MSCs
transplant team on a regular basis. The HUC-MSCs transplant process
was performed according to the study by Dalous et al
(12).
Therapeutic effect
To the best of our knowledge there are no published
criteria to measure therapeutic efficacy in the treatment of
HUC-MSCs for neurological disorders. The HAI was applied for the
evaluation of treatment efficacy (11). The HAI is a rating scale developed
by Hauser et al (11) to
assess mobility by evaluating the time and degree of assistance
required to walk 25 feet. The scores ranged between 0 (asymptomatic
and fully active) and 10 (bedridden) as follows: 0 = asymptomatic,
fully active; 1 = walks normally, however, reports fatigue that
interferes with athletic or other demanding activities; 2 =
abnormal gait or episodic imbalance, gait disorder is noticed by
family and friends, able to walk 25 feet (8 meters) in ≤10 sec; 3 =
walks independently, able to walk 25 feet in ≤20 sec; 4 = requires
unilateral support (cane or single crutch) to walk, walks 25 feet
in ≤20 sec; 5 = requires bilateral support (canes, crutches or
walker) and walks 25 feet in ≤25 sec or requires unilateral support
but requires >20 sec to walk 25 feet; 6 = requires bilateral
support and >20 sec to walk 25 feet, may use wheelchair on
occasion; 7 = walking limited to several steps with bilateral
support, unable to walk 25 feet, may use wheelchair for the
majority of activities; 8 = restricted to wheelchair, able to
transfer self independently; 9 = restricted to wheelchair, unable
to transfer self independently; 10 = bedridden.
Results
Autonomic improvement of spinal cord
injury
Administration of HUC-MSCs via intrathecal routes
was well tolerated during the clinical treatment course. Out of 88
patients, technical difficulties were encountered in 20 patients
(23%), 12 of which required general anesthesia supplementation,
three required Taylor’s approach and five required multiple
attempts for the localization of subarachnoid space. In total, 10
patients suffered from postprocedural headache, which was relieved
within 24 h with analgesics, hydration and rest; 3 patients had
low-grade fever lasting for 24 h; 3 patients had lower back pain
and 2 patients had lower limb pain, which was responded to within
24 h of symptomatic treatment. On long-term follow-up, functional
indices improved in 50 (31.67%) patients, including 15 patients
with spinal cord injury, 10 with cerebral palsy, 10 with
post-traumatic brain syndrome, 5 with post-brain infarction
syndrome, 5 with spinocerebellar ataxias and 5 with motor neuron
disease. Patients with cerebral palsy and post-traumatic brain
syndrome demonstrated improvement in muscle tone, rigidity and
spasm (Table I).
Out of the 30 spinal cord injury patients, 20 had a
previous history of spinal surgery. In 15 spinal cord injury
patients with HAI scale improvement, 10 patients had an injury
period of <1 year, whereas 5 had an injury period of >1 year.
A total of 15 patients showed improvement in motor power (10 with
an injury period of <1 year and 5 >1 year). Prior to
treatment, all these patients were HAI grade 9. Following HUC-MSC
transplantation, one was grade 4, two were grade 5, three were
grade 6 and four were grade 7. A marked improvement was observed in
four bedridden patients who were able to walk with the help of a
walker (HAI grade 9-HAI grade 4/5). In 12 patients, autonomic
improvement was observed, including 8 patients with an injury
period of <1 year. Of these, three became catheter-free and two
required intermittent catheterization. In addition, 3 patients
showed improvement in bowel sensations and sweating. A mixed motor
and autonomic improvement was observed in 8 of 30 patients
(Table II).
 | Table IISpinal cord injury clinical
profile. |
Table II
Spinal cord injury clinical
profile.
| Condition | Number |
|---|
| Number of
patients | 38 |
| Pattern of
injury | 12 |
| Complete
transection | 22% |
| Nontransection | 78% |
| Injury to treatment
duration | 4 months to 2
years |
| Clinical and
functional benefit |
| <1 year | 13 (35.3%) |
| >1 year | 6 (18.7%) |
| Pattern of motor
improvement according to HAIa |
| <1 year | 13 |
| Posttransplant
grade | |
| 4 | 2 |
| 5 | 3 |
| 6 | 2 |
| 7 | 3 |
| >1 year | 5 |
| Posttransplant
grade |
| 6 | 3 |
| 7 | 1 |
| 8 | 1 |
| Autonomic
improvement | 12 |
| <1 year | 8 |
| Catheter free | 2 |
| Intermittent
catheterization | 3 |
| Bowel sensations
and sweating | 2 |
| >1 year | 4 |
| Intermittent
catheterization | 3 |
| Improved bladder
tone | 1 |
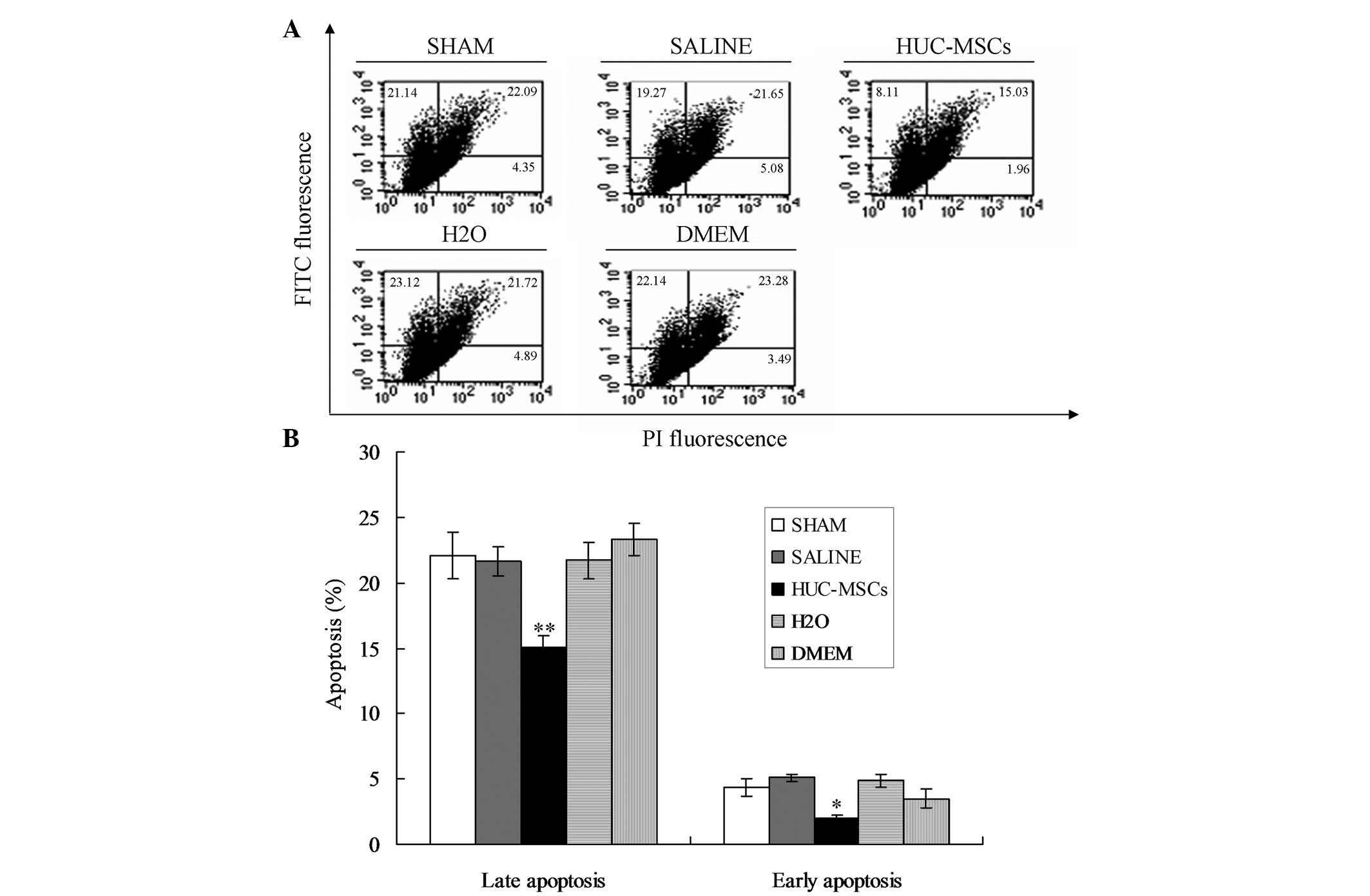
HUC-MSC transplantation inhibits
apoptosis
In order to investigate the therapeutic effect of
HUC-MSCs on neurological disorders, HUC-MSCs were transplanted into
mice. The results indicated that HUC-MSCs were able to
significantly inhibit apoptosis of neurocytes compared with the
other groups (Fig. 1;
P<0.01).
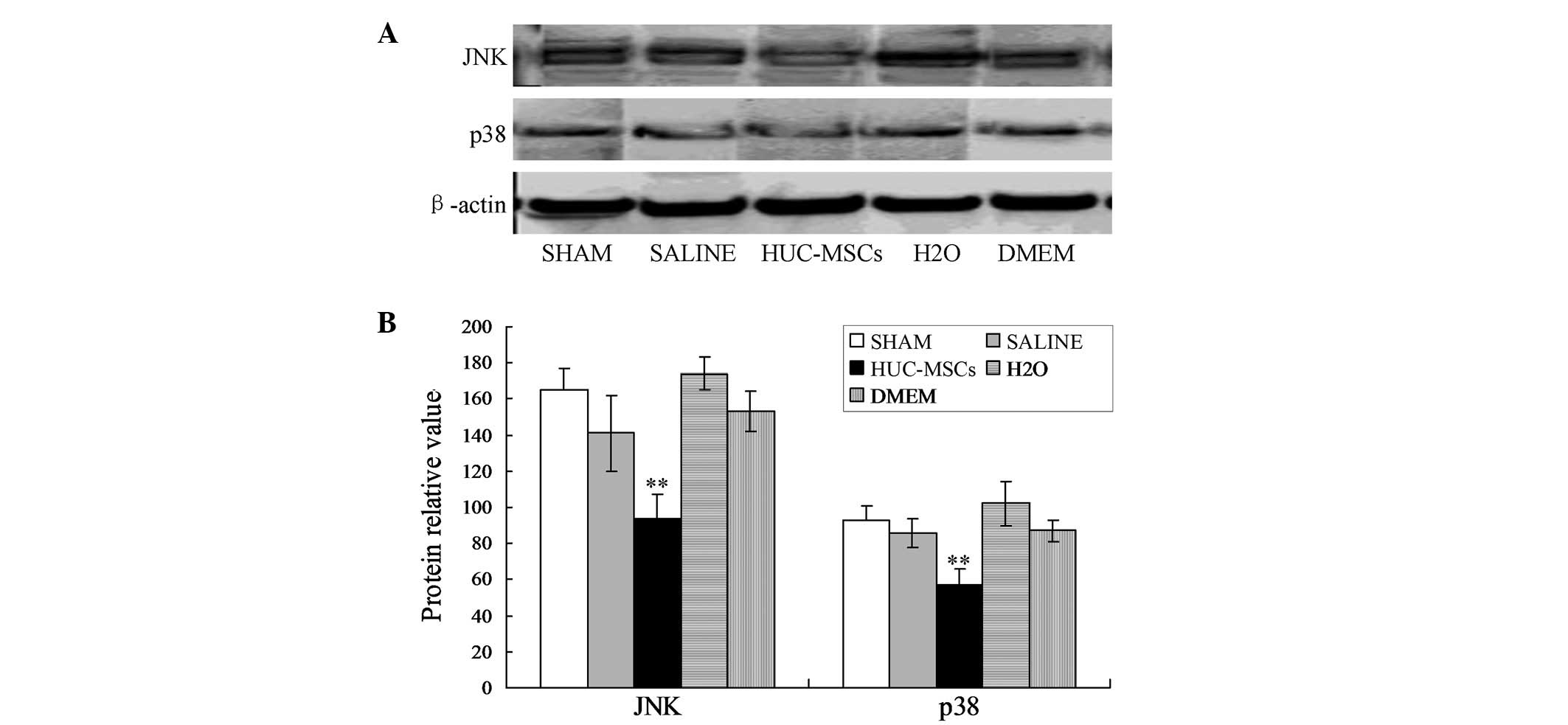
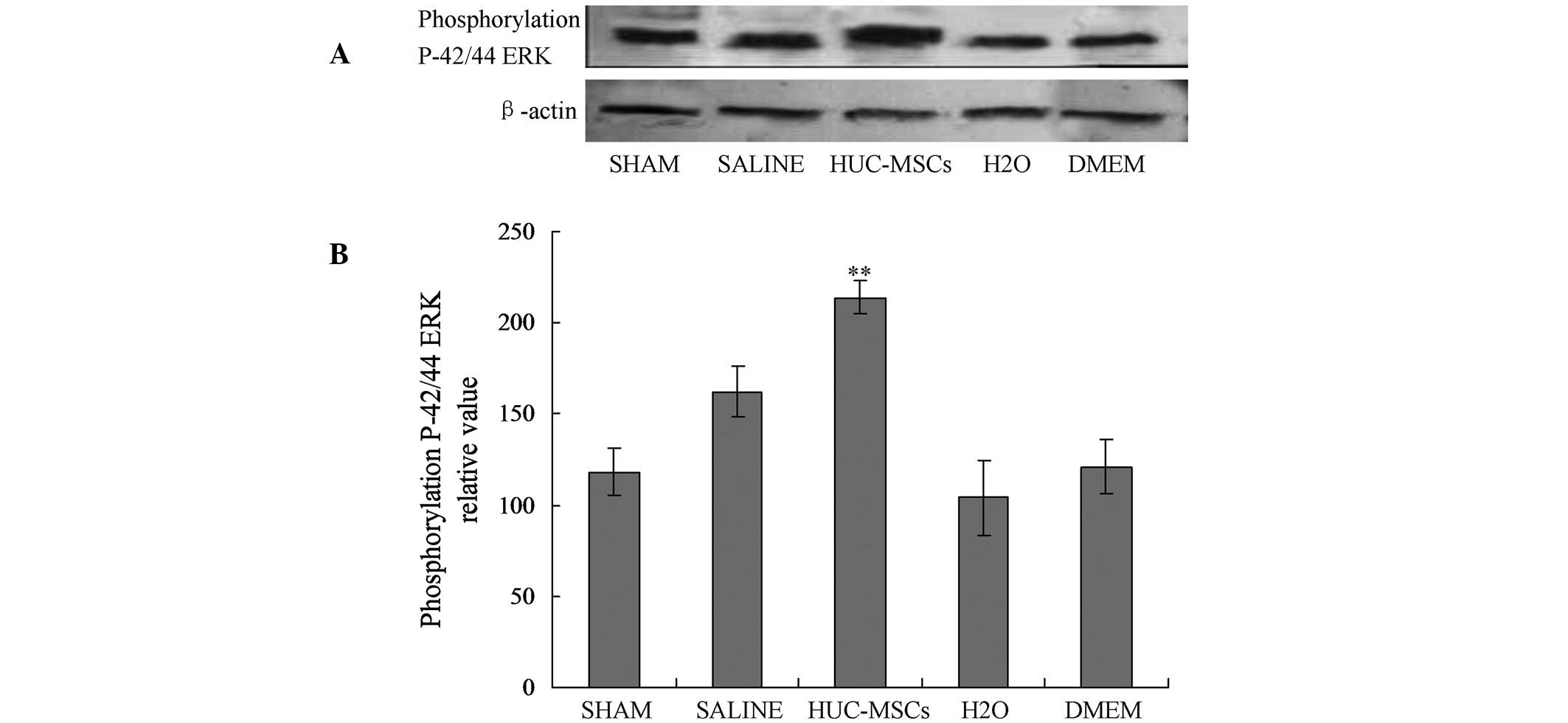
HUC-MSCs inhibit apoptosis via the
mitogen-activated protein kinase (MAPK) and
extracellular-signal-regulated kinase (ERK)1/2 pathways
The activation of the other two members of MAPKs,
c-Jun N-terminal kinase and p38, decreased the rate of apoptosis
following treatment with HUC-MSCs (Fig. 2). On the basis of these
observations, the effect of normothermic ischemia-reperfusion
stress on the different members of the MAPK family were
investigated. The expression of phospho-p 44/42 ERK1/2 was
significantly higher in the liver of animals treated with HUC-MSCs
(Fig. 3).
Discussion
Mesenchymal stem cells (MSCs) are highly
proliferative in vitro, have multilineage differentiation
potential and are able to be obtained from various tissue sources,
including the bone marrow (BM), adipose tissue and umbilical cord
blood. The BM is the main source for isolation of MSCs and
BM-derived MSCs (BM-MSCs) have been studied extensively. However,
BM harvesting is a highly invasive method for the donors.
Furthermore, the multipotent differentiation potential, maximal
lifespan of BM-MSCs and proliferation efficiency are correlated
with the age of the patients. In addition, MSCs can be isolated
from umbilical cord Wharton’s jelly and differentiate into
different types of cells, including adipocytes, osteocytes,
chondrocytes and neurons. Compared with the MSCs isolated from
other tissues, UC-MSCs can differentiate into many types of cell,
including blood, nerve and bone cells. Furthermore, this type of
MSC does not express the major histocompatibility complex (MHC)
class II (HLA-DR) antigens (13),
which is expressed in other types of MSCs. Several studies have
indicated that UC-MSCs retain their viable status, and are not
rejected even four months following transplantation. Therefore,
immune suppression is unnecessary for UC-MSCs transplantation,
which suggests that UC-MSCs are a feasible and stable cell source
for transplantation. Notably, the umbilical cord can be easily
obtained and isolated. Thus, UC-MSCs transplantation may act as a
potential MSC cell source for tissue engineering.
Several studies have demonstrated that the
transplantation of UC-MSCs is a potential therapeutic strategy for
the treatment of central nervous system diseases, including
cerebrovascular disease, nerve degenerative diseases, spinal cord
injury and cerebral palsy. The transplantation path in cell therapy
is diverse (14–16) and mainly includes the intervention
path, local implantation, intravenous route and lumbar puncture
way. Migration toward pathology is the first critical step in
UC-MSCs engagement during regeneration and it is hypothesized that
the inflammatory response itself guides the behavior of potentially
reparative UC-MSCs. It has been revealed that introducing UC-MSCs
into the subarachnoid space of the spinal cord transports the cells
through CSF and allows more efficient delivery of cells to the
injured area of the central nervous system compared with the
intravenous route.
Intrathecal administration of UC-MSCs is safe, less
invasive and a more convenient procedure involving no surgery.
However, the efficacy of UC-MSCs transplantation is limited by the
grafting method. The mechanical process of grafting may decrease
the viability and survival of the transplanted MSCs. Therefore, for
the transplantation of MSCs it is necessary to improve the
viability and survival of UC-MSCs prior to transplantation
(14). The present study examined
the viability of MSCs using the trypan blue exclusion assay. The
patient should be positioned to ensure the intervertebral spaces
are detectable for lumbar puncture for transplantation of cells
into the CSF.
At present, the optimized method for injecting cells
is to slowly deliver a fixed volume at a constant rate. The
quantity and density of transplanted MSCs are critical to ensure an
adequate number of cells for grafting and optimal survival. For the
injection procedure, the needle tip should be left in the same
position after the cells have been injected.
Notably, UC-MSCs cell transplantation was able to
inhibit apoptosis (Fig. ). It is well established that cell
apoptosis can trigger neurological diseases and degenerative
diseases. In the present study, UC-MSCs improved the disease status
through inhibiting the apoptotic process of the cells.
In the present study, a significant number of
patients with spinal cord injury were included. A lumbar puncture
was performed in these patients, however, this was technically
difficult (17–20) due to the
following reasons: pathological scoliosis and positional
difficulties. Certain other factors, including cognitive,
behavioral and communication problems as well as coexisting
diseases and the specific drug therapy methods, affected the
anesthetic management. In addition, other problems affected the
transplantation of MSCs, including gastroesophageal reflux,
electrolyte imbalance and pulmonary aspiration in the present
study. Furthermore, the positioning of the patients for the
transplantation was difficult as analgesia and inadequate
anesthesia may lead to increased muscle tone and spasm. Thus,
judicious use of an anesthetic agent intraoperatively was critical
to ensure a relaxed peri-operative and post-operative period.
Following intraspinal injection of UC-MSCs in this patient
population, certain patients (n=13) suffered from postprocedural
headache. This was possibly due to numerous reasons, including the
alteration in CSF circulation, leakage of CSF and the use of a
large-bore spinal needle, which was essential to prevent any
mechanical damage to the cells during infusion. Furthermore, 3
patients suffered from lower back pain and 2 patients had lower
limb pain, possibly due to nerve root injuries; however, they
recovered quickly. All the side effects resolved within 24 h with
no long-term sequelae.
In conclusion, the present study indicated that
subarachnoid transplantation with UC-MSCs in neurological disorders
may be a promising therapy, which is relatively safe, simple to
perform and has no long-term adverse effects. However, certain
studies have provided different conclusions, and thus this method
requires further investigation so that the potential of this
therapy may be fully realized.
Acknowledgements
The authors would like to thank the Professional
associates for their assistance in obtaining patient data
throughout the present study. This study was supported by Zhejiang
Provincial Qianjiang talent plan (grant no. 2012R10041), China
National Funds for Young Scientists (grant no. 81100241), the
Zhejiang Provincial Natural Science Foundation (grant no.
Y2110033), The Ph.D. Programs Foundation of the Ministry of
Education of China (grant no. 20110101120119), the Science and
Technology Department of Zhejiang Province Public Technology
Research and the social development project (grant no.
2013C33131).
References
|
1
|
Wang L, et al: Protective effect of
transplanted bone marrow-derived mesenchymal stem cells on
pancreatitis-associated lung injury in rats. Mol Med Rep.
6:287–292. 2012.PubMed/NCBI
|
|
2
|
Trivanović D, Kocić J, Mojsilović S, et
al: Mesenchymal stem cells isolated from peripheral blood and
umbilical cord Wharton’s jelly. Srp Arh Celok Lek. 41:178–186.
2013. View Article : Google Scholar
|
|
3
|
Titomanlio L, et al: Stem cell therapy for
neonatal brain injury: perspectives and challenges. Ann Neurol.
70:698–712. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Han D, Wang Z, et al: Clinical
analysis of the treatment of spinal cord injury with umbilical cord
mesenchymal stem cells. Cytotherapy. 15:185–191. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Ji H, Zhou J, et al: Therapeutic
potential of umbilical cord mesenchymal stromal cells
transplantation for cerebral palsy: a case report. Case Rep
Transplant. 2013:1463472013.PubMed/NCBI
|
|
6
|
O’Hare E and Young PJ: Childhood spinal
muscular atrophy and stem cell research: Is cellular replacement
therapy the answer? (Review). Mol Med Rep. 2:3–5. 2009.
|
|
7
|
Lim JY, Jeong CH, et al: Therapeutic
effects of human umbilical cord blood-derived mesenchymal stem
cells after intrathecal administration by lumbar puncture in a rat
model of cerebral ischemia. Stem Cell Res Ther. 2:382011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kang EJ, Lee YH, Kim MJ, et al:
Transplantation of porcine umbilical cord matrix mesenchymal stem
cells in a mouse model of Parkinson’s disease. J Tissue Eng Regen
Med. 7:169–182. 2013. View
Article : Google Scholar
|
|
9
|
Zhang XJ, Luan ZG and Ma XC: shRNAs
targeting high-mobility group box-1 inhibit E-selectin expression
via homeobox A9 in human umbilical vein endothelial cells. Mol Med
Rep. 7:1251–1256. 2013.PubMed/NCBI
|
|
10
|
Gu Z, Akiyama K, Ma X, et al:
Transplantation of umbilical cord mesenchymal stem cells alleviates
lupus nephritis in MRL/lpr mice. Lupus. 19:1502–1514. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hauser SL, Dawson DM, et al: Intensive
immunosuppression in progressive multiple sclerosis. A randomized,
three-arm study of high-dose intravenous cyclophosphamide, plasma
exchange, and ACTH. N Engl J Med. 308:173–180. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalous J, Larghero J and Baud O:
Transplantation of umbilical cord-derived mesenchymal stem cells as
a novel strategy to protect the central nervous system: technical
aspects, preclinical studies, and clinical perpectives. Pediatr
Res. 71:482–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Glass JD, Boulis NM, et al: Lumbar
intraspinal injection of neural stem cells in patients with
amyotrophic lateral sclerosis: results of a phase I trial in 12
patients. Stem Cells. 30:1144–1151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang H, Yang Y, et al: Programming of
human umbilical cord mesenchymal stem cells in vitro to promote
pancreatic gene expression. Mol Med Rep. 8:769–774. 2013.PubMed/NCBI
|
|
15
|
Lepore AC: Intraspinal cell
transplantation for targeting cervical ventral horn in amyotrophic
lateral sclerosis and traumatic spinal cord injury. J Vis Exp. Sep
18–2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wiley CA and Achim C: Human
immunodeficiency virus encephalitis is the pathological correlate
of dementia in acquired immunodeficiency syndrome. Ann Neurol.
36:673–676. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu W, et al: Chitooligosaccharides and
N-acetyl-D-glucosamine stimulate peripheral blood mononuclear
cell-mediated antitumor immune responses. Mol Med Rep. 6:385–390.
2012.PubMed/NCBI
|
|
18
|
Ciardi A, et al: The involvement of the
cerebral cortex in human immunodeficiency virus encephalopathy: a
morphological and immunohistochemical study. Acta Neuropathol.
81:51–59. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cursio R, Gugenheim J, Ricci JE, et al: A
caspase inhibitor fully protects rats against lethal normothermic
liver ischemia by inhibition of liver apoptosis. FASEB J.
13:253–261. 1999.PubMed/NCBI
|